Back to Journals » International Medical Case Reports Journal » Volume 16
Metagenomics as New Tool for Diagnosis of Scrub Typhus: Two Case Reports
Authors Ling Y , Hu X, Zheng G, Ye W, Yuan K, Ye L, Huang W, Tian B, Gu B
Received 22 July 2023
Accepted for publication 20 September 2023
Published 27 September 2023 Volume 2023:16 Pages 617—622
DOI https://doi.org/10.2147/IMCRJ.S431864
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Yong Ling,1,* Xuejiao Hu,1,* Guansheng Zheng,1 Weitao Ye,2 Kaixuan Yuan,1 Long Ye,1 Weiye Huang,3 Benshun Tian,1 Bing Gu1
1Department of Clinical Laboratory Medicine, Guangdong Provincial People’s Hospital (Guangdong Academy of Medical Sciences), Southern Medical University, Guangzhou, Guangdong, 510000, People’s Republic of China; 2Department of Radiology, Guangdong Provincial People’s Hospital (Guangdong Academy of Medical Sciences), Southern Medical University, Guangzhou, Guangdong, 510000, People’s Republic of China; 3Department of Pathology, Guangdong Provincial People’s Hospital (Guangdong Academy of Medical Sciences), Southern Medical University, Guangzhou, Guangdong, 510000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Benshun Tian; Bing Gu, Department of Laboratory Medicine, Guangdong Provincial People’s Hospital, 106 Zhongshan 2nd Road, Yuexiu District, Guangzhou, Guangdong, 510000, People’s Republic of China, Email [email protected]; [email protected]
Abstract: Scrub typhus is a vector-borne infectious disease caused by Orientia tsutsugamushi. Accurate and timely diagnosis at the early infection stage could save the patients’ lives. Traditional technologies were limited to rapidly and successfully detecting Orientia tsutsugamushi due to poor specificity, especially in the condition of atypical symptoms. The technology of Metagenomic next-generation sequencing (mNGS) is amenable to finding the real pathogen because it holds potential as a diagnostic platform for unbiased pathogen identification and precision medicine. Herein, we reported two clinical case reports relative to the Orientia tsutsugamushi infection diagnosed by mNGS. We hope these two cases will improve clinical diagnosis.
Keywords: scrub typhus, metagenomic next-generation sequencing, Orientia tsutsugamushi
Introduction
Scrub typhus, a kind of zoonotic bacterial infection, is becoming a threat to public health,1 which usually occurs to field operators, especially those living in remote and rural areas.2 Scrub typhus is caused by Orientia tsutsugamushi that is a kind of bacteria of gram-negative coccobacillus,3 the latent period is usually 5 to 14 days after exposure, main clinical manifestations of scrub typhus may be associated with high fever, rash, eschar, headache, vomiting, and other non-specific symptoms.4 Among these clinical symptoms, fever is the most common symptom.5 An eschar is considered a unique diagnostic clue.6 According to previous literature, depending on the geographical area and study, eschar was found in 1% and 97% of patients,7 therefore, it is difficult to diagnose those patients without eschar. The mNGS, also known as unbiased NGS or clinical metagenomics, is a technology where all the nucleic acid (DNA or RNA) of a specimen is sequenced in parallel. mNGS uses high-throughput sequencing of nucleic acids extracted from biological samples, and uses bioinformatics for comparison and analysis to obtain information on the types and abundance of microorganisms contained in the samples, the detection comprehensively covered more than 16,000 different pathogens. In this study, We reported two cases of scrub typhus detected by mNGS in detail.
Methods
The negative quality control we used in mNGS was the human genome, and the reference was the plant-derived Arabidopsis thaliana genome. The source of clinical sample used was collected from the peripheral venous blood of the two patients.
