Back to Journals » International Journal of Nanomedicine » Volume 19
Metal Nanoparticles: Advanced and Promising Technology in Diabetic Wound Therapy
Authors Zheng Q, Chen C, Liu Y, Gao J, Li L, Yin C, Yuan X
Received 9 August 2023
Accepted for publication 14 December 2023
Published 26 January 2024 Volume 2024:19 Pages 965—992
DOI https://doi.org/10.2147/IJN.S434693
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Dr Xing Zhang
Qinzhou Zheng,1,* Cuimin Chen,2,* Yong Liu,3 Jie Gao,2,* Luxin Li,1 Chuan Yin,4 Xiaohuan Yuan1
1College of Life Science, Mudanjiang Medical University, Mudanjiang, People’s Republic of China; 2Changhai Clinical Research Unit, Shanghai Changhai Hospital, Naval Medical University, Shanghai, 200433, People’s Republic of China; 3Center for Comparative Medicine, Mudanjiang Medical University, Mudanjiang, People’s Republic of China; 4Department of Gastroenterology, Shanghai Changzheng Hospital, Naval Medical University, Shanghai, 200003, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Chuan Yin, Department of Gastroenterology, Shanghai Changzheng Hospital, Naval Medical, University, Shanghai, 200003, People’s Republic of China, Email [email protected] Xiaohuan Yuan, College of Life Science, Mudanjiang Medical University, Mudanjiang, 157001, Heilongjiang, People’s Republic of China, Tel/Fax +86-453-6984647, Email [email protected]
Abstract: Diabetic wounds pose a significant challenge to public health, primarily due to insufficient blood vessel supply, bacterial infection, excessive oxidative stress, and impaired antioxidant defenses. The aforementioned condition not only places a significant physical burden on patients’ prognosis, but also amplifies the economic strain on the medical system in treating diabetic wounds. Currently, the effectiveness of available treatments for diabetic wounds is limited. However, there is hope in the potential of metal nanoparticles (MNPs) to address these issues. MNPs exhibit excellent anti-inflammatory, antioxidant, antibacterial and pro-angiogenic properties, making them a promising solution for diabetic wounds. In addition, MNPs stimulate the expression of proteins that promote wound healing and serve as drug delivery systems for small-molecule drugs. By combining MNPs with other biomaterials such as hydrogels and chitosan, novel dressings can be developed and revolutionize the treatment of diabetic wounds. The present article provides a comprehensive overview of the research progress on the utilization of MNPs for treating diabetic wounds. Building upon this foundation, we summarize the underlying mechanisms involved in diabetic wound healing and discuss the potential application of MNPs as biomaterials for drug delivery. Furthermore, we provide an extensive analysis and discussion on the clinical implementation of dressings, while also highlighting future prospects for utilizing MNPs in diabetic wound management. In conclusion, MNPs represent a promising strategy for the treatment of diabetic wound healing. Future directions include combining other biological nanomaterials to synthesize new biological dressings or utilizing the other physicochemical properties of MNPs to promote wound healing. Synthetic biomaterials that contain MNPs not only play a role in all stages of diabetic wound healing, but also provide a stable physiological environment for the wound-healing process.
Keywords: metal nanoparticles, wound healing, diabetic wound, drug delivery, antibacterial activity
Introduction
Diabetes mellitus (DM) is a prevalent endocrine disorder characterized primarily by elevated blood glucose levels resulting from either insulin resistance or diminished insulin secretion.1 The occurrence of DM in the adult population is expected to grow by 69% in developing nations and 20% in affluent countries by 2030 in comparison to 2010.2 The number of Americans with diabetes is expected to triple.3
Prolonged hyperglycemia can cause a variety of organ lesions in diabetic patients, including diabetic nephropathy, skin ulcers, retinopathy, and neuropathy.4 In diabetes, the failure of wound healing is attributed to macrovascular and microvascular lesions, leading to complications that are a major cause of increased diabetes-related morbidity and mortality.5 Moreover, the high glucose environment of the wound makes the wound susceptible to infection, which also leads to slow and worse wound recovery.
The current treatment methods mainly include blood glucose control, surgical debridement, skin transplantation, wound dressing change and other conventional treatment methods. However, conventional treatments only clean the wound and do not effectively promote the healing of diabetic wounds.
Therefore, advancing treatment of diabetic wounds requires the addition of innovative biomaterials and bioactive substances. These novel interventions are designed to accelerate wound healing and minimize unattractive scar formation by means of anti-inflammatory, antioxidant, and antimicrobial agents.6 Among the various emerging technologies, MNPs have shown great potential in promoting diabetic wound healing.7 MNPs effectively promote diabetic wound healing due to their wide range of antimicrobial activities and other biological properties. Moreover, the special physical and chemical properties of MNPs, including their acoustic, optical, and electrical properties, also play an important role in the wound healing measurement process. MNPs also possess properties that differ from nanoparticles, including a large specific surface area, uniform distribution, and the ability to the modify surface morphology. These remarkable properties propel MNPs as an important component in the synthesis of biomaterials for diabetic wound healing.8 In the realm of therapeutic drug delivery, MNPs offer multiple avenues for effective drug administration. They can be dispersed or encapsulated within metal shells, or covalently attached to their surfaces, enabling efficient drug delivery to targeted diseases. Moreover, MNPs also serve as effective multimodal contrast agents, facilitating disease diagnosis through various modalities such as fluorescence and magnetic resonance imaging. These versatile properties of MNPs render them invaluable in both therapeutic and diagnostic applications.9,10 MNPs also facilitate intelligent drug release, such as the ability to be externally triggered by infrared radiation or magnetic fields, thereby achieving multi-stimulus-responsive drug release.11,12 This property provides effective targeting to the complex healing process of diabetic wounds, allowing progress toward accelerated healing at different stages of wound healing.13
Recently, the unique physicochemical properties of MNPs have attracted more and more attention in the study of diabetic wound healing. There are numerous reviews mainly focused on a certain application (anti-inflammatory, antibacterial, etc.) of single MNPs (AgNPs, CuNPs) or summaries of the new preparation technology of certain MNPs (green synthesis), but a comprehensive and systematic review is lacking. We hope to comprehensively and systematically elaborate the role, mechanism and clinical application of MNPs in the treatment of diabetic wounds. To comprehensively elucidate the mechanism of MNPs in diabetic wound healing, we carefully selected MNPs that have been extensively studied and utilized, and systematically summarized the direct pro-healing effects of these MNPs on diabetic wounds as well as their indirect effects when combined with other biomaterials.14–17 Based on these premises, we present a comprehensive review of the latest advancements in MNPs research and elucidate their potential role in enhancing diabetic wound healing through angiogenesis, drug delivery, and intelligent release mechanisms. Furthermore, we summarize and discuss the clinical applications of MNPs along with their associated adverse effects. Finally, propose avenues to deepen the potential of MNPs in the study of diabetic wounds in the future, and make reasonable assumptions.
Wound Healing Mechanisms
Structure and Function of the Skin
The skin is composed of the epidermis and dermis, which sit above subcutaneous tissue. The skin is the body’s primary defense against external pathogens and other factors, making it an important organ. The epidermis, consisting of numerous keratinocytes, serves as a barrier to maintain water balance in the body. The dermis lies between the epidermis and subcutaneous tissue and is composed of connective tissue made of extracellular matrix (ECM) and fibroblasts. Collagen and elastin secretion maintains the mechanical properties and elasticity of the skin. Additionally, the dermis contains blood vessels, nerve endings, hair follicles, and other tissues. The subcutaneous tissue below the dermis is made up of loose connective tissue and fat providing a myxoid nature that permits fluid absorption.18
Wound Healing Process
A skin wound is the destruction of or damage to the normal anatomical structure and function of the skin. The destruction of the integrity of the epithelial cells or the destruction of the full thickness of the skin may extend to deeper structures, such as blood vessels, muscles, etc.19
The key steps in the healing process are shown in Figure 1. Platelet aggregation and inflammation start immediately after injury, and neutrophil infiltrates and macrophage differentiation can be seen at the injury site between days 1 and 4 after injury. On days 4 to 21, granulation tissue and neovascularization are apparent. Collagen and skin remodeling and scar tissue removal occur at the wound site after 21 days. The process can be roughly summarized as the four stages of wound healing, closely linked but overlapping: (1) hemostasis, (2) inflammation, (3) proliferation, and (4) remodeling.20,21
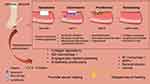 |
Figure 1 Physiological process of wound healing. Comparison of factors promoting wound healing and delaying wound healing. |
Platelets form fibrin clots at the injury site immediately after an injury,22 inducing the synthesis of growth factors and promoting the proliferation and migration of cells.23 Mast cells release serotonin and histamine, triggering endothelial cells to open up their membrane junctions for unrestricted migration of immune cells.24 Platelets release cytokines that contribute to localized inflammation and facilitate the formation of granulation tissue, including platelet-derived growth factor (PDGF), transforming growth factor-β (TGF-β), epidermal growth factor (EGF), and fibroblast growth factor (FGF).25 Macrophages phagocytose microbial debris, secrete various cytokines and growth factors, and stimulate the formation of granulation tissue.26–28 Angiogenesis, fibroblast migration, and ECM production lead to wound healing.24,27
In the proliferative phase, new blood vessels grow from existing vessels, collagen is deposited, granulation tissue forms, cells grow, and the wound heals.29 IL-4 promotes fibroblast differentiation and induces matrix synthesis.30 When the remodeling period begins, the wound begins to contract. The induction of ECM production begins with tissue granulation and collagen degradation and continues with the production of new collagen. Collagen is remodeled and rearranged along the lines of tension. Finally, old fibroblasts are removed by apoptosis, and new fibroblasts and ECM are generated.31
The Process of Diabetic Wound Healing
In normal physiological conditions, that the injured body follows the natural course of wound healing. However, certain circumstances or abnormal factors can disrupt this process, leading to the development of chronic wounds, such as diabetic wounds. Several factors contribute to the transformation of diabetic wounds into chronic wounds that pose challenges for effective healing, including. (1) excessive proinflammatory macrophages, (2) microbial interference, and (3) blocked angiogenesis.32
Macrophages play a key role in wound healing, and abnormal macrophage polarization in diabetic wounds leads to prolonged wound inflammation. Upon traumatic stimulation, the number of macrophages involved in the inflammatory response increases rapidly, and these cells polarize into classically activated M1 (proinflammatory) subtypes or activated M2 (healing promotion) subtypes.33 This heterogeneity in the cell population is due to their different responses to microenvironmental signals, described as macrophage polarization.34 During the initial stages of inflammation, the predominant M1 subtype of macrophages emerges. These M1 macrophages play a vital role in combating bacterial infections, inducing the phagocytosis of pathogens, eliminating neutrophils, clearing tissue debris, and releasing cytokines like tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6). As tissue remodeling progresses, there is a rise in the M2 macrophages populations.35 These M2 macrophages are primarily responsible for releasing growth factors such as TGF-β and insulin-like growth factor, which actively contribute to tissue regeneration and remodeling.36 Furthermore, studies have revealed that the impaired recruitment of M2 macrophages hampers diabetic wound healing, while the extraction of TGF-β from these macrophages promotes both wound healing and angiogenesis. The impaired activation of macrophages by the nuclear receptor PPARγ has been reported in diabetic wounds, leading to the inability of PPARγ to facilitate wound healing through downregulation of pro-inflammatory factors.37 Macrophages isolated from peritoneal exudates in streptozotocin-induced diabetic mice demonstrate reduced TNF-α release and an increased production of nitric oxide (NO).38 These results suggest that hyperglycemia increases the production of M1 macrophages and decreases the generation of the M2 macrophages resulting in a prolonged inflammatory stage and wound healing failure.
Angiogenesis is an important step in wound healing, and the interaction between related cells and cytokines during angiogenesis is the key to successful blood vessel formation.39 Therefore, diabetes-induced angiogenesis impairment is another obstacle to regenerating cells at the wound site. Insufficient wound angiogenesis in people with diabetes is manifested by reduced endothelial cell proliferation and a reduced reaction between cells and growth factors.40 When a wound causes hypoxia, VEGF generated by macrophages, fibroblasts, and epithelial cells actives and phosphorylates eNOS in the bone marrow. This boost in NO levels causes endothelial progenitor cells (EPCs) to mobilize into the bloodstream. The EPCs assist in cellular homing to the site of injury by the chemokine SDF-1α, where they take part in neovasculogenesis.41 Gallagher et al demonstrated that impaired eNOS phosphorylation in the bone marrow and reduced SDF-1α expression in epithelial cells and fibroblasts, observed in a diabetic mouse model (right), resulted in the inability of VEGF to induce eNOS phosphorylation and activation, thereby limiting EPC mobilization from the bone marrow into circulation and reaching the wound site42 (Figure 2). One study revealed that fibroblasts extracted from diabetic mice have a lower migration rate and decreased VEGF expression,43 indicating that hyperglycemia damages cell function and inhibits blood vessel formation. In contrast, impaired angiogenesis in diabetic mice with increased VEGF expression is due to decreased VEGF signal transduction and increased sVEGFR-1 expression, which inhibits angiogenesis.44 Both studies highlight the importance of proper VEGF levels for successful wound healing.45 When VEGF does not operate properly (by decreased expression or decreased signaling), angiogenesis is impaired, and wound healing is delayed.
Common wound treatments that promote rapid healing include controlling infection, promoting skin perfusion recovery, and minimizing inflammation. However, due to the complexity of diabetes, treatment for diabetic wounds is limited. Traditional dressings remain the primary treatment for diabetic wounds, but the treatment process is long, and the prognosis is not optimal. Thus, researchers have aimed to improve diabetic wound healing by introducing MNPs into wound treatment methods. The subsequent section delineates the pivotal role of diverse MNPs in facilitating wound healing, particularly in the context of diabetic wound healing.
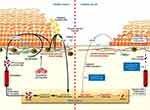 |
Figure 2 Comparison of angiogenesis during wound healing in healthy versus diabetic subjects. Used with permission of American Society for Clinical Investigation, from Brem H, Tomic-Canic M. Cellular and molecular basis of wound healing in diabetes. J Clin Invest. 2007;117(5):1219–1222; permission conveyed through Copyright Clearance Center, Inc.41 |
Research and Application of MNPs in Wound Healing
The repair process of diabetic wounds is characterized by slower and more intricate healing compared to normal wounds, thus necessitating the use of materials that can effectively stimulate rapid and complete healing to promote diabetic wound healing. The materials should additionally demonstrate excellent biocompatibility, be non-toxic to living organisms, and promote a moist wound environment. With the advancement of nanotechnology, MNPs have been demonstrated to possess unique physical and chemical properties that exhibit significant effects on diabetic wound healing. These MNPs directly stimulate wound healing. They also function as indirect carriers in the wound-healing process. Thus, we focus on some of the most commonly studied and clinically used MNPs and their oxides and summarize their effects at the critical stage of wound healing while exploring the potential application of MNPs in combination with other biomaterials as nanomedicine and nanocarriers for treating diabetes wounds.