Case Presentation 1
The patient, a 57-year-old male, a rural resident occupying in farming, had 2 years of history of hypertension and gout, was delivered to the Department of Nephrology due to high urinary creatinine increase (1221.75 μmol/L). His vital signs are as follows, BP: 143/83mmHg, RR:18 bpm, HR: 90 bpm, while frequent urination (4 to 5 times a day) and weight loss of 4 kilograms in a month, no abnormal status in other systems, no eschar. Laboratory blood tests demonstrated white blood cell count: 5.05×109/L, hemoglobin concentration: 82g/L, platelet count: 215×109/L, C reactive protein (CRP): 38.9mg/L, Creatine kinase (CK): 54U/L, creatine kinase isoenzyme MB: 11.7U/L, urine routine urine protein ++, urine albumin ++, 24 hours total urine albumin: 353.1mg/24h, stool routine occult blood test +, transferrin + (immune method). He was initially diagnosed with chronic kidney disease stage 5 with anemia, chronic nephritis syndrome, gouty arthritis, and hypertension grade 3.
The patient developed chills and fever during the hemodialysis process on the 6th day of hospitalization. The highest body temperature was 37.5°C. After the patient returned to the ward, he developed obvious chills at the bedside. At that time, the body temperature was measured at 39.5°C. The infection related to the dialysis catheter was suspected. After assessment of PCT, blood culture, and other indicators, the hemodialysis catheter was removed temporarily, and bacterial and fungal cultures were performed. The patient has a persistent high fever, up to 40°C, and the body temperature did not drop even after being given physical cooling such as alcohol and ice, or an oral drug such as ibuprofen. Besides, it still does not work either using cefoperazone sodium/sulbactam sodium injection (1500mg intravenous infusion q12h) or vancomycin injection (500mg intravenous infusion q3d).
Fortunately, we received an mNGS report from the laboratory, which demonstrated Orientia tsutsugamushi in peripheral blood (Figure 1). Simultaneously, a comprehensive skin examination was performed where there was no obvious eschar or lymphadenopathy. The previous anti-infective regimen was discontinued and changed to 100mg doxycycline tablets orally q12h and moxifloxacin tablets 400mg orally qd. After five days of changing antibiotic treatment, the patient’s symptoms abated (Figure 2). Considering that the anti-infection is effective, we continued suggesting the patient keep the current anti-infective regimen after his discharge. Finally, the patient recovered uneventfully after anti-infective therapy.
 |
Figure 1 Necleotide Position along Orientia tsutsugamushi Genome. |
 |
Figure 2 A flowchart describing all of the steps leading to the conclusion of Orientia tsutsugamushi. |
Case Presentation 2
The patient, a 76-year-old female with a medical history of high pressure, was bitten by an unknown insect while applying pesticides in a farmyard 11 days ago and the bitten wound was not treated thereafter. One week ago, she developed symptoms of recurrent low-grade fever and aching limbs and then visited the Second People’s Hospital of Huadu District. However, the symptoms did not abate, then she was delivered to Guangdong Provincial People’s Hospital for further treatment.
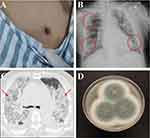
The vital signs on admission were T: 37°C, BP: 102/68mmHg, RR:18 bpm, HR: 101 bpm. On physical examination, there was a notable eschar on the anterior chest wall (Figure 3A). Computed tomography (CT) and X-ray of the chest showed multiple exudations from bilateral lungs (Figure 3B and C). The clinical laboratory results are as follows, CRP: 1584g/L, D-dimer: 9650ng/L, Plasma fibrinogen content: 1.36g/L, Activated partial thromboplastin time: 54.5s, total bilirubin: 57.4μmo/L, Binding bilirubin: 37.4μmo1/L, Alanine aminotransferase: 78U/L. Empirical anti-infection (tazobactam-piperacillin) and anti-inflammatory (injectable cefoperazone sodium/sulbactam sodium and moxifloxacin sodium chloride) treatment was performed. However, the patient still had a fever and the infection index was gradually elevated. The bronchial luma of the right main bronchus and each of the bronchial segments were unobtrusively examined by bronchoscopy, and the mucous membranes were severely congested with erosion and secretions, which were rinsed and collected for etiological mNGS examination. The mNGS results showed the Orientia tsutsugamushi pathogen. The patient was diagnosed with scrub typhus and received anti-infective treatment with doxycycline. After two-day treatment, The index of infection began to decline progressively.