AuNPs
Because of their plasmon resonance, biocompatibility, and antioxidant and antibacterial capabilities, AuNPs play a unique role in biomedical applications.46,47 The antibacterial properties of AuNPs have been exploited to reduce the chance of diabetic wound infection in the study of materials for enhancing diabetic wound healing. AuNPs are also used as carriers to load genes or growth factors to the cells or even the nucleus of the wound site to create a normal wound-healing environment.
The main antimicrobial mechanism of AuNPs is ATP depletion via membrane potential changes and ATP synthase inhibition, which blocks energy metabolism and leads to bacterial death (Figure 3A).48 According to the study conducted by Cui et al, the presence of AuNPs led to the suppression of oxidative phosphorylation pathways, specifically affecting F-type ATP synthase and ATP levels. The researchers observed a significant reduction in the activity of F-type ATP synthase and the expression of ATP in the membrane proteins of Escherichia coli when exposed to AuNPs. Additionally, other studies have demonstrated that bactericidal antibiotics induce cell death by causing an accumulation of excessive hydroxyl radicals. This pathway is closely associated with oxidative damage and results in the depletion of NADH and the tricarboxylic acid cycle.49 We performed NAD [+] cycling assays and determined ROS levels. The NAD [+]/NADH ratio of AuNP-treated E. coli was almost unchanged from that of untreated E. coli., suggesting that AuNPs kill bacteria without inducing oxidative stress. Oxidative stress can lead to the death of normal cells in the body (Figure 3B). These results indicate that the low cytotoxicity of AuNPs is because AuNPs may kill bacteria through nonoxidative stress pathways rather than oxidative stress pathways.50
 |
Figure 3 (A) Changes in membrane potential and inhibition of ATPase activity led to a decrease in cellular metabolism. (B) AuNPs kill bacteria by inhibiting the enzyme ATP synthase. Notes: (A) Reprinted with permission from Dove Medical Press. Vimbela G, Ngo SM, Fraze C, Yang L, Stout DA. Antibacterial properties and toxicity from metallic nanomaterials. IJN. 2017;12:3941–3965. Creative Commons.48 (B) Reprinted from . 33(7). Cui Y, Zhao Y, Tian Y, Zhang W, X L, Jiang X. The molecular mechanism of action of bactericidal gold nanoparticles on Escherichia coli. 2327–2333, Copyright (2012), with permission from Elsevier.50 |
The incorporation of exogenous reducing agents, capping agents and stabilizing agents during the synthesis of conventional AuNPs introduces potential toxicities or adverse effects that may compromise their utility. Recently, researchers developed a green synthesis method, which permits AuNPs to be synthesized at mild pressures, temperatures, and pH to avoid many harmful properties. The green AuNPs synthesized by Boomi et al, using an aqueous extract of Acalypha indica as a carrier accelerated the inflammatory response and promoted collagen remodeling. The authors also found that the AuNPs synthesized by this method interacted with bacterial cell membranes and exhibited a high antibacterial activity and a high antioxidant activity at a concentration of 100μg/mL. The combined effect of these factors greatly diminishes the likelihood of wound infection and facilitates the process of wound healing.51 Annamalai et al employed a green synthesis method to create AuNPs using an extract derived from the leaves of the medicinal plant Euphorbia hirta. The AuNPs exhibited excellent antibacterial properties against Escherichia coli, Pseudomonas aeruginosa, and Klebsiella pneumoniae.52 In similar studies, AuNPs synthesized from the extract of the leaves of the insulin-like plant Astragalus membranaceus demonstrated similarly good antioxidant activities, and the antioxidant and anti-inflammatory properties increased over time.53 The augmented antibacterial efficacy of AuNPs may be attributed to the synergistic effect between the plant extracts and AuNPs.48 AuNPs synthesized from plant extracts not only contribute to the antibacterial action of AuNPs, but also play different functions in different plant extracts. Due to the diversity of plant extracts, AuNPs synthesized from plant extracts may have different roles. AuNPs can also selectively inhibit lipopolysaccharide-induced (LPS-induced) cytokine production by, downregulating IL-1β-induced epithelial cell proliferation.54 Barathmanikanth created AuNPs using biosynthesis techniques and found that AuNPs (2.5 mg/kg in diabetic mice) created conditions for controlling hyperglycemia by inhibiting ROS formation and lipid peroxidation and scavenging free radicals. The AuNPs synthesized from the extract promoted glucose consumption and produced insulin-like results. Furthermore, the AuNPs demonstrated no toxicity to vital organs and sustained control of blood glucose and lipids.55
Due to the abundance of positive charges on their surface, AuNPs serve as a versatile drug delivery platform, as their surface can be readily modified with various medications. Pornpattananangkul et al developed chitosan-coated AuNPs-stabilized phospholipid liposomes (Vancomycin AuChi-Liposome) as a drug delivery platform for antibiotics (Figure 4A). They combined positively charged AuNPs with negatively charged liposomes to create a stable liposomal composite that prevents them from fusing and reduces the rate at which the encapsulated antibiotics are released. The stability of AuNPs to liposomes leads to the sustained release of vancomycin, thus vancomycin shows a sustained and highly toxic inhibitory effect on bacteria. The α-toxin secreted by S. aureus specifically binds to the outer membrane of susceptible cells.56 The rapid poring of liposomes by the binding of the toxin causes liposome cleavage and triggers the release of the antibiotic (vancomycin) from AuNP-stable liposomes to inhibit the growth of S. aureus.57
Li et al used 4,6-diamino-2-pyrimidinethiol (DAPT)-coated AuNPs (D-Au NPs) to treat intestinal E. Coli-induced bacterial infection.58,59 Due to the negative charge on the surface of proteins, AuNPs can be modified by proteins, stabilize the structure and function of proteins, and be delivered to the site of action. KGF binds to 60 nm gold nanoparticles (GNPs) and poly (ethylene glycol) thiolates to produce KGF-GNPs, which means that KGF can be stably applied to wounds with high efficiency.60 CD spectroscopy has been widely recognized and used as a method to measure changes in protein secondary structure.61 In this study, the KGF secondary structure did not change after KGF-GNP crosslinking, as measured by CD. In addition, KGF molecules are tightly clustered on the surface of GNPs, making it easier to simultaneously target nearby KGF receptors (Figure 4B). The secondary structure of KGF-GNPs exhibits exceptional resilience against harsh conditions, including elevated temperatures, acidic environments, and enzymatic degradation. The slow release rate of KGF, as demonstrated in vitro, showcases its stability and biological activity. When KGF-GNPs were utilized on wounds in diabetic mice, they expedited the healing process and promoted re-epithelialization. The expression of key factors like COL-I, α-SMA, and TGF-β1 were significantly augmented, indicating the positive impact of KGF-GNPs. Wang et al developed a gene delivery system (AuNPs@LL37) by grafting AuNPs onto antimicrobial peptides LL37 as carriers, which acted as both an antimicrobial agent and a nuclear translocation facilitator62 (Figure 4C). The cellular uptake, intracellular distribution, and in vitro bactericidal efficacy of AuNPs@LL37 were investigated to elucidate its mechanism of gene delivery. Incorporating AuNPs into LL37 enhanced the composite stability and antibacterial properties while reducing toxicity. The negatively charged surface of MRSA attracted the complex formed by AuNPs@LL37, leading to observed lesions and collapse on MRSA surfaces. AuNPs@LL37 was also bound with plasmid DNA expressing VEGF forming an effective complex termed as AuNPs@LL37/pDNA. In vivo studies using a diabetic wound healing model showed that the complex formed by AuNPs@LL37/pDNA successfully enhanced wound healing efficacy by promoting increased VEGF expression within nuclei along with accelerated angiogenesis. Similarly, Chen et al demonstrated that AuNPs could be used to promote angiogenesis and for gene delivery. AuNPs instantly open the skin’s cuticle and increase the absorption of specific compounds through the skin. The authors discovered that the combination of EGCG and alpha lipoic acid antioxidants with AuNPs effectively regulated inappropriate inflammation, oxidative stress, and vascular injury. This synergistic activity significantly expedited diabetic wound healing through precise anti-inflammatory and angiogenic modulation, wherein AuNPs functioned as an adjuvant to augment cutaneous absorption and enhance antioxidant efficacy.63
In the aforementioned scenarios, AuNPs were demonstrated to serve a dual role in promoting wound healing. Firstly, they facilitate the efficient healing of infected wounds through their inherent antibacterial properties. Secondly, green synthesis methods employed to synthesize AuNPs using various plant extracts, not only augment their antibacterial activity but also expedite the resolution of inflammation during diabetic wound healing via anti-inflammatory and antioxidant mechanisms. Consequently, this broadens the scope of AuNPs beyond their conventional application as metal-based antimicrobials.
 |
Figure 4 (A) Vancomycin-loaded liposomes protect them by adsorbing chitosan-coated AuNPs on their surfaces to prevent them from fusing with each other or with bacterial membranes. When bacterial toxins bind to liposomes, the toxins create pores in the liposomes and release vancomycin to kill bacteria. Reprinted with permission from Pornpattananangkul D, Zhang L, Olson S, et al. Bacterial toxin-triggered drug release from gold nanoparticle-stabilized liposomes for the treatment of bacterial infection. J Am Chem Soc. 2011;133(11):4132–4139. Copyright © 2011, American Chemical Society.57 (B) Hypothesized mechanism of KGF-GNPs on protein stability. Used with permission of Future Medicine Ltd. Li S, Tang Q, Xu H, et al. Improved stability of KGF by conjugation with gold nanoparticles for diabetic wound therapy. Nanomedicine. 2019;14(22):2909–2923.60 Permission conveyed through Copyright Clearance Center, Inc. (C) AuNPs@LL37/pDNAs preparation and antibacterial diagram; AuNPs@LL37/pDNAs cell uptake and cell-to-cell gene delivery to optimize the therapeutic effect of bacterial infected DFUs. Used with permission of Royal Society of Chemistry. Wang S, Yan C, Zhang X, et al. Antimicrobial peptide modification enhances the gene delivery and bactericidal efficiency of gold nanoparticles for accelerating diabetic wound healing. Biomater Sci. 2018;6(10):2757–2772.62 Permission conveyed through Copyright Clearance Center, Inc. |
AgNPs
AgNPs have become the most widely studied and used MNPs due to their obvious anti-inflammatory properties and effective inhibition of drug-resistant bacteria.64 Numerous studies have described the effect of AgNPs or AgNPs combined with other composite materials on diabetic wound healing. As shown in Table 1, some of these dressings containing AgNPs have been widely used in clinical practice.65 The antibacterial mechanisms of AgNPs encompass the ROS-dependent pathway, protein binding and inactivation, as well as inhibition of DNA replication (Figure 5).17,66 The Ag+ released by AgNPs binds mercaptan to proteins and enzyme sets (-SH) found on the cell surface, leading to cell membrane instability and a disruption of ATP synthesis pathways.67 AgNPs subsequently adhere to the membrane wall, leading to the formation of pores through which they penetrate the bacteria and interact with intracellular components or sulfur-containing proteins.66 The collapse of the proton motive force and inhibition of ATP generation occur upon disruption of the bacterial cell wall and membrane.68
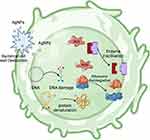 |
Figure 5 Schematic representation of the antimicrobial mechanism of AgNPs, describing the ROS-dependent pathway, DNA damage, protein denaturation and enzymatic inactivation of the antimicrobial action of AgNPs. Adapted from Xu L, Wang YY, Huang J, Chen CY, Wang ZX, Xie H. Silver nanoparticles: synthesis, medical applications and biosafety. Theranostics. 2020;10(20):8996–9031. Creative Commons.17 |
 |
Table 1 The Mechanism Underlying the Diabetic Wound Healing of MNPs |
The Ag+ released by AgNPs causes the intracellular formation of a wide spectrum of membrane-reactive ROS, which eventually causes cell death due to oxidative stress.48 During Ag treatment, Ag+ may produce superoxide radicals (the primary form of ROS) by damaging enzymes in the respiratory chain. This damage may be caused by Ag+-mercaptan interactions.95 The observed mechanism demonstrates the broad-spectrum antibacterial activity of AgNPs against diverse bacterial strains, encompassing both gram-positive and gram-negative bacteria, which possess distinct cell structures.2 The antibacterial activity of AgNPs is attributed to the simultaneous involvement of multiple mechanisms of action. Ag+ demonstrates a remarkable synergistic effect, exhibiting promising antimicrobial activity against a wide range of drug-resistant bacteria.96
To regulate the abrupt discharge of AgNPs, which leads to an elevated local concentration of Ag+ and cytotoxicity, we utilized the gradual degradation characteristics of the hydrogel to achieve a controlled release effect. The chitosan and dextran within the AgNP@CNDM hydrogel create an internal network channel for absorbing wound exudates, while also allowing for the gradual degradation the chitosan to release Ag+ ions that can act on bacterial cell membranes, inhibiting inflammatory responses and accelerating wound healing in diabetic infections.97 Similarly, the chitosan/Ca-AlGNP/AgNP hydrogel also utilizes the biodegradation function of chitosan to gradually release Ca2+ from AgNPs and Ca alginate nanoparticles within the hydrogel, thereby facilitating hemostasis acceleration and achieving continuous sterilization.98 In further investigation, the sustained release properties of the hydrogel were utilized to introduce fresh blood into the chitosan/Ca-AlgNP/AgNP hydrogel. By harnessing the growth factors present in the blood, an artificial enrichment of these growth factors within the hydrogel was achieved, thereby enhancing wound healing99 (Figure 6A–C). Wang et al utilized alginate-based hydrogels containing hemin (protoporphyrin IX with a ferric iron ion) and AgNPs to enhance antibacterial activity and ROS scavenging, while also promoting cell attachment and growth.100 Similarly, AgNPs were synthesized from aloe leaf extracts using biosynthetic techniques. These green MNPs showed satisfactory inhibition of Staphylococcus aureus. A mixture of green AgNPs and photobiomodulation (PBM) encourages cell migration to diabetic wounds and exerts antibacterial effects.70 PBM leads to the proliferation of cell migration activity and increases collagen production in diabetic human skin fibroblasts.101 By activating cellular processes, PBM assists in treating diabetic wounds.72 The biogenic AgNPs also exhibit potent antibacterial activity against the most prevalent wound infection pathogens, namely Pseudomonas aeruginosa and Staphylococcus aureus.