However, the patient developed a fever of unknown origin though doxycycline plus moxifloxacin were administered for 5 days. Fortunately, with the culture result of the broncho alveolar lavage fluid (BALF), the Aspergillus fumigatus, a kind of fungi, was found (Figure 3D) Voriconic needle was recommended to be given the first dose of 400mg, followed by 200mg q12h intravenous drip. After one day of antifungal treatment, the patient’s shortness of breath abated significantly. Considering the effectiveness of anti-fungal infection, the current anti-infection regimen was recommended to continue. Unfortunately, on the second day of treatment, the patient developed septic shock accompanied by high fever, increasing infection indicators (CRP and PCT), and clammy limbs. The patient was in critical condition with severe lung infection, severe respiratory failure, and poor oxygenation index. At the request of her family, she was discharged from the hospital.
Discussion
With the development of gene sequencing methods, mNGS technology has played a good role in disease diagnosis. At present, the laboratory methods for diagnosis of scrub typhus in China are mainly based on serological and molecular biology experiments, and the proportion of laboratory diagnoses is only 4.7% in the last 11 years.8 Indirect immunofluorescence testing (IFA) is the gold standard for diagnosing tsutsugamushi disease,7 while it requires trained testing personnel and specialized equipment.9 Enzyme-linked immunosorbent assay showed good sensitivity and specificity, but it was easily affected by the time and quality of specimen collection.10 Though the Weil-Felix technology is the cheapest and most readily available serological test, it is less specific and sensitive.11 Molecular diagnostic methods such as Polymerase chain reaction (PCR) are the most commonly used method for diagnosing tsutsugamushi disease, However, in the case of diagnosing tsutsugamushi disease without eschar, it is not easy to choose PCR for diagnosis. Pathogen culturing is difficult and requires professional personnel and experimental centers, which is limited to carry out on a large scale.12 The mNGS is an open analysis and diagnosis system that can objectively detect nucleic acid sequences of pathogenic microorganisms in clinical specimens (including viruses, bacteria, fungi, parasites, etc.) directly by high-throughput sequencing of nucleic acids in clinical samples, and then compare and analyze with the database. At present, mNGS can detect more than 8000 pathogens, and the detection sequence does not require specific amplification, and is not affected by the use of antibiotics, with high specificity and sensitivity, especially suitable for the diagnosis of acute and severe infections. Liu et al reported that mNGS was significantly superior to traditional detection methods such as Weil-Felix technology, IFA, qPCR, and germiculture in the early diagnosis of scrub typhus.13 More importantly, mNGS can be exploited as an effective detection method to diagnose rickettsial disease without typical clinical symptoms. Li et al and Wu et al respectively reported a case in which traditional etiological tests were negative while scrub typhus without eschar was successfully diagnosed by mNGS.14,15 Liu et al reported a case of scrub typhus with a high D-dimer level and urinary tract infection as the manifestation of atypical clinical symptoms diagnosed by mNGS, indicating that mNGS is an effective method to identify atypical clinical manifestations of infection.16 A cohort study by Liu et al showed that the sensitivity of mNGS to detect Orientia tsutsugamushi was 100%, much higher than that of fluorescence quantitative polymerase chain reaction (11.1%). The sensitivity results were consistent with the results of this study, emphasizing that mNGS could be routinely used for the early diagnosis of infectious diseases, especially for infections that are difficult to identify by traditional diagnostic methods.13 Orientia tsutsugamushi is an obligatory intracellular bacterium, and once its sequence is detected by mNGS, it should be considered as a possible pathogenic agent.17
In this case of unknown fever, mNGS could be chosen to find the answer. Of note, as the second case report depicted, the patient still developed a high fever though the patient was verified as a tsutsugamushi-associated infection via mNGS. We should conduct further identification based on the patient’s clinical manifestations (pulmonary infection), and traditional measures such as bronchoscopy, to ultimately determine the final cause of the patient’s fever.
Conclusion
mNGS could play a critical role in the diagnosis of fever caused by clinical unknown origin such as scrub typhus. It’s important to note that we could not just rely on mNGS to make the final judgment, in some cases, the patient’s clinical presentation and other clinically assisted diagnostic methods to make a comprehensive judgment should also be considered.
Ethics and Consent
Patient 1 and Patient 2 both consented to the publication of the case and have signed the consent form. All procedures carried out comply with the ethical standards of the Responsible Committee on Human Experimentation (institutional and national) and the principles of the Declaration of Helsinki. Informed consents were obtained from the patients for being included in this report. The authors confirm that both patients provided written informed consent for their case details and images to be published. The two case reports were approved by Guangdong Provincial People’s Hospital (KY-N-2022-003-03).