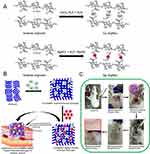 |
Figure 6 (A–C) Illustration of the synthesis of calcium alginate nanoparticles (Ca-AlgNPs) and AgNPs and the promotion of wound healing by combining Ca-AlgNPs and AgNPs on a gel and injecting them into wounds of diabetic mice. Reprinted from Int J Biol Macromol.Volume178. Choudhary M, Chhabra P, Tyagi A, Singh H. Scar free healing of full thickness diabetic wounds: a unique combination of silver nanoparticles as antimicrobial agent, calcium alginate nanoparticles as hemostatic agent, fresh blood as nutrient/growth factor supplier and chitosan as base matrix. 41–52. Copyright © 2021, with permission from Elsevier B.V.99 |
AgNPs facilitate the transition from the inflammatory phase to the proliferative phase of diabetic wounds, thereby promoting wound healing. This is achieved through the suppression of proinflammatory cytokine secretion and expression. Singla and colleagues utilized bamboo cellulose nanocrystals loaded with AgNPs to develop nanobiocomposite (NC) hydrogels, which demonstrated a remarkable ability to expedite wound healing in diabetic individuals. These NCs effectively inhibit the expression of proinflammatory cytokines and growth factors, further enhancing the healing process.71 The acceleration of the healing process can be attributed to a reduction in inflammation, early proliferation, and enhanced collagen formation. In a study conducted by Frankova et al, the effects of AgNPs on normal dermal fibroblasts (NHDFs) and normal epidermal keratinocytes (NHEKs) were examined. The findings revealed that AgNPs have the potential to promote wound healing by suppressing the secretion of pro-inflammatory cytokines such as IL-8, IL-6, IL-12, and TNF-α by NHDFs and NHEKs, particularly when the wound is not infected.102 Rangasamy et al prepared nanoparticles (AgPyNPs) composed of silver-pyridoxine complexes using the antioxidant properties of pyridoxine.103 They observed that AgPyNPs induced interleukin-8 (IL-8) secretion to enhance the immune response of macrophages to eliminate microorganisms. At the same time, the antioxidant properties of pyridoxine reduce cytotoxicity by reducing the production of ROS in macrophages. In addition, AgPyNPs induce IL-8 to increase wound reepithelialization by stimulating keratinocyte proliferation and migration.72 Therefore, we speculate that AgNPs accelerate the wound healing rate in a hyperglycemic environment where the wound site poorly heals heal due to a prolonged inflammatory period.
Compared with other MNPs, AgNPs are the most widely used in wound healing and entered clinical use earlier. Their excellent antibacterial activity, especially their ability to inhibit drug-resistant bacteria, makes AgNPs widely popular. However, with the deepening of other MNP studies, AgNPs play a single role in diabetic wound healing. However, Fathil et al successfully formed AgLTF by complexing AgNPs with LTF and utilized this for the targeted therapy of diabetic wounds by loading DsiRNA.104 The negatively charged DsiRNA was adsorbed onto AgLTF to silence the prostaglandin transporter (PGT) and promote wound healing. Although this study did not employ AgNPs as a drug carrier, the successful adsorption of DsiRNA onto AgLTF demonstrates the potential use of AgNPs as carriers in future applications beyond their antibacterial properties. In the future, AgNPs have the potential to serve as highly efficient antibacterial agents and carriers for protein or nucleic acid adsorption, thereby modifying the wound microenvironment and facilitating diabetic wound healing.
CuNPs
The application of Cu2+ in biomaterials has a long history, beginning with contraceptive IUDs. Cu2+ demonstrates antibacterial properties similar to those of many other metal ions,105 making them widely applicable. It can be argued that rapid control of wound infection can also promote faster healing. Chatterjee reported a novel method for synthesizing stable CuNPs that are highly efficient at bacterial cell filamentation and cell killing. In addition to inducing membrane lysis and intracellular production of reactive ROS that inhibit bacterial growth and division, CuNPs contribute to the remodeling of bacterial lipopolysaccharides and promote antibacterial membrane activity by reducing cell–cell aggregation and matrix instability.74 Different CuNP synthetic methods and conditions produce NPs with variable antibacterial effects. Furthermore, the antibacterial efficacy of CuNPs was significantly augmented by the photothermal response of Cu.
Veerapandian et al successfully synthesized mixed glucosamine-functionalized CuNPs (GLCN-CuNPs) and observed that the antibacterial activity of GLCN-CuNPs surpassed that of conventional CuNPs. The in vitro antibacterial activity of GLCN-CuNPs under UV irradiation was higher than that of GLCN-CuNPs and kanamycin without UV exposure. These results may be due to the modification of GLCN-CuNPs surfactant metal nanometers, promoting the production of oxygen-free radicals and their derived structural activities.75 Similarly, the concentration and size of the crystal structure of CuNPs also impact the antibacterial activity. Hassan et al observed increased bacterial inhibition with increasing concentrations of CuO nanocrystals, which are known to produce ROS. These ROS interact with the cell membrane to produce free radicals, which enter the cell and damage its internal contents. Eventually, this ROS disruption causes bacterial cells to leak.106 The antibacterial effects of CuONPs and CuNPs are also quite different. The antibacterial activity of CuNPs surpasses that of CuONPs, potentially due to the enhanced electron transfer between bacteria and CuNPs. The interaction between MN bacteria and positively charged MNPs facilitates electron transfer and disruption of bacterial membranes. Akhavan et al compared the interaction between CuONPs and CuNPs on bacteria and found that CuNPs were more successful in inhibiting bacterial growth. More direct interactions between CuNPs and bacterial strains may lead to greater infiltration and rupture of bacterial membranes. Cell membrane rupture can lead to cellular enzyme dysfunction and, ultimately, cell death.107 CuONPs synthesized via a green method using Ficus religiosa leaf extract show long-lasting inhibitory activity against human pathogens. CuONPs also inhibit the survival of pathogenic bacteria in albino rat wounds and enhance wound healing activity.108 Studies on the antibacterial properties of CuNPs reveal that CuNPs induce bacterial membrane lysis and promote ROS production in bacteria through the action of positively charged CuNPs and negatively charged bacterial membranes. The photothermal responsiveness of Cu may also catalyze the surface activity of CuNPs under UV light to enhance their antimicrobial activity.
Metal-organic frameworks (MOFs) have emerged as promising drug delivery carriers in biomedicine, thanks to their remarkably porous internal structure. However, their potential application is hindered by their inherent instability in protein solutions, posing challenges to their effective utilization.109 Zhang et al used the high porosity of HKUST-1 to adsorb NO and created a micropatterned scaffold containing NO@HKUST-1 (NO@HPG scaffold) in the core layer of the nanofibers to stably load NO onto HKUST-1 (Figure 7C and D). They found that NO@HPG scaffolds released Cu2+ and NO at sustained low concentrations, synergistically promoting hematopoiesis and collagen deposition. Simultaneously, the release of Cu2+ and NO from the NO@HPG scaffold attenuated the inflammatory response and facilitated the transition of wounds from the inflammatory phase to the proliferative phase (Figure 7E).77,110 Hyperglycemia in diabetic patients can impede endogenous NO synthesis, thereby contributing to the delayed healing of diabetic wounds.111 Therefore, augmenting endogenous NO synthesis or administering exogenous NO can hinder inflammation, foster angiogenesis and stimulate collagen deposition during the process of diabetic wound healing.112 Xiao et al synthesized HKUST-1 NPs embedded in PPCN (H-HKUST-1) by embedding Cu metal-organic frameworks (HKUST-1 NPs) into an antioxidant thermoresponsive citrate-based hydrogel (PPCN). This hydrogel effectively reduced the abrupt release of Cu2+ and cytotoxicity. Hydrogels also increase the stability of HKUST-1 NPs in physiological protein solutions, allowing them to be applied without losing their function due to premature degradation. In addition, the slow release of Cu2+ from H-HKUST-1 stimulated wound angiogenesis and promoted collagen synthesis and deposition in diabetic mice, thereby increasing epidermal regeneration (Figure 7A).113 Zhang et al demonstrated that surface coating of Cu-based MOF effectively retarded the release of Cu2+ and enhanced the in vivo stability of Cu-based MOF within a microenvironment.114 In a subsequent study, surface modification techniques were used to increase the hydrophobicity of Cu-based metal-organic frameworks (Cu-MOFs) to ensure the stability of Cu-MOFs in protein solutions. They therefore used folic acid to modify the surface of Cu-MOFs to increase the hydrophobicity of Cu-MOFs and synthesized folic acid-modified HKUST-1 (F-HKUST-1). The study demonstrated that modification with folic acid not only achieved a slow release of Cu2+ but was also less cytotoxic and safer than the conventional fluorine-containing hydrophobic coating F-HKUST-1. Based on the performance of the HKUST-1 NPs described above, F-HKUST-1 also promotes angiogenesis and collagen synthesis in wounds (Figure 7B).115 To facilitate angiogenesis during the early stages of wound healing, HKUST-1 was engineered by incorporating biotin and combining it with an acellular dermal matrix (ADM) scaffold to create a specialized biological niche for cellular attachment and proliferation (known as ADM-B-HKUST-1), which serves as a conducive environment for hosting mesenchymal stem cells (MSCs). By releasing Cu2+ and promoting MSC adhesion, ADM-B-HKUST-1 demonstrates potential in addressing angiogenic disorders associated with diabetic wounds.116 Stabilizing the CuNPs structure by embedding it in hydrogels or modifying the surface of MOFs to release Cu2+ slowly stimulates collagen deposition at the wound site at a more suitable concentration than traditional CuNPs. Therefore, the Cu-based organic framework serves as a dual-functional platform for wound healing; not only does it act as a drug carrier to promote wound healing, but the released Cu2+ participates in treating the refractory wounds associated with diabetes mellitus (Figure 7C). In future studies, this vector can be used to load proteins, growth factors, genes or even living cells to increase the range of applications of Cu-based MOFs.
 |
Figure 7 (A) Schematic illustration of PPCN binding to H-HKUST-1 and its effect on the wound. Adapted from Xiao J, Chen S, Yi J, Zhang HF, Ameer GA. A cooperative copper metal-organic framework-hydrogel system improves wound healing in diabetes. Adv Funct Mater. 2017;27(1):1604872113 © 2016, WILEY - VCH Verlag GmbH & Co. KGaA, Weinheim.Permission conveyed through Copyright Clearance Center, Inc. (B) Comparison of wound healing between folic acid modified HKUST-1 and the other two groups. Reprinted with permission from Xiao J, Zhu Y, Huddleston S, et al. Copper metal-organic framework nanoparticles stabilized with folic acid improve wound healing in diabetes. ACS Nano. 2018;12(2):1023–1032. Copyright © 2018, American Chemical Society.115 (C and D) The NO@HPG scaffold promotes angiogenesis, collagen deposition and inhibition of the inflammatory response in diabetic mice through the slow release of Cu2+ and NO. A NO sustained-release system with core-shell structure was designed by electrospinning using a Cu-based organic‒organic framework (HKUST-1) loaded with NO. To promote wound healing in diabetic mice. (E) Synthesis of NO@HKUST-1 and synthesis of NO@HKUST-1/PCL/Gel (NO@HPG) scaffolds acting on wounds. Figures 7C-E were Reprinted with permission from Zhang P, Li Y, Tang Y, et al. Copper-based metal-organic framework as a controllable nitric oxide-releasing vehicle for enhanced diabetic wound healing. ACS Appl Mater Interfaces. 2020;12(16):18319–18331. Copyright © 2020, American Chemical Society.110 |
Cu2+ exhibits the potential to stimulate angiogenesis117 and augment the expression of skin-related proteins in diabetic wounds.118 Huang et al utilized electrospinning to fabricate a radially structured fibrous membrane composed of a blend of polycaprolactone (PCL) and polyvinylpyrrolidone (PVP), incorporating copper peroxide nanoparticles (CPs) as an agent for chemo-kinetic therapy (CDT).119 The most innovative aspect lies in the in-situ generation of H2O2 within the acidic environment of diabetic wounds for the Fenton reaction, which effectively eliminates wound bacteria. Simultaneously, Cu2+ released enhances in vitro angiogenesis by modulating human umbilical vein endothelial cell (HUVEC) behavior. Alizadeh et al demonstrated that at non-toxic concentrations, CuNPs promoted endothelial cell proliferation and migration.79 As previously stated, the thermal sensitivity of Cu enhances its antibacterial properties and facilitates the healing process of wounds. Could the photothermal responsiveness of Cu also stimulate wound proliferation and angiogenesis by stimulating the rapid release of Cu2+? Tao et al developed a composite hydrogel composed of methacrylate-modified gelatin and N, N-bis (allyl) cysteamine (BACA)-chelated CuNPs synthesized via radical polymerization and photosensitization. Using the local surface plasmon resonance (LSPR) effect, CuNPs effectively converted energy from near-infrared laser irradiation (808 nm) into local heat for photothermal treatment (Figure 8A). The photothermal properties of CuNPs permit the rapid release of antibacterial Cu2+. In addition, the Cu2+ that is released has the potential to promote the proliferation of NIH-3T3 fibroblasts and facilitate neovascularization, while minimizing any inflammatory reactions73 (Figure 8B). Exploiting thermal properties to improve wound healing is an active area of research. For example, Wang et al created a tissue-engineered membrane to treat tumors and regenerate skin tissue. Using an improved electrostatic spinning method, the authors incorporated Cu2S nanoflowers into biopolymer fibers and successfully prepared micropatterned nanocomposite films. The Cu2+ released after the degradation of polymer scaffolds promoted skin cell growth and capillary formation.120 VEGF expression is known to be inhibited in diabetic wounds.121 Enhancing the Cu2+ concentration at the site of injury stimulates VEGF expression to facilitate angiogenesis and promote connexin expression during the process of wound healing.76 For example, Borkow et al prepared wound-healing dressing in CuO that induced the production of VEGF and HIF-1α. VEGF plays a significant role in promoting angiogenesis, while HIF-1 aids in the induction of protein expression related to the formation of the vascular network and wound recovery.122 Cu also contributes to wound healing wounds by promoting the stability of ECM protein synthesis, including fibrinogen and collagen.78
In comparison to other MNPs, CuNPs exhibit the ability to promote angiogenesis, reduce wound susceptibility, and significantly enhance diabetic wound healing. Unlike AuNPs and AgNPs, which facilitate wound healing by eradicating bacteria, CuNPs not only possess antibacterial properties but also directly stimulate VEGF expression to expedite wound healing. The utilization of Cu-based MOFs as drug delivery carriers or photothermal therapy for augmenting the antibacterial activity of CuNPs can expand their application to promote wound healing.
 |
Figure 8 (A) The CuNPs embedded in the hydrogel (BACA/Cu NPs/Gel‐MA hydrogel) generate ROS combined with photothermal treatment to kill bacteria and promote wound healing. (B) Schematic diagram of CuNPs embedded in hydrogels and synergistic antibacterial activity by photothermal therapy and ROS production under visible light irradiation at 808 nm. Used with permission of Royal Society of Chemistry from Tao B, Lin C, Deng Y, et al. Copper-nanoparticle-embedded hydrogel for killing bacteria and promoting wound healing with photothermal therapy. J Mater Chem B. 2019;7(15):2534–2548; permission conveyed through Copyright Clearance Center, Inc.73 |
ZnNPs
Zinc oxide nanoparticles (ZnONPs) influence diabetic wound healing by at least two mechanisms. They exert hypoglycemic effects and demonstrate the same antibacterial, antioxidant, and anti-inflammatory effects as other MNPs.
Zn promotes liver sugar production by acting on the insulin signaling pathway, thereby enhancing glucose utilization. Furthermore, it inhibits intestinal glucose absorption, thus reducing blood glucose and inhibiting glucagon secretion,123 reducing gluconeogenesis and glycogenolysis. Umrani et al developed a drug containing ZnONPs that had a significant antidiabetic effect, improving glucose tolerance, increasing serum insulin, and lowering blood glucose.124 Research has indicated that the promotion of wound angiogenesis, re-epithelialization, and keratinocyte migration can be facilitated by Zn.90
Yadav et al created ZnONPs via a green synthesis method using Portulaca Oleracea extract and studied their effect on wound healing. ZnONPs showed strong antioxidant and anti-inflammatory activities in vitro. Because of the reduced oxidative stress and inflammatory response, the topical administration of ZnONPs to damaged dermal tissue promoted the rapid synthesis and deposition of collagen fibers, as well as the restoration of tissue granulation and epithelial lining, resulting in faster wound closure and healing.125 The size of ZnONPs may affect their antibacterial activity84 by altering their ability to disrupt the bacterial cell membrane and promote oxidative stress in bacteria.126 Synthesized ZnONPs (with an average particle diameter of approximately 12 nm) inhibit 100% of bacterial growth at a concentration of 1.0×10−2 to 3.0×10−3 M. This is a result of the E. coli membrane destruction, causing an increase in permeability and resulting in the accumulation of NPs within both the bacterial membrane and cytoplasmic regions.84 ZnONPs also inhibit and inactivate Campylobacter jejuni cell growth, possibly via the destruction of the Campylobacter cell membrane and oxidative stress.85 The ZnONPs-loaded sodium alginate-gum acacia hydrogels (SAGA-ZnONPs), formed by crosslinking the hydroxyl groups of sodium alginate and gum acacia polymers with glutaraldehyde’s aldehyde group, exhibit potent antibacterial activity and facilitate fibroblast migration at the appropriate concentrations.127
ZnONPs may also demonstrate anti-inflammatory properties during infection or after chemical exposure.86 They exert antibacterial activity via adsorption-induced membrane damage and ROS-mediated cytotoxicity.128 The anti-inflammatory activities of ZnONPs include decreasing mast cell proliferation and LPS-induced COX-2 production and blocking the expression of proinflammatory factors. ZnONPs also inhibit caspase-1, an IL-1 converting enzyme that promotes mast cell activity.87 The generation of malondialdehyde, a well-known indicator of oxidative stress, is also inhibited by ZnONPs in a dose-dependent manner.86 ZnNPs inhibit mast cell proliferation by regulating p53 protein levels. Prolonged p53 activation is demonstrated to induce tumorigenic inflammation through the release of high-mobility histone 1.88 ZnONPs exhibit a dose-dependent inhibition of COX-2 activation in LPS-treated macrophages. LPS promotes the release of prostaglandin E2 (PGE2), a promoter of inflammation, by activating COX-2 gene expression. However, ZnONPs demonstrate no direct effect on PGE2 expression. Likewise, ZnONPs effectively suppress the production of macrophage-derived nitric oxide (NO) by downregulating the expression of inducible nitric oxide synthase, thus mitigating the potentially harmful effects of inflammatory responses on cells. In a study conducted by Nagajyothiet et al, ZnONPs were synthesized using the root extract of Paeonia tenuifolia, revealing that ZnONPs not only enhanced the generation of oxygen free radicals but also inhibited the expression of proinflammatory factors. These findings indicate that ZnONPs possess remarkable anti-inflammatory and antioxidant properties, thereby promoting the cellular activities associated with wound healing.128
Compared to other types of MNPs, ZnNPs not only effectively eliminate bacteria to facilitate wound healing but also expedite the inflammatory phase by inhibiting the expression of pro-inflammatory factors, thus accelerating the overall wound-healing process. Moreover, ZnNPs also play a vital role in biomaterial synthesis. Qian et al engineered a hydrogel using glycyrrhizic acid (GA) and induced the self-assembly of ZnNPs, combined with photo-crosslinked methyl acrylated silk fibroin (SF) to create a promising biomaterial.89 This hybrid hydrogel enhances mechanical properties and injectability, and is notable for its non-cytotoxic formulation achieved by incorporating ZnNPs. The addition of ZnNPs further allows for leveraging their anti-inflammatory properties to regulate macrophage polarization. Hu et al via a carrier-based approach, utilized a zinc-based nanoscale metal-organic framework (NMOF) to develop an ROS-responsive hydrogel. This hydrogel encapsulated BR@Zn-BTB nanoparticles, resulting in an improved drug loading capacity and controlled release of both the drug and Zn2+.59 This approach synergistically enhances the antibacterial and anti-inflammatory effects of ZnNPs and BR.
Based on the studies mentioned above, the incorporation of ZnNPs not only enhances the safety and immunomodulatory functions of biomaterials but also promotes wound healing through synergistic drug action. Thus, ZnNPs promote diabetic wound healing. In addition, because of the special role of Zn in the insulin pathway, ZnNPs can be used to change the blood glucose environment at the site of diabetes to promote wound healing. This is a method that should be developed in future research.
TiNPs
The photocatalytic properties of TiNPs, specifically TiO2, are responsible for its antibacterial effects. When exposed to ultraviolet light, TiO2 generates ROS that effectively eliminates 100% of microorganisms. Additionally, the growth and viability of bacteria are influenced by positively charged nanomaterials through alterations in their permeability. The bactericidal action is further facilitated by the highly reactive ROS produced during the photocatalytic processes involving TiO2.80 nTiO2/PU nanocomposite films produce hydroxyl radicals when exposed to the photocatalytic activity of sunlight. Hydroxyl free radicals kill bacteria via CoA reduction, but nTiO2/PU nanocomposite films do not show antibacterial activity in the absence of illumination.81 The investigation of nanocatalysts represents a novel and promising research area in the field of wound healing and remodeling. Lopez-goerne et al synthesized TiO2–SiO2 submicron mesoporous particles with varying sizes and shapes via the sol-gel method, which contained ultras mall platinum nanoparticles possessing catalytic properties. These nanoparticles stimulated althy cell proliferation while simultaneously combating multiple bacterial infections. In addition, the catalytic nanoparticles speed up wound healing, which could reduce the risk of delayed diabetic wound healing leading to amputation.129
Upon encountering foreign bacteria or pathogens, Toll-like receptors (TLRs) present on the surface of titanium dioxide nanoparticle (TiO2NP) platelets are triggered. TLR stimulation enhanced the proinflammatory response of platelets and the expression of p-selectin.82 PSG1 (P-selectin glycol protein) is produced by neutrophils and monocytes and interacts with p-selectin to create O2 (a reactive oxygen species) in macrophages, resulting in oxidative stress. Studies reveal that blood platelet counts decrease after incubation with TiO2NPs.82,83 In contrast to other MNPs, the antibacterial action of TiNPs is via photocatalysis and promotes the proinflammatory response of platelets to kill bacteria. TiNPs may therefore have low biotoxicity in normal cells. Thus, TiNPs should be more widely used in the study of promoting wound healing.
FeNPs
The antibacterial mechanism of iron oxide nanoparticles (FeONPs) may be related to the ROS production induced by FeO. The vitamin B2-functionalized FeO nanozyme trio functions to decrease bacterial viability while also scavenging ROS to protect cells from oxidative damage.92 These properties are extremely beneficial in controlling bacterial infections during wound healing. Arakha et al investigated the impact of the FeONP-bacterial interface interaction mode on antimicrobial susceptibility to FeONPs, and found that chitosan-coated FeONPs exhibited significantly enhanced antibacterial activity. It was also revealed that the FeONP-chitosan coating enhanced ROS production at the interface, thus providing antibacterial activity.91
FeONPs have also demonstrated magnetic and catalytic capabilities.130 Exosomes derived from stem cells have been identified as potent mediators that enhance wound healing by promoting angiogenesis and re-epithelialization through the modulation of miR-21-3p.131 Moreover, MNPs such as Fe3O4 nanoparticles and Fe2O3 nanoparticles are magnetic, exerting a weak magnetic force on cells under a static magnetic field and promoting tissue regeneration.132 Wu et al used mesenchymal stem cells from bone marrow to create novel exosomes and confirmed that the amplification of miR-21-5p increased angiogenesis and glial cell migration in the presence of Fe3O4 nanoparticles and a static magnetic field.133
CeO2NPs
Cesium oxide NPs (CeO2NPs) has the same broad-spectrum antibacterial activity as other MNPs. Positively charged CeO2NPs adsorb well to negatively charged bacterial cells and penetrates into them and inactivates bacterial enzymes, resulting in the production of ROS. ROS further damages nucleic acids and proteins, eventually leading to bacterial cell death.134 When encountering polysaccharide-encapsulated bacteria, CeO2NPs interacts with the extracellular bacterial environment and produces ROS, which directly destroys the cell wall and enters the cell to inactivate nucleic acids and proteins for bactericidal purposes.135
CeO2NPs are known as free radical scavengers.93 Ghaznavi et al discovered that CeO2NPs protect against the oxidative stress caused by high glucose levels.136 Chigurupati found that a local application of water-soluble CeO2NPs increased the proliferation and migration of fibroblasts and keratinocytes in mice, expediting entire cutaneous wound healing. Water-soluble CeO2NPs also infiltrated the wound tissue and reduced oxidative damage to cell membrane proteins.94 Hirst et al revealed that CeO2NPs scoured ROS and inhibited the production of inducible nitric oxide synthase (iNOS), an inflammatory mediator, in J774A.1 mouse macrophages.137 We speculate that the antioxidant mechanism of CeO2NPs occurs because the nanoparticles act as direct antioxidants, limiting ROS production, or that these nanoparticles may directly reduce ROS production levels.
Similar to CuNPs, CeO2NPs can also induce the formation of blood vessels in wounds. Das et al demonstrated that the small size of CeO2NPs and their higher surface concentration of Ce3+/Ce4+ promoted the development of tubular structures in endothelial cells. They also observed that CeO2NPs decreased intracellular oxygen levels while temporarily stimulating HIF-1α to translocate into the nucleus and up-regulate the expression of angiogenic proteins.94 Augustine et al developed electrospun poly (3-hydroxybutyrate co-3-hydroxyvalerate) PHBV membranes containing different concentrations of CeO2NPs (0.5%, 1%, 2%, or 4%w/w) for the treatment of diabetic wounds. They found that CeO2NPs in PHBV/nCeO2 significantly increased angiogenesis and facilitated keratinocyte migration.138
Adverse Effects of MNPs
One challenge remaining in MNP research is biotoxicity. If the concentration of metal ions is too high or there is a sudden release of many metal ions, there will be high cytotoxicity and irreversible damage to the liver and kidney. During the treatment of diabetic wounds, long-term skin contact may cause MNPs to enter the blood circulation and eventually deposit in the liver and kidney organs. A large accumulation of MNPs can trigger the inflammatory response and oxidative stress of normal liver and kidney cells, leading to cell death.139,140 Chen et al observed elevated serum aspartate aminotransferase (AST) and alanine transaminase (ALT) levels in mice treated with TiO2NPs at high doses, leading to liver cell necrosis, apoptosis, and liver fibrosis.141 The serum ALT level in mice treated with ZnNPs increased significantly, indicating liver necrosis.142 Jain et al reported that 24 hours after injecting FeO into male Sprague–Dawley rats, serum AST levels significantly increased, leading to adverse liver reactions after acute exposure.143 Nosrati et al identified a potential nephrotoxic risk following repeated oral administration of AgNPs in mice.144 Subsequent staining of the kidneys from mice exposed to varying doses of AgNPs for 28 consecutive days revealed infiltration of inflammation into the capillaries within the kidney tubules, which was accompanied by an increased expression of genes associated with apoptosis in renal interstitial and epithelial cells. These findings suggest that the accumulation of high levels of AgNPs may induce inflammatory reactions with normal cells, leading to nephrotoxicity. This underscores the importance of strict dosage control when utilizing MNPs. There are also many studies evaluating the toxicity of CeO2NPs.These studies reveal that long-term exposure to high concentrations of CeO2NPs resulted in genetic damage (DNA damage to white blood cells and hepatocytes) and biochemical changes (changes in serum lactate dehydrogenase and reduced glutathione levels in the brain) in rats.145,146
Application of Metal-Based Nanoparticles to Biomaterials
As highlighted earlier, the delayed healing of diabetic wounds is attributed to the multiple stages involved in wound healing. Therefore, a dressing for diabetic wound healing needs to fulfill multiple functions simultaneously and prevent excessive accumulation of MNPs. Numerous studies have reported that hydrogels and microneedles can achieve controlled and sustained release of drugs and MNPs, thereby reducing the concentration of metal ions at the wound site. Furthermore, we have comprehensively summarized the utilization of MNPs as nanocarriers for the treatment of diabetic wounds. MNPs exhibit the ability to encapsulate drugs and synergistically collaborate with therapeutic agents, thereby enabling precise drug delivery and multifunctionality in wound dressings.
Dressings Containing Metal-Based Nanomaterials
Hydrogels
Hydrogels, which are formed by the physical or chemical cross-linking of 3D polymer networks, exhibit high absorptivity and adjustable properties, rendering them optimal carriers for drug delivery systems.147 By modifying the properties of hydrogels, the rate of drug release can be effectively controlled, enabling the release of MNPs at non-cytotoxic concentrations. Hydrogels are also one of the most commonly used dressings for wound healing. The cytotoxicity caused by the sudden release of MNPs is a major challenge in the treatment of diabetic wounds with MNPs. To control the sudden release of MNPs, which leads to the increase of local MNP concentration and cell poisoning death, we use the slow degradation characteristics of hydrogels to MNP achieve the effect of slow release.
The aforementioned p(NIPAM-co-hemin)/ALG-EDA hydrogel,100 AgNP@CNDM hydrogel,97 the SF/GA/Zn hybrid hydrogel89 and chitosan/Ca-AlgNP/AgNP hydrogel98 demonstrate the capability of sustained release, which ensures a suitable concentration of metal ions for bactericidal activity in wounds. Simultaneously, these hydrogels exhibit an enhanced mechanical strength and adhesion rate, enabling optimal wound coverage and continuous moisturization to promote wound healing. Moreover, these hydrogels not only facilitate the controlled release of metal ions but also safeguard the structural integrity of MNPs. The discussions on HKUST-1 NPs113 and BR@Zn-BTB/Gel59 reveal that the hydrogel not only preserves the structural integrity of MNPs but also facilitates MOFs to accommodate more drugs, thereby improving the drug loading efficiency and enabling the controlled release of metal ions. Furthermore, the hydrogel enhances the therapeutic efficacy of diabetic wounds treated with MNPs and drugs.
Microneedles
Diabetic wounds are susceptible to infection, and the prolonged infectious state facilitates bacterial proliferation and the formation of bacterial biofilms within the wound.148 Bacterial biofilms are self-synthesizing substrates of hydrated extracellular polymeric substances (EPS) produced by bacteria.149 The presence of biofilm hinders the efficacy of conventional antibacterial methods, such as gels, fiber membranes, and creams, to eradicate bacteria residing within the biofilm.150 Therefore, we chose to take advantage of a new penetrating drug delivery method, namely the physical penetration of biofilms, to deliver antibacterial agents to the bacterial community to achieve the bactericidal effect.151
Bacteria-sensitive nanoparticles (NPs) and doxycycline (DOX) loaded in soluble microneedles (MNs) have been used to treat wound infections.152 The MNs penetrate the biofilm, and as the MNs dissolve, the internal NPs and DOX are released. Inspired by this, other researchers have combined the antibacterial properties of MNPs with microneedles, using microneedles to precisely deliver MNPs to wound infections. For instance, Permana et al synthesized AgNPs using chitosan-modified green tea extract and assembled them into soluble microneedles. Compared with the control cream containing AgNPs, the number of bacteria in the infected wound was significantly reduced, which effectively achieved the purpose of sterilization and promoting wound recovery.148 Yang et al utilized hyaluronic acid (HA) microneedles incorporating cerium/zinc-based nanomaterials (ZCO) for the treatment of diabetic wounds. Upon penetration of the biofilm, the Zn2+ and Ce3+/4+ ions within the microneedle are gradually released through the action of hyaluronidase in the wound, thereby exerting bactericidal effects while also exhibiting scavenging properties against ROS. This dual functionality effectively mitigates inflammatory reactions within the wound, facilitating wound remodeling and cellular proliferation, ultimately leading to a significantly accelerated healing rate in diabetic wounds.153 Zeng et al similarly used HA microneedles as a tool for penetrating infected wound biofilms, which were loaded with dimethylglyoxyglycine (DMOG) encapsulated in PCN-224 MOF nanoparticles (DMOG@PCN-224 NPs) and co-loaded with antibiotic meropenem (MEM). The laser-activated PCN-224 NPs (Zr6) converted O2 to a single oxygen state (1O2) and acted together with MEM to sterilize the bacteria, greatly reducing the antibiotic dose without reducing the bacterial killing effect. Over time, DMOG@PCN-224 NPs gradually degrade to release DMOG, which competitively inhibits prolyl hydroxylase and promotes wound tissue remodeling and angiogenesis.154 The use of microneedles might increase the penetration of MNPs into the wound. Due to the safety of microneedles, there is no need to worry about the biotoxicity of MNPs to the user.151
Biomaterials for MNPs Combined Drugs
The remarkable attributes of high surface area, photothermal responsiveness, and versatility enable the physical or chemical cross-linking of MNPs with other biomaterials to form drug-delivery carriers.155 MNPs deliver the drug to the wound after loading the drug, and play a synergistic role with the drug to promote wound healing when the drug is released. MNPs are useful for the delivery of drugs, small molecular proteins, nucleic acids, etc.156
The growth factor KGF was loaded with AuNPs as a carrier to produce KGF-GNPs nanoparticles,61 which enabled KGF to maintain biological activity under a harsh environment and significantly accelerated the reepithelialization of diabetic wounds. AuNPs@LL37 binds to plasmid DNA to form AuNPs@LL37/pDNAs,62 and VEGF is successfully transfected, while AuNPs still play the role of the antibacterial agent. More typical are MOF applications, such as NO@HKUST-1,77,110 ADM-B-HKUST-1,116 and BR@Zn-BTB.59 By modifying the surface of MOFs, large pores in MOFs can be loaded with NO, drugs and even living cells. MOFs serve as a carrier platform to deliver these agents to the corresponding location in the wound and release the agent in a controlled manner to enhance the healing ability of the wound. Yu et al constructed a nanocarrier using Haase and 3-aminophenylboronic acid (APBA), which can be loaded with gentamicin (Gen) to form HB/Ag/g nanoparticles after the addition of AgNPs. AgNPs and Gen are simultaneously released at the wound site, acting synergistically on the infected wound. The simultaneous use of AgNPs and Gen not only reduces the amount of AgNPs but also enhances the antibacterial activity of this nanoparticle.157
The Mechanism Underlying the Diabetic Wound Healing Ability of MNPs
MNPs have several therapeutic effects on diabetic wounds, including promoting wound angiogenesis and exhibiting antibacterial, anti-inflammatory, and anti-oxidative properties. Numerous studies have demonstrated the exceptional performance of metal-based nanomaterials in these aspects through various means (Table 1) (Figure 9).
Antioxidant Activity
Bacteria can cause wound infections and delay healing by increasing proinflammatory cytokines.16,158 MNPs can come in contact with bacteria and cause death.74,159 MNPs, in particular, have advantageous physicochemical properties that result in high antibacterial activity.48 According to the above, we summarized the antibacterial function of biomaterials containing MNPs. Examples include HPAMAM-N(CH3)2/AuNP,69 vancomycin AuChi-liposome,56 chitosan/Ca-AlgNP/AgNP hydrogel,99 Ag/PAAc nanogel,160 GLCN-CuNPs75, CuONPs107, ZnONPs,84 SAGA-ZnONPs,127 nTiO2/PU nanocomposite films,129 and FeONPs.92 These contain MNPs that interact with bacterial cell walls, significantly increasing the permeability of bacterial cell membranes. Eventually, the bacteria die because they cannot regulate membrane transport properly.161 Some researchers have innovatively applied photothermal therapy (PTT) to effectively eliminate bacteria and promote wound healing.120 He et al, utilized polyhexamethylenediuanidine (PHMB) to modify AuNPs. In the near-infrared light, the formation of bacterial biofilm was synergically inhibited, and the bacteria were quickly removed to heal the wound.59 Molybdenum bronze nanoparticles (SMB NPs) with sonodynamic therapeutic effects were used in combination with a second near-infrared (NIR-II) photothermal effect.162 NIR-II stimulation of SMB NPs produced an extremely strong oxidase mimetic activity and photothermal conversion efficiency, and promoted bacteria to produce excessive reactive oxygen species to eliminate bacteria in deep infected wounds. The same monolayer HE MXenes prepared from transition metals with high entropy and low Gibbs free energy showed extremely strong bactericidal effects and minimal side effects after irradiation through NIR-II.163 The implementation of external stimuli, such as light, heat, and sound, to modify the microstructure of MNPs significantly amplifies their bactericidal effect. These remarkable advancements have unveiled the extensive clinical potential of MNPs in combating drug-resistant bacterial infections and facilitating the healing process of infected wounds.
Anti-Inflammatory Mechanism
Some MNPs enhance the transition of the inflammatory phase by promoting macrophages to M2 polarization and eliminating the ROS production induced by high glucose environment. Some even inhibit cellular pro-inflammatory factor expression, reducing inflammation and accelerating wound healing.164 Because of their small size, MNPs easily enter the blood, interact with proteins in the plasma, and are surrounded by these cellular elements.165 Some small MNPs enter the cell through pores or ion channels in the cell membrane and activate M2-type macrophages.75 At higher concentrations, the smallest MNPs are easily entoxified by most cell vesicles.166 M2 macrophages limit the inflammatory response and promote the transition from the inflammatory phase to the proliferative phase in diabetic wounds. We found that reducing ROS production and blocking proinflammatory cytokine production were the main mechanisms by which almost all MNPs inhibited inflammation and accelerated wound healing. MNPs block the build-up of inflammation in cells. For example, AuNPs,63 FeONPs,92 Y2ONPs and CeO2NPs93,136 enhance the anti-inflammatory and antioxidant capacity of diabetic wounds by reducing ROS production and blocking the production of proinflammatory cytokines.
An Angiogenesis Promotion
MNPs induce up-regulation of pro-angiogenic growth factors in diabetic wounds, thereby stimulating cell proliferation. Due to the dysregulated release of cytokines, including EGF, VEGF, TNF-α, and IL-6, in individuals with diabetes, angiogenesis and cell migration are hindered. Several MNPs have demonstrated remarkable efficacy in promoting angiogenesis, a critical process for enhancing wound healing in diabetic patients. For instance, ZnONPs induce endothelial cell migration by producing NO via the MAPK/Akt/eNOS pathway.90,167 AuNPs,168 CuONPs122 and HKUST-1NPs113 release metal ions to stimulate the production of angiogenic related cytokines. The surface valence of CeO2NPs stimulates endothelial cells to form tubular structures and also promotes angiogenesis by stabilizing the expression of HIF-1α in endothelial cells.168 In addition to angiogenesis, MNPs, such as KGF-GNPs,60 AuNPs@LL37,62 and F-HKUST-1,115 may also promote diabetic wound healing by carrying cytokines or genes that stimulate cell migration and collagen deposition.
Recently, the focus on promoting diabetic wound healing has shifted towards enhancing angiogenesis in these wounds, coupled with antibacterial, anti-inflammatory, and anti-oxidation properties. However, the research on metal-based nanomaterials has surpassed the exploration of individual MNPs’ functions. Presently, researchers are delving into various strategies to functionalize MNPs, cross-link them with biomaterials, employ external stimuli (eg, light, magnetic, ultrasound) to activate MNPs, or combine them with bimetallic nanoparticles. Consequently, the application of MNPs in the treatment of diabetic wounds extends beyond the enhancement of their antibacterial properties. Numerous studies have significantly advanced our understanding of the antioxidant and angiogenesis functions of metal-based nanomaterials. Additionally, other unexplored or challenging MNPs, such as Pd or cerium nanoparticles, are being investigated for their remarkable healing effects in the treatment of diabetic wounds.
Clinical Application of MNPs
Current treatments for chronic nonhealing wounds, such as debridement, negative-pressure wound therapy, and skin grafting, are often costly, burdensome, and associated with discomfort. In contrast, dressings containing MNPs provide a superior and more convenient solution for promoting wound healing compared to traditional treatments.
Currently, multiple MNPs-containing dressings utilized in clinical treatment have undergone clinical validation. Below, we discuss and summarize some of the dressings in clinical trials and already on the market (Table 2). Mehdi Teimouri conducted clinical trials on the use of an Agicoat silver Nano-Crystalline Dressing for wound healing caused by fractures. Patients treated with this dressing experienced less pain and a significantly shorter wound healing time compared to those treated with Gaz Vaseline.169 In a clinical trial assessing the efficacy of CuO-impregnated foam polymer dressings in preventing postoperative wound infection, patients using the Cu dressings had significantly lower rates of wound infection compared to those using non-Cu dressings.170 In the controlled clinical trial using the SilvrSTAT Gel, a significant reduction in wound size was observed after 2 weeks of use and almost complete healing was achieved after 8 weeks.171 This study effectively demonstrated the beneficial impact of MNPs in the process of wound healing in diabetic patients. The clinical application of commercially available dressings containing MNPs has also been reported. Lee et al conducted a comparative study on the application of various dressings for wounds infected with methicillin-resistant Staphylococcus aureus (MRSA). Acticoat™. The dressings released Ag+ for a longer period of time and caused less inflammation at the wound site. As a result, the frequency of dressing change is greatly reduced and wound healing is accelerated.172 Acticoat™ on diabetic wounds in a randomized clinical trial effectively promoted wound healing without any observed adverse effects, thereby demonstrating the efficacy and safety of Acticoat™ for treating diabetic wounds.173 In addition, MedCu Technologies is a sterile wound dressing impregnated internally with CuO particles. In studies of noninfected diabetic wounds, this dressing reduced inflammation at the wound site and stimulate wound angiogenesis and epithelial formation.174 Moreover, both Biatain ®Ag175 and AQUACEL® Ag176 effectively killed bacteria to protect wounds from infection and reduce patient pain during dressing changes, which is beneficial for wound prognosis and patient experience.
 |
Table 2 Clinical Application and Clinical Trials of Metal in Wound Healing |
At the same time, we also summarized the latest patents on dressings containing MNPs (Table 3). The AMP and CeONs were encapsulated in GelMA-DOPA hydrogels by Cheng et al. The synergistic antibacterial activity of AMP and CeONs was harnessed, while the CeONs effectively eliminated ROS generated during wound inflammation upon release. This dual action resulted in bacterial eradication and suppression of inflammation within the wound.177 Similarly, a multi-enzyme-like activity exhibiting multifunctional hydrogel made up of mussel-inspired carbon dot reduced-Ag (CDs/AgNPs) and Cu/Fe-nitrogen-doped carbon (Cu,Fe-NC) was designed. This hydrogel promotes wound healing by utilizing the synergistic antibacterial properties of Cu and AgNPs and nanozyme in the hydrogel to remove ROS.178 Au-AgNCs@ GSH and Cu-Ag nanocluster composite hydrogel are the latest designed dressings. Their common feature is the use of double MNPs to achieve the effect of antibacterial activity enhancement.
 |
Table 3 Patents on Dressings Containing MNPs |
Clinical trials and practical applications demonstrate the effectiveness of MNP-containing dressings in absorbing wound secretions and releasing metal ions for antimicrobial therapy. No accumulation of MNPs was found in the wound. These findings provide strong evidence for the safety and efficacy of these dressings in practical applications. By promoting wound healing and reducing patient pain, these dressings play a vital role in improving patient outcomes.
Challenges and Future Prospects
In summary, our findings demonstrate that MNPs possess antibacterial, antioxidant, anti-inflammatory, and pro-angiogenic activities, thereby accelerating the healing process of diabetic wounds. However, it is important to note that MNPs are easily released into body fluids in large quantities, leading to direct tissue damage caused by a metal ion attack. This presents a significant challenge for the application of MNPs. Through further research and improvement in the preparation technology of MNPs, we aim to achieve non-biologically toxic concentrations in vivo. These advancements will enable the safe and efficient release of MNPs into the body while maximizing their precise effects. Nevertheless, it should be acknowledged that long-term accumulation of MNPs in vivo may still result in a certain degree of cytotoxicity. Therefore, we are faced with the challenge of enhancing the therapeutic efficacy of MNPs using current technologies while simultaneously expediting their elimination from the body and maintaining a balance between the therapeutic benefits and production costs.166,172,174
Therefore, in the future, it is imperative to not only expand the application range of MNPs but also streamline and optimize their production and utilization in biomaterials. Additionally, exploring novel MNPs with multifaceted effects should be a prospective avenue for research. Furthermore, synergistic combinations of multiple MNPs or integrating MNPs with diverse biomaterials can be employed at different stages of diabetic wound healing to ensure enhanced functionality while minimizing potential complications.
Conclusion
Compared to normal wounds, the complex pathophysiological environment of diabetic wounds presents greater challenges to the healing process. Here, we not only provide a comprehensive overview of the roles played by different types of MNPs in diabetic wound healing but also explore investigations into their significant effects on the intricate mechanisms involved, with a particular focus on their anti-inflammatory, anti-oxidative, anti-bacterial, and pro-angiogenic properties. Additionally, considering the high surface area and adsorbability of MNPs, we summarize their potential as drug carriers for efficient delivery and their synergistic combination with other biomaterials. The utilization of MNPs not only enhances biocompatibility but also ensures sustained and safe therapeutic effects on diabetic wounds through controlled release mechanisms. These innovative investigations deepen our understanding of the potential applications of MNPs in treating diabetic wounds while providing novel research directions for exploring therapeutic mechanisms involving these nanoparticles.
In the future, with the synthesis of an increasing number of MNPs and their integration with biomaterials in diabetic wound research, not only will the healing rate of diabetic wounds be enhanced, but also more MNPs-containing bio-materials will transition from research to clinical application, thereby illuminating the healing process of diabetic wounds.
Funding
This work was supported by the Natural Science Foundation of China (82072051), Central Finance supports Local Colleges and Universities Talent Development Funding from Heilongjiang Provincial Department of Finance (2020GSP09), and Torch Program of Mudanjiang Medical College Science Foundation (2022-MYHJ-006).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Ezhilarasu H, Vishalli D, Dheen ST, Bay BH, Srinivasan DK. Nanoparticle-based therapeutic approach for diabetic wound healing. Nanomaterials (Basel). 2020;10(6):E1234. doi:10.3390/nano10061234
2. Vijayakumar V, Samal SK, Mohanty S, Nayak SK. Recent advancements in biopolymer and metal nanoparticle-based materials in diabetic wound healing management. Int J Biol Macromol. 2019;122:137–148. doi:10.1016/j.ijbiomac.2018.10.120
3. He K, Hemmila MR, Cain-Nielsen AH, Machado-Aranda DA, Frydrych LM, Delano MJ. Complications and resource utilization in trauma patients with diabetes. PLoS One. 2019;14(8):e0221414. doi:10.1371/journal.pone.0221414
4. Miyajima K, Hui Teoh S, Yamashiro H, et al. Effects on glycemic control in impaired wound healing in spontaneously diabetic Torii (SDT) fatty rats. Med Arch. 2018;72(1):4–8. doi:10.5455/medarh.2018.72.4-8
5. Tam JCW, Lau KM, Liu CL, et al. The in vivo and in vitro diabetic wound healing effects of a 2-herb formula and its mechanisms of action. J Ethnopharmacol. 2011;134(3):831–838. doi:10.1016/j.jep.2011.01.032
6. Shu B, Xie JL, Xu YB, et al. Effects of skin-derived precursors on wound healing of denervated skin in a nude mouse model. Int J Clin Exp Pathol. 2015;8(3):2660–2669.
7. Korzeniewska E, Szczęsny A, Lipiński P, Dróżdż T, Kiełbasa P, Miernik A. Prototype of a textronic sensor created with a physical vacuum deposition process for staphylococcus aureus detection. Sensors (Basel). 2020;21(1):E183. doi:10.3390/s21010183
8. Wang J, Li J, Wang Y, Gao M, Zhang X, Yang P. Development of versatile metal–organic framework functionalized magnetic graphene core–shell biocomposite for highly specific recognition of glycopeptides. ACS Appl Mater Interfaces. 2016;8(41):27482–27489. doi:10.1021/acsami.6b08218
9. Sun Y, Wang D, Xu L, et al. Fluorescent small Au nanodots prepared from large Ag nanoparticles for targeting and imaging cancer cells. RSC Adv. 2015;5(64):52088–52094.
10. Wang C, Yao Y, Song Q. Gold nanoclusters decorated with magnetic iron oxide nanoparticles for potential multimodal optical/magnetic resonance imaging. J Mater Chem C. 2015;3(23):5910–5917. doi:10.1039/C5TC00290G
11. Feng Q, Zhang Y, Zhang W, et al. Tumor-targeted and multi-stimuli responsive drug delivery system for near-infrared light induced chemo-phototherapy and photoacoustic tomography. Acta Biomater. 2016;38:129–142. doi:10.1016/j.actbio.2016.04.024
12. Feng Q, Zhang Y, Zhang W, et al. Programmed near-infrared light-responsive drug delivery system for combined magnetic tumor-targeting magnetic resonance imaging and chemo-phototherapy. Acta Biomater. 2017;49:402–413. doi:10.1016/j.actbio.2016.11.035
13. Wang F, Zhang W, Li H, Chen X, Feng S, Mei Z. How effective are nano-based dressings in diabetic wound healing? A comprehensive review of literature. Int J Nanomed. 2022;17:2097–2119. doi:10.2147/IJN.S361282
14. Ermini ML, Voliani V. Antimicrobial nano-agents: the copper age. ACS Nano. 2021;15(4):6008–6029. doi:10.1021/acsnano.0c10756
15. Kalantari K, Mostafavi E, Afifi AM, et al. Wound dressings functionalized with silver nanoparticles: promises and pitfalls. Nanoscale. 2020;12(4):2268–2291. doi:10.1039/c9nr08234d
16. Wang L, Hu C, Shao L. The antimicrobial activity of nanoparticles: present situation and prospects for the future. IJN. 2017;12:1227–1249. doi:10.2147/IJN.S121956
17. Xu L, Wang YY, Huang J, Chen CY, Wang ZX, Xie H. Silver nanoparticles: synthesis, medical applications and biosafety. Theranostics. 2020;10(20):8996–9031. doi:10.7150/thno.45413
18. Nosrati H, Khodaei M, Alizadeh Z, Banitalebi-Dehkordi M. Cationic, anionic and neutral polysaccharides for skin tissue engineering and wound healing applications. Int J Biol Macromol. 2021;192:298–322. doi:10.1016/j.ijbiomac.2021.10.013
19. Wang J, Windbergs M. Functional electrospun fibers for the treatment of human skin wounds. Eur J Pharm Biopharm. 2017;119:283–299. doi:10.1016/j.ejpb.2017.07.001
20. Stadelmann WK, Digenis AG, Tobin GR. Physiology and healing dynamics of chronic cutaneous wounds. Am J Surg. 1998;176(2A Suppl):26S–38S. doi:10.1016/s0002-9610(98)00183-4
21. Ho J, Hantash B. The principles of wound healing. Exp Rev Dermatol. 2014;8:639–658. doi:10.1586/17469872.2013.857161
22. Pastar I, Stojadinovic O, Yin NC, et al. Epithelialization in wound healing: a comprehensive review. Adv Wound Care (New Rochelle). 2014;3(7):445–464. doi:10.1089/wound.2013.0473
23. Evans ND, Oreffo ROC, Healy E, Thurner PJ, Man YH. Epithelial mechanobiology, skin wound healing, and the stem cell niche. J Mech Behav Biomed Mater. 2013;28:397–409. doi:10.1016/j.jmbbm.2013.04.023
24. Rajendran NK, Kumar SSD, Houreld NN, Abrahamse H. A review on nanoparticle based treatment for wound healing. J Drug Delivery Sci Technol. 2018;44:421–430. doi:10.1016/j.jddst.2018.01.009
25. Guo S, Dipietro LA. Factors affecting wound healing. J Dent Res. 2010;89(3):219–229. doi:10.1177/0022034509359125
26. Behm B, Babilas P, Landthaler M, Schreml S. Cytokines, chemokines and growth factors in wound healing. J Eur Acad Dermatol Venereol. 2012;26(7):812–820. doi:10.1111/j.1468-3083.2011.04415.x
27. Li J, Chen J, Kirsner R. Pathophysiology of acute wound healing. Clin Dermatol. 2007;25(1):9–18. doi:10.1016/j.clindermatol.2006.09.007
28. Pakyari M, Farrokhi A, Maharlooei MK, Ghahary A. Critical role of transforming growth factor beta in different phases of wound healing. Adv Wound Care (New Rochelle). 2013;2(5):215–224. doi:10.1089/wound.2012.0406
29. Atala A, Irvine DJ, Moses M, Shaunak S. Wound healing versus regeneration: role of the tissue environment in regenerative medicine. MRS Bull. 2010;35(8):597–606. doi:10.1557/mrs2010.528
30. Fertin C, Nicolas JF, Gillery P, Kalis B, Banchereau J, Maquart FX. Interleukin-4 stimulates collagen synthesis by normal and scleroderma fibroblasts in dermal equivalents. Cell Mol Biol. 1991;37(8):823–829.
31. Hinz B. Formation and function of the myofibroblast during tissue repair. J Invest Dermatol. 2007;127(3):526–537. doi:10.1038/sj.jid.5700613
32. Lazarus GS, Cooper DM, Knighton DR, et al. Definitions and guidelines for assessment of wounds and evaluation of healing. Arch Dermatol. 1994;130(4):489–493. doi:10.1001/archderm.1994.01690040093015
33. Basu Mallik S, Jayashree BS, Shenoy RR. Epigenetic modulation of macrophage polarization- perspectives in diabetic wounds. J diabet complicat. 2018;32(5):524–530. doi:10.1016/j.jdiacomp.2018.01.015
34. Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32(5):593–604. doi:10.1016/j.immuni.2010.05.007
35. Mantovani A, Biswas SK, Galdiero MR, Sica A, Locati M. Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol. 2013;229(2):176–185. doi:10.1002/path.4133
36. Willenborg S, Lucas T, van Loo G, et al. CCR2 recruits an inflammatory macrophage subpopulation critical for angiogenesis in tissue repair. Blood. 2012;120(3):613–625. doi:10.1182/blood-2012-01-403386
37. Kim SY, Nair MG. Macrophages in wound healing: activation and plasticity. Immunol Cell Biol. 2019;97(3):258–267. doi:10.1111/imcb.12236
38. Han G, Ceilley R. Chronic wound healing: a review of current management and treatments. Adv Ther. 2017;34(3):599–610. doi:10.1007/s12325-017-0478-y
39. Sun C, Sun L, Ma H, et al. The phenotype and functional alterations of macrophages in mice with hyperglycemia for long term. J Cell Physiol. 2012;227(4):1670–1679. doi:10.1002/jcp.22891
40. Tahergorabi Z, Khazaei M. Imbalance of angiogenesis in diabetic complications: the mechanisms. Int J Prev Med. 2012;3(12):827. doi:10.4103/2008-7802.104853
41. Brem H, Tomic-Canic M. Cellular and molecular basis of wound healing in diabetes. J Clin Invest. 2007;117(5):1219–1222. doi:10.1172/JCI32169
42. Gallagher KA, Liu ZJ, Xiao M, et al. Diabetic impairments in NO-mediated endothelial progenitor cell mobilization and homing are reversed by hyperoxia and SDF-1α. J Clin Invest. 2007;117(5):1249–1259. doi:10.1172/JCI29710
43. Lerman OZ, Galiano RD, Armour M, Levine JP, Gurtner GC. Cellular dysfunction in the diabetic fibroblast. Am J Pathol. 2003;162(1):303–312. doi:10.1016/S0002-9440(10)63821-7
44. Hazarika S, Dokun AO, Li Y, Popel AS, Kontos CD, Annex BH. Impaired angiogenesis after hindlimb ischemia in type 2 diabetes mellitus: differential regulation of vascular endothelial growth factor receptor 1 and soluble vascular endothelial growth factor receptor 1. Circ Res. 2007;101(9):948–956. doi:10.1161/CIRCRESAHA.107.160630
45. Kendall RL, Thomas KA. Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc Natl Acad Sci USA. 1993;90(22):10705–10709. doi:10.1073/pnas.90.22.10705
46. Dreaden EC, Alkilany AM, Huang X, Murphy CJ, El-Sayed MA. The golden age: gold nanoparticles for biomedicine. Chem Soc Rev. 2012;41(7):2740–2779. doi:10.1039/c1cs15237h
47. Mohamed MM, Fouad SA, Elshoky HA, Mohammed GM, Salaheldin TA. Antibacterial effect of gold nanoparticles against Corynebacterium pseudotuberculosis. Int J Vet Sci Med. 2017;5(1):23–29. doi:10.1016/j.ijvsm.2017.02.003
48. Vimbela G, Ngo SM, Fraze C, Yang L, Stout DA. Antibacterial properties and toxicity from metallic nanomaterials. IJN. 2017;12:3941–3965. doi:10.2147/IJN.S134526
49. Kohanski MA, Dwyer DJ, Hayete B, Lawrence CA, Collins JJ. A Common Mechanism of Cellular Death Induced by Bactericidal Antibiotics. Cell. 2007;130(5):797–810. doi:10.1016/j.cell.2007.06.049
50. Cui Y, Zhao Y, Tian Y, Zhang W, X L, Jiang X. The molecular mechanism of action of bactericidal gold nanoparticles on Escherichia coli. Biomaterials. 2012;33(7):2327–2333. doi:10.1016/j.biomaterials.2011.11.057
51. Boomi P, Ganesan R, Prabu Poorani G, et al. Phyto-engineered gold nanoparticles (AuNPs) with potential antibacterial, antioxidant, and wound healing activities under in vitro and in vivo conditions. Int J Nanomed. 2020;15:7553–7568. doi:10.2147/IJN.S257499
52. Annamalai A, Christina VLP, Sudha D, Kalpana M, Lakshmi PTV. Green synthesis, characterization and antimicrobial activity of Au NPs using Euphorbia hirta L. leaf extract. Colloids Surf., B. 2013;108:60–65. doi:10.1016/j.colsurfb.2013.02.012
53. Wei SC, Chang L, Huang CC, Chang HT. Dual-functional gold nanoparticles with antimicrobial and proangiogenic activities improve the healing of multidrug-resistant bacteria-infected wounds in diabetic mice. Biomater Sci. 2019;7(11):4482–4490. doi:10.1039/c9bm00772e
54. Kingston M, Pfau JC, Gilmer J, Brey R. Selective inhibitory effects of 50-nm gold nanoparticles on mouse macrophage and spleen cells. J Immunotoxicol. 2016;13(2):198–208. doi:10.3109/1547691X.2015.1035819
55. BarathManiKanth S, Kalishwaralal K, Sriram M, et al. RAesneatrcih-oxidant effect of gold nanoparticles restrains hyperglycemic conditions in diabetic mice. J Nanobiotechnol. 2010;8:16. doi:10.1186/1477-3155-8-16
56. Song L, Hobaugh MR, Shustak C, Cheley S, Bayley H, Gouaux JE. Structure of staphylococcal alpha-hemolysin, a heptameric transmembrane pore. Science. 1996;274(5294):1859–1866. doi:10.1126/science.274.5294.1859
57. Pornpattananangkul D, Zhang L, Olson S, et al. Bacterial toxin-triggered drug release from gold nanoparticle-stabilized liposomes for the treatment of bacterial infection. J Am Chem Soc. 2011;133(11):4132–4139. doi:10.1021/ja111110e
58. Li J, Cha R, Zhao X, et al. Gold Nanoparticles Cure Bacterial Infection with Benefit to Intestinal Microflora. ACS Nano. 2019;13(5):5002–5014. doi:10.1021/acsnano.9b01002
59. He X, Dai L, Ye L, et al. A vehicle‐free antimicrobial polymer hybrid gold nanoparticle as synergistically therapeutic platforms for Staphylococcus aureus infected wound healing. Adv Sci. 2022;9(14):2105223. doi:10.1002/advs.202105223
60. Li S, Tang Q, Xu H, et al. Improved stability of KGF by conjugation with gold nanoparticles for diabetic wound therapy. Nanomedicine. 2019;14(22):2909–2923. doi:10.2217/nnm-2018-0487
61. Miles AJ, Wallace BA. Circular dichroism spectroscopy of membrane proteins. Chem Soc Rev. 2016;45(18):4859–4872. doi:10.1039/C5CS00084J
62. Wang S, Yan C, Zhang X, et al. Antimicrobial peptide modification enhances the gene delivery and bactericidal efficiency of gold nanoparticles for accelerating diabetic wound healing. Biomater Sci. 2018;6(10):2757–2772. doi:10.1039/C8BM00807H
63. Chen SA, Chen HM, Yao YD, Hung CF, Tu CS, Liang YJ. Topical treatment with anti-oxidants and Au nanoparticles promote healing of diabetic wound through receptor for advance glycation end-products. Eur J Pharm Sci. 2012;47(5):875–883. doi:10.1016/j.ejps.2012.08.018
64. Nosrati H, Heydari M, Tootiaei Z, Ganjbar S, Khodaei M. Delivery of antibacterial agents for wound healing applications using polysaccharide-based scaffolds. J Drug Delivery Sci Technol. 2023;84:104516. doi:10.1016/j.jddst.2023.104516
65. Chaloupka K, Malam Y, Seifalian AM. Nanosilver as a new generation of nanoproduct in biomedical applications. Trends Biotechnol. 2010;28(11):580–588. doi:10.1016/j.tibtech.2010.07.006
66. Agnihotri S, Mukherji S, Mukherji S. Immobilized silver nanoparticles enhance contact killing and show highest efficacy: elucidation of the mechanism of bactericidal action of silver. Nanoscale. 2013;5(16):7328. doi:10.1039/c3nr00024a
67. Dizaj SM, Lotfipour F, Barzegar-Jalali M, Zarrintan MH, Adibkia K. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater Sci Eng C. 2014;44:278–284. doi:10.1016/j.msec.2014.08.031
68. Lok CN, Ho CM, Chen R, et al. Proteomic analysis of the mode of antibacterial action of silver nanoparticles. J Proteome Res. 2006;5(4):916–924. doi:10.1021/pr0504079
69. Zhang Y, Peng H, Huang W, Zhou Y, Yan D. Facile preparation and characterization of highly antimicrobial colloid Ag or Au nanoparticles. J Colloid Interface Sci. 2008;325(2):371–376. doi:10.1016/j.jcis.2008.05.063
70. Ayuk SM, Houreld NN, Abrahamse H. Collagen production in diabetic wounded fibroblasts in response to low-intensity laser irradiation at 660 nm. Diabetes Technol Ther. 2012;14(12):1110–1117. doi:10.1089/dia.2012.0125
71. Singla R, Soni S, Patial V, et al. In vivo diabetic wound healing potential of nanobiocomposites containing bamboo cellulose nanocrystals impregnated with silver nanoparticles. Int J Biol Macromol. 2017;105(Pt 1):45–55. doi:10.1016/j.ijbiomac.2017.06.109
72. Dhilip Kumar SS, Houreld NN, Abrahamse H. Selective laser efficiency of green-synthesized silver nanoparticles by aloe arborescens and its wound healing activities in normal wounded and diabetic wounded fibroblast cells: in vitro studies. IJN. 2020;15:6855–6870. doi:10.2147/IJN.S257204
73. Tao B, Lin C, Deng Y, et al. Copper-nanoparticle-embedded hydrogel for killing bacteria and promoting wound healing with photothermal therapy. J Mater Chem B. 2019;7(15):2534–2548. doi:10.1039/c8tb03272f
74. Chatterjee AK, Chakraborty R, Basu T. Mechanism of antibacterial activity of copper nanoparticles. Nanotechnology. 2014;25(13):135101. doi:10.1088/0957-4484/25/13/135101
75. Veerapandian M, Sadhasivam S, Choi J, Yun K. Glucosamine functionalized copper nanoparticles: preparation, characterization and enhancement of anti-bacterial activity by ultraviolet irradiation. Chem Eng J. 2012;209:558–567. doi:10.1016/j.cej.2012.08.054
76. Das D, Nath BC, Phukon P, Dolui SK. Synthesis and evaluation of antioxidant and antibacterial behavior of CuO nanoparticles. Colloids Surf., B. 2013;101:430–433. doi:10.1016/j.colsurfb.2012.07.002
77. Kobayashi Y. The regulatory role of nitric oxide in proinflammatory cytokine expression during the induction and resolution of inflammation. J Leukoc Biol. 2010;88(6):1157–1162. doi:10.1189/jlb.0310149
78. Borkow G, Gabbay J, Dardik R, et al. Molecular mechanisms of enhanced wound healing by copper oxide-impregnated dressings. Wound Repair Regener. 2010;18(2):266–275. doi:10.1111/j.1524-475X.2010.00573.x
79. Alizadeh S, Seyedalipour B, Shafieyan S, Kheime A, Mohammadi P, Aghdami N. Copper nanoparticles promote rapid wound healing in acute full thickness defect via acceleration of skin cell migration, proliferation, and neovascularization. Biochem Biophys Res Commun. 2019;517(4):684–690. doi:10.1016/j.bbrc.2019.07.110
80. Haldorai Y, Shim JJ. Novel chitosan-TiO 2 nanohybrid: preparation, characterization, antibacterial, and photocatalytic properties. Polym Compos. 2014;35(2):327–333. doi:10.1002/pc.22665
81. Charpentier PA, Burgess K, Wang L, Chowdhury RR, Lotus AF, Moula G. Nano-TiO2/polyurethane composites for antibacterial and self-cleaning coatings. Nanotechnology. 2012;23(42):425606. doi:10.1088/0957-4484/23/42/425606
82. Sonmez O, Sonmez M. Role of platelets in immune system and inflammation. Porto Biomed J. 2017;2(6):311–314. doi:10.1016/j.pbj.2017.05.005
83. Seisenbaeva GA, Fromell K, Vinogradov VV, et al. Dispersion of TiO2 nanoparticles improves burn wound healing and tissue regeneration through specific interaction with blood serum proteins. Sci Rep. 2017;7(1):15448. doi:10.1038/s41598-017-15792-w
84. Brayner R, Ferrari-Iliou R, Brivois N, Djediat S, Benedetti MF, Fiévet F. Toxicological impact studies based on Escherichia coli bacteria in ultrafine ZnO nanoparticles colloidal medium. Nano Lett. 2006;6(4):866–870. doi:10.1021/nl052326h
85. Xie Y, He Y, Irwin PL, Jin T, Shi X. Antibacterial activity and mechanism of action of zinc oxide nanoparticles against Campylobacter jejuni. Appl Environ Microbiol. 2011;77(7):2325–2331. doi:10.1128/AEM.02149-10
86. Mittal M, Siddiqui MR, Tran K, Reddy SP, Malik AB. Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal. 2014;20(7):1126–1167. doi:10.1089/ars.2012.5149
87. Agarwal H, Nakara A, Shanmugam VK. Anti-inflammatory mechanism of various metal and metal oxide nanoparticles synthesized using plant extracts: a review. Biomed Pharmacother. 2019;109:2561–2572. doi:10.1016/j.biopha.2018.11.116
88. Kim MH, Seo JH, Kim HM, Jeong HJ. Aluminum-doped zinc oxide nanoparticles attenuate the TSLP levels via suppressing caspase-1 in activated mast cells. J Biomater Appl. 2016;30(9):1407–1416. doi:10.1177/0885328216629822
89. Qian Y, Zheng Y, Jin J, et al. Immunoregulation in diabetic wound repair with a photoenhanced glycyrrhizic acid hydrogel scaffold. Adv Mater. 2022;34(29):2200521. doi:10.1002/adma.202200521
90. Lin PH, Sermersheim M, Li H, Lee PHU, Steinberg SM, Ma J. Zinc in wound healing modulation. Nutrients. 2017;10(1):E16. doi:10.3390/nu10010016
91. Arakha M, Pal S, Samantarrai D, et al. Antimicrobial activity of iron oxide nanoparticle upon modulation of nanoparticle-bacteria interface. Sci Rep. 2015;5:14813. doi:10.1038/srep14813
92. Gu Y, Huang Y, Qiu Z, et al. Vitamin B2 functionalized iron oxide nanozymes for mouth ulcer healing. Sci China Life Sci. 2020;63(1):68–79. doi:10.1007/s11427-019-9590-6
93. Garzón-Manjón A, Aranda-Ramos A, Melara-Benítez B, et al. Simple synthesis of biocompatible stable CeO 2 Nanoparticles as antioxidant agents. Bioconjugate Chem. 2018;29(7):2325–2331. doi:10.1021/acs.bioconjchem.8b00300
94. Chigurupati S, Mughal MR, Okun E, et al. Effects of cerium oxide nanoparticles on the growth of keratinocytes, fibroblasts and vascular endothelial cells in cutaneous wound healing. Biomaterials. 2013;34(9):2194–2201. doi:10.1016/j.biomaterials.2012.11.061
95. Park HJ, Kim JY, Kim J, et al. Silver-ion-mediated reactive oxygen species generation affecting bactericidal activity. Water Res. 2009;43(4):1027–1032. doi:10.1016/j.watres.2008.12.002
96. Silver S. Bacterial silver resistance: molecular biology and uses and misuses of silver compounds. FEMS Microbiol Rev. 2003;27(2–3):341–353. doi:10.1016/S0168-6445(03)00047-0
97. Shi G, Chen W, Zhang Y, Dai X, Zhang X, Wu Z. An antifouling hydrogel containing silver nanoparticles for modulating the therapeutic immune response in chronic wound healing. Langmuir. 2019;35(5):1837–1845. doi:10.1021/acs.langmuir.8b01834
98. Zielińska D, Struszczyk MH, Madej-Kiełbik L, et al. Design of New-generation usable forms of topical haemostatic agents containing Chitosan. Molecules. 2017;22(12):2240. doi:10.3390/molecules22122240
99. Choudhary M, Chhabra P, Tyagi A, Singh H. Scar free healing of full thickness diabetic wounds: a unique combination of silver nanoparticles as antimicrobial agent, calcium alginate nanoparticles as hemostatic agent, fresh blood as nutrient/growth factor supplier and chitosan as base matrix. Int J Biol Macromol. 2021;178:41–52. doi:10.1016/j.ijbiomac.2021.02.133
100. Wang YY, Addisu KD, Gebrie HT, et al. Multifunctional thermosensitive hydrogel based on alginate and P(NIPAM-co-HEMIN) composites for accelerated diabetic wound healing. Int J Biol Macromol. 2023;241:124540. doi:10.1016/j.ijbiomac.2023.124540
101. Masood N, Ahmed R, Tariq M, et al. Silver nanoparticle impregnated chitosan-PEG hydrogel enhances wound healing in diabetes induced rabbits. Int J Pharm. 2019;559:23–36. doi:10.1016/j.ijpharm.2019.01.019
102. Franková J, Pivodová V, Vágnerová H, Juráňová J, Ulrichová J. Effects of silver nanoparticles on primary cell cultures of fibroblasts and keratinocytes in a wound-healing model. J Appl Biomater Funct Mater. 2016;14(2):e137–142. doi:10.5301/jabfm.5000268
103. Paul A, Ju H, Rangasamy S, Shim Y, Song JM. Nanosized silver (II) pyridoxine complex to cause greater inflammatory response and less cytotoxicity to RAW264.7 macrophage cells. Nanoscale Res Lett. 2015;10(1):140. doi:10.1186/s11671-015-0848-9
104. Fathil MAM, Katas H. Antibacterial, Anti-biofilm and pro-migratory effects of double layered hydrogels packaged with lactoferrin-DsiRNA-silver nanoparticles for chronic wound therapy. Pharmaceutics. 2023;15(3):991. doi:10.3390/pharmaceutics15030991
105. Chauhan PS, Shrivastava V, Tomar RS. Biofabrication of copper nanoparticles: a next-generation antibacterial agent against wound-associated pathogens. Turk J Pharm Sci. 2018;15(3):238–247. doi:10.4274/tjps.52724
106. Hassan MS, Amna T, Yang OB, El-Newehy MH, Al-Deyab SS, Khil MS. Smart copper oxide nanocrystals: synthesis, characterization, electrochemical and potent antibacterial activity. Colloids Surf., B. 2012;97:201–206. doi:10.1016/j.colsurfb.2012.04.032
107. Akhavan O, Ghaderi E. Cu and CuO nanoparticles immobilized by silica thin films as antibacterial materials and photocatalysts. Surf Coat Technol. 2010;205(1):219–223. doi:10.1016/j.surfcoat.2010.06.036
108. Sankar R, Baskaran A, Shivashangari KS, Ravikumar V. Inhibition of pathogenic bacterial growth on excision wound by green synthesized copper oxide nanoparticles leads to accelerated wound healing activity in Wistar Albino rats. J Mater Sci Mater Med. 2015;26(7):214. doi:10.1007/s10856-015-5543-y
109. Della Rocca J, Liu D, Lin W. Nanoscale metal-organic frameworks for biomedical imaging and drug delivery. Acc Chem Res. 2011;44(10):957–968. doi:10.1021/ar200028a
110. Zhang P, Li Y, Tang Y, et al. Copper-based metal-organic framework as a controllable nitric oxide-releasing vehicle for enhanced diabetic wound healing. ACS Appl Mater Interfaces. 2020;12(16):18319–18331. doi:10.1021/acsami.0c01792
111. Su CH, Li WP, Tsao LC, et al. Enhancing microcirculation on multitriggering manner facilitates angiogenesis and collagen deposition on wound healing by photoreleased NO from hemin-derivatized colloids. ACS Nano. 2019;13(4):4290–4301. doi:10.1021/acsnano.8b09417
112. Champeau M, Póvoa V, Militão L, et al. Supramolecular poly(acrylic acid)/F127 hydrogel with hydration-controlled nitric oxide release for enhancing wound healing. Acta Biomater. 2018;74:312–325. doi:10.1016/j.actbio.2018.05.025
113. Xiao J, Chen S, Yi J, Zhang HF, Ameer GA. A cooperative copper metal-organic framework-hydrogel system improves wound healing in diabetes. Adv Funct Mater. 2017;27(1):1604872. doi:10.1002/adfm.201604872
114. Zhang W, Hu Y, Ge J, Jiang HL, Yu SH. A facile and general coating approach to moisture/water-resistant metal-organic frameworks with intact porosity. J Am Chem Soc. 2014;136(49):16978–16981. doi:10.1021/ja509960n
115. Xiao J, Zhu Y, Huddleston S, et al. Copper metal-organic framework nanoparticles stabilized with folic acid improve wound healing in diabetes. ACS Nano. 2018;12(2):1023–1032. doi:10.1021/acsnano.7b01850
116. Děng Z. A biotin-stabilized HKUST-1ADM scaffold for facil.pdf; 2023.
117. Gopal A, Kant V, Gopalakrishnan A, Tandan SK, Kumar D. Chitosan-based copper nanocomposite accelerates healing in excision wound model in rats. Eur J Pharmacol. 2014;731:8–19. doi:10.1016/j.ejphar.2014.02.033
118. Mandinov L, Mandinova A, Kyurkchiev S, et al. Copper chelation represses the vascular response to injury. Proc Natl Acad Sci U S A. 2003;100(11):6700–6705. doi:10.1073/pnas.1231994100
119. Huang Y, Qi L, Liu Z, et al. Radially electrospun fibrous membrane incorporated with copper peroxide nanodots capable of self-catalyzed chemodynamic therapy for angiogenesis and healing acceleration of diabetic wounds. ACS Appl Mater Interfaces. 2023:
120. Wang X, Lv F, Li T, et al. Electrospun micropatterned nanocomposites incorporated with Cu2S nanoflowers for skin tumor therapy and wound healing. ACS Nano. 2017;11(11):11337–11349. doi:10.1021/acsnano.7b05858
121. Romano Di Peppe S, Mangoni A, Zambruno G, et al. Adenovirus-mediated VEGF(165) gene transfer enhances wound healing by promoting angiogenesis in CD1 diabetic mice. Gene Ther. 2002;9(19):1271–1277. doi:10.1038/sj.gt.3301798
122. Imtiyaz HZ, Simon MC. Hypoxia-inducible factors as essential regulators of inflammation. Curr Top Microbiol Immunol. 2010;345:105–120. doi:10.1007/82_2010_74
123. Jansen J, Karges W, Rink L. Zinc and diabetes--clinical links and molecular mechanisms. J Nutr Biochem. 2009;20(6):399–417. doi:10.1016/j.jnutbio.2009.01.009
124. Umrani RD, Paknikar KM. Zinc oxide nanoparticles show antidiabetic activity in streptozotocin-induced Type 1 and 2 diabetic rats. Nanomedicine. 2014;9(1):89–104. doi:10.2217/nnm.12.205
125. Yadav E, Singh D, Yadav P, Verma A. Ameliorative effect of biofabricated ZnO nanoparticles of Trianthema portulacastrum Linn. on dermal wounds via removal of oxidative stress and inflammation. RSC Adv. 2018;8(38):21621–21635. doi:10.1039/C8RA03500H
126. Huang Z, Zheng X, Yan D, et al. Toxicological effect of ZnO nanoparticles based on bacteria. Langmuir. 2008;24(8):4140–4144. doi:10.1021/la7035949
127. Raguvaran R, Manuja BK, Chopra M, et al. Sodium alginate and gum acacia hydrogels of ZnO nanoparticles show wound healing effect on fibroblast cells. Int J Biol Macromol. 2017;96:185–191. doi:10.1016/j.ijbiomac.2016.12.009
128. Nagajyothi PC, Cha SJ, Yang IJ, Sreekanth TVM, Kim KJ, Shin HM. Antioxidant and anti-inflammatory activities of zinc oxide nanoparticles synthesized using Polygala tenuifolia root extract. J Photochem Photobiol B Biol. 2015;146:10–17. doi:10.1016/j.jphotobiol.2015.02.008
129. López-Goerne T, Ramírez P, Alvarez D, et al. Physicochemical properties and in vivo evaluation of Pt/TiO 2 –SiO 2 nanopowders. Nanomedicine. 2018;13(17):2171–2185. doi:10.2217/nnm-2018-0078
130. Cormode DP, Gao L, Koo H. Emerging biomedical applications of enzyme-like catalytic nanomaterials. Trends Biotechnol. 2018;36(1):15–29. doi:10.1016/j.tibtech.2017.09.006
131. Hu Y, Rao SS, Wang ZX, et al. Exosomes from human umbilical cord blood accelerate cutaneous wound healing through miR-21-3p-mediated promotion of angiogenesis and fibroblast function. Theranostics. 2018;8(1):169–184. doi:10.7150/thno.21234
132. Li X, Wei J, Aifantis KE, et al. Current investigations into magnetic nanoparticles for biomedical applications. J Biomed Mater Res A. 2016;104(5):1285–1296. doi:10.1002/jbm.a.35654
133. Wu D, Kang L, Tian J, et al. Exosomes derived from bone mesenchymal stem cells with the stimulation of Fe3O4 nanoparticles and static magnetic field enhance wound healing through upregulated miR-21-5p. Int J Nanomed. 2020;15:7979–7993. doi:10.2147/IJN.S275650
134. Kannan SK, Sundrarajan M. A green approach for the synthesis of a cerium oxide nanoparticle: characterization and antibacterial activity. Int J Nanosci. 2014;13(03):1450018. doi:10.1142/S0219581X14500185
135. Zholobak NM, Ivanov VK, Shcherbakov AB. Chapter 12 - Interaction of nanoceria with microorganisms. In: Grumezescu AM, editor. Nanobiomaterials in Antimicrobial Therapy. William Andrew Publishing; 2016:419–450. doi:10.1016/B978-0-323-42864-4.00012-9
136. Ghaznavi H, Najafi R, Mehrzadi S, et al. The neuro-protective effects of cerium and yttrium oxide nanoparticles on high glucose-induced oxidative stress and apoptosis in undifferentiated PC12 cells. Neurological Res. 2015;37(7):624–632.
137. Nosrati H, Heydari M, Khodaei M. Cerium oxide nanoparticles: synthesis methods and applications in wound healing. Mater Today Bio. 2023;23:100823. doi:10.1016/j.mtbio.2023.100823
138. Augustine R, Hasan A, Patan NK, et al. Cerium oxide nanoparticle incorporated electrospun Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) membranes for diabetic wound healing applications. ACS Biomater Sci Eng. 2020;6(1):58–70. doi:10.1021/acsbiomaterials.8b01352
139. Yao Y, Zang Y, Qu J, Tang M, Zhang T. The toxicity of metallic nanoparticles on liver: the subcellular damages, mechanisms, and outcomes. Int j Nanomed. 2019;14:8787–8804. doi:10.2147/IJN.S212907
140. Sengul AB, Asmatulu E. Toxicity of metal and metal oxide nanoparticles: a review. Environ Chem Lett. 2020;18(5):1659–1683. doi:10.1007/s10311-020-01033-6
141. Wang J, Zhou G, Chen C, et al. Acute toxicity and biodistribution of different sized titanium dioxide particles in mice after oral administration. Toxicol Lett. 2007;168(2):176–185. doi:10.1016/j.toxlet.2006.12.001
142. Chen J, Dong X, Zhao J, Tang G. In vivo acute toxicity of titanium dioxide nanoparticles to mice after intraperitioneal injection. J Appl Toxicol. 2009;29(4):330–337. doi:10.1002/jat.1414
143. Jain TK, Reddy MK, Morales MA, Leslie-Pelecky DL, Labhasetwar V. Biodistribution, clearance, and biocompatibility of iron oxide magnetic nanoparticles in rats. Mol Pharm. 2008;5(2):316–327. doi:10.1021/mp7001285
144. Thakur N, Manna P, Das J. Synthesis and biomedical applications of nanoceria, a redox active nanoparticle. J Nanobiotechnol. 2019;17(1):84. doi:10.1186/s12951-019-0516-9
145. Kumari M, Kumari SI, Grover P. Genotoxicity analysis of cerium oxide micro and nanoparticles in Wistar rats after 28 days of repeated oral administration. Mutagenesis. 2014;29(6):467–479. doi:10.1093/mutage/geu038
146. Ranjbar A, Ghasemi H, Abedian A, Kheiripour N. Cerium oxide nanoparticle modulates hepatic damage, inflammatory and oxidative stress biomarkers in a dose-dependent manner: an in vivo study of rat liver. Nanomed J. 2018;5(4). doi:10.22038/nmj.2018.05.00009
147. Xing Y, Zeng B, Yang W. Light responsive hydrogels for controlled drug delivery. Front Bioeng Biotechnol. 2022;10:1075670. doi:10.3389/fbioe.2022.1075670
148. Permana AD, Anjani QK, Utomo E, et al. Selective delivery of silver nanoparticles for improved treatment of biofilm skin infection using bacteria-responsive microparticles loaded into dissolving microneedles. Mater Sci Eng C. 2021;120:111786. doi:10.1016/j.msec.2020.111786
149. Flemming HC, Wingender J. The biofilm matrix. Nat Rev Microbiol. 2010;8(9):623–633. doi:10.1038/nrmicro2415
150. Waghule T, Singhvi G, Dubey SK, et al. Microneedles: a smart approach and increasing potential for transdermal drug delivery system. Biomed Pharmacother. 2019;109:1249–1258. doi:10.1016/j.biopha.2018.10.078
151. Bariya SH, Gohel MC, Mehta TA, Sharma OP. Microneedles: an emerging transdermal drug delivery system. J Pharm Pharmacol. 2012;64(1):11–29. doi:10.1111/j.2042-7158.2011.01369.x
152. Permana AD, Mir M, Utomo E, Donnelly RF. Bacterially sensitive nanoparticle-based dissolving microneedles of doxycycline for enhanced treatment of bacterial biofilm skin infection: a proof of concept study. Int J Pharm X. 2020;2:100047. doi:10.1016/j.ijpx.2020.100047
153. Yang J, Chu Z, Jiang Y, et al. Multifunctional hyaluronic acid microneedle patch embedded by cerium/zinc-based composites for accelerating diabetes wound healing. Adv. Healthcare Mater. 2023;12(24):2300725. doi:10.1002/adhm.202300725
154. Zeng Y, Wang C, Lei K, et al. Multifunctional MOF-based microneedle patch with synergistic chemo-photodynamic antibacterial effect and sustained release of growth factor for chronic wound healing. Adv. Healthcare Mater. 2023;12(19):2300250. doi:10.1002/adhm.202300250
155. Pang Q, Jiang Z, Wu K, Hou R, Zhu Y. Nanomaterials-based wound dressing for advanced management of infected wound. Antibiotics (Basel). 2023;12(2):351. doi:10.3390/antibiotics12020351
156. Luther DC, Huang R, Jeon T, et al. Delivery of drugs, proteins, and nucleic acids using inorganic nanoparticles. Adv Drug Deliv Rev. 2020;156:188–213. doi:10.1016/j.addr.2020.06.020
157. Yu N, Wang X, Qiu L, et al. Bacteria-triggered hyaluronan/AgNPs/gentamicin nanocarrier for synergistic bacteria disinfection and wound healing application. Chem Eng J. 2020;380:122582. doi:10.1016/j.cej.2019.122582
158. Konturek PC, Brzozowski T, Konturek SJ, Kwiecien S, Dembinski A, Hahn EG. Influence of bacterial lipopolysaccharide on healing of chronic experimental ulcer in rat. Scand J Gastroenterol. 2001;36(12):1239–1247. doi:10.1080/003655201317097065
159. Zhou Y, Kong Y, Kundu S, Cirillo JD, Liang H. Antibacterial activities of gold and silver nanoparticles against Escherichia coli and bacillus Calmette-Guérin. J Nanobiotechnol. 2012;10(1):19. doi:10.1186/1477-3155-10-19
160. Choi JB, Park JS, Khil MS, et al. Characterization and antimicrobial property of poly(acrylic acid) nanogel containing silver particle prepared by electron beam. Int J Mol Sci. 2013;14(6):11011–11023. doi:10.3390/ijms140611011
161. Sondi I, Salopek-Sondi B. Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria. J Colloid Interface Sci. 2004;275(1):177–182. doi:10.1016/j.jcis.2004.02.012
162. He X, Hou J, Sun X, et al. NIR‐II photo‐amplified sonodynamic therapy using sodium molybdenum bronze nanoplatform against subcutaneous Staphylococcus Aureus infection. Adv Funct Mater. 2022;32(38):2203964. doi:10.1002/adfm.202203964
163. He X, Qian Y, Wu C, et al. Entropy‐mediated high‐entropy MXenes nanotherapeutics: NIR‐II‐enhanced intrinsic oxidase mimic activity to combat methicillin‐resistant Staphylococcus Aureus infection. Adv Mater. 2023;35(26):2211432. doi:10.1002/adma.202211432
164. Kuhn DA, Vanhecke D, Michen B, et al. Different endocytotic uptake mechanisms for nanoparticles in epithelial cells and macrophages. Beilstein J Nanotechnol. 2014;5:1625–1636. doi:10.3762/bjnano.5.174
165. Vinluan RD, Zheng J. Serum protein adsorption and excretion pathways of metal nanoparticles. Nanomedicine. 2015;10(17):2781–2794. doi:10.2217/nnm.15.97
166. Simko M, Fiedeler U, Gazsó A, Nentwich M The impact of nanoparticles on cellular functions; November 9, 2011. http://hw.oeaw.ac.at/nanotrust-dossier.
167. Nosrati H, Aramideh khouy R, Nosrati A, et al. Nanocomposite scaffolds for accelerating chronic wound healing by enhancing angiogenesis. J Nanobiotechnol. 2021;19(1):1. doi:10.1186/s12951-020-00755-7
168. Hao D, Zhang G, Gong Y, Ma Z. Development and biological evaluation of cerium oxide loaded polycaprolactone dressing on cutaneous wound healing in nursing care. Mater Lett. 2020;265:127401. doi:10.1016/j.matlet.2020.127401
169. Teimouri M, Lalehzar SS. Evaluation of the therapeutic effect of dressing containing Silver (Ag coat) in the process of healing skin blisters caused by limb fractures: a clinical trial study. BMC Surg. 2023;23:101. doi:10.1186/s12893-023-02012-8
170. Arendsen LP, Thakar R, Bassett P, Sultan AH. The impact of copper impregnated wound dressings on surgical site infection following caesarean section: a double blind randomised controlled study. Eur J Obstetrics Gynecol Reprod Biol. 2020;251:83–88. doi:10.1016/j.ejogrb.2020.05.016
171. Essa MS, Ahmad KS, Zayed ME, Ibrahim SG. Comparative study between silver nanoparticles dressing (SilvrSTAT Gel) and conventional dressing in diabetic foot ulcer healing: a prospective randomized study. Int J Low Extrem Wounds. 2023;22(1):48–55. doi:10.1177/1534734620988217
172. Erring M, Gaba S, Mohsina S, Tripathy S, Sharma RK. Comparison of efficacy of silver-nanoparticle gel, nano-silver-foam and collagen dressings in treatment of partial thickness burn wounds. Burns. 2019;45(8):1888–1894. doi:10.1016/j.burns.2019.07.019
173. Abdollahimajd F, Pourani MR, Mahdavi H, Mirzadeh H, Younespour S, Moravvej H. Efficacy and safety of chitosan-based bio-compatible dressing versus nanosilver (ActicoatTM) dressing in treatment of recalcitrant diabetic wounds: a randomized clinical trial. Dermatol Ther. 2022;35(9):e15682. doi:10.1111/dth.15682
174. Melamed E, Rovitsky A, Roth T, Assa L, Borkow G. Stimulation of healing of non-infected stagnated diabetic wounds by copper oxide-impregnated wound dressings. Medicina. 2021;57(10):1129. doi:10.3390/medicina57101129
175. Senet P, Bause R, Jørgensen B, Fogh K. Clinical efficacy of a silver-releasing foam dressing in venous leg ulcer healing: a randomised controlled trial: clinical efficacy of a silver-releasing foam dressing in venous leg ulcer healing. Int Wound J. 2014;11(6):649–655. doi:10.1111/iwj.12022
176. Kuo FC, Chen B, Lee MS, Yen SH, Wang JW. AQUACEL® Ag surgical dressing reduces surgical site infection and improves patient satisfaction in minimally invasive total knee arthroplasty: a prospective, randomized, controlled study. Biomed Res Int. 2017;2017:1–8. doi:10.1155/2017/1262108
177. Cheng H, Shi Z, Yue K, et al. Sprayable hydrogel dressing accelerates wound healing with combined reactive oxygen species-scavenging and antibacterial abilities. Acta Biomater. 2021;124:219–232. doi:10.1016/j.actbio.2021.02.002
178. Zhu J, Han Q, Li Q, et al. A multi-enzyme-like activity exhibiting mussel-inspired nanozyme hydrogel for bacteria-infected wound healing. Biomater Sci. 2023;11(8):2711–2725. doi:10.1039/D2BM02004A
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