Funding
This research was supported by the Research Foundation for Advanced Talents of Guangdong Provincial People’s Hospital (KY012023289) and the Key R & D Program of Jiangsu Province (BE2020646).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Kelly DJ, Fuerst PA, Ching WM, Richards AL. Scrub typhus: the geographic distribution of phenotypic and genotypic variants of Orientia tsutsugamushi. Clin Infect Dis. 2009;48(Suppl 3):S203–230. doi:10.1086/596576
2. Walker DH, Fishbein DB. Epidemiology of rickettsial diseases. Eur J Epidemiol. 1991;7(3):237–245. doi:10.1007/BF00145672
3. Paris DH, Shelite TR, Day NP, Walker DH. Unresolved problems related to scrub typhus: a seriously neglected life-threatening disease. Am J Trop Med Hyg. 2013;89(2):301–307. doi:10.4269/ajtmh.13-0064
4. Mahajan SK. Scrub typhus. J Assoc Physicians India. 2005;53:954–958.
5. Tsay RW, Chang FY. Serious complications in scrub typhus. J Microbiol Immunol Infect. 1998;31(4):240–244.
6. Xu G, Walker DH, Jupiter D, et al. A review of the global epidemiology of scrub typhus. PLoS Negl Trop Dis. 2017;11(11):e0006062. doi:10.1371/journal.pntd.0006062
7. Koraluru M, Bairy I, Varma M, Vidyasagar S. Diagnostic validation of selected serological tests for detecting scrub typhus. Microbiol Immunol. 2015;59(7):371–374. doi:10.1111/1348-0421.12268
8. Xin HL, Yu JX, Hu MG, et al. Evaluation of scrub typhus diagnosis in China: analysis of nationwide surveillance data from 2006 to 2016. Infect Dis Poverty. 2019;8(1):59. doi:10.1186/s40249-019-0566-0
9. Gautam R, Parajuli K, Tshokey T, et al. Diagnostic evaluation of IgM ELISA and IgM Immunofluorescence assay for the diagnosis of Acute Scrub Typhus in central Nepal. BMC Infect Dis. 2020;20(1):138. doi:10.1186/s12879-020-4861-y
10. Qi Y, Yin Q, Shao Y, et al. Development of a rapid and visual nucleotide detection method for a Chinese epidemic strain of Orientia tsutsugamushi based on recombinase polymerase amplification assay and lateral flow test. Int J Infect Dis. 2018;70:42–50. doi:10.1016/j.ijid.2018.03.003
11. Patricia KA, Hoti SL, Kanungo R, et al. Improving the diagnosis of Scrub typhus by combining groEL based polymerase chain reaction and IgM ELISA. J Clin Diagn Res. 2017;11(8):DC27–DC31. doi:10.7860/JCDR/2017/26523.10519
12. Ajapakse S, Weeratunga P, Sivayoganathan S, Fernando SD. Clinical manifestations of scrub typhus. Trans R Soc Trop Med Hyg. 2017;111(2):43–54. doi:10.1093/trstmh/trx017
13. Liu X, Zhang Y, Zhang J, et al. The early diagnosis of scrub typhus by metagenomic next-generation sequencing. Front Public Health. 2021;9:755228. doi:10.3389/fpubh.2021.755228
14. Li J, Chen CW, Zou FQ, et al. Diagnosing scrub typhus without eschar: a case report using metagenomic next-generation sequencing (mNGS). Ann Transl Med. 2021;9(14):1190. doi:10.21037/atm-21-3015
15. Wu J, Wu YM, Huang M. Metagenomic next-generation sequencing helped diagnose scrub typhus without eschar: a case report. Int J Infect Dis. 2020;90:1–4. doi:10.1016/j.ijid.2019.10.020
16. Liu MF, Liu Y, Xu DR, Wan L-G, Zhao R. mNGS helped diagnose scrub typhus presenting as a urinary tract infection with high D-dimer levels: a case report. BMC Infect Dis. 2021;21(1):1219. doi:10.1186/s12879-021-06889-9
17. Li N, Cai QQ, Miao Q. High-throughput metagenomics for identification of pathogens in the clinical settings. Small Methods. 2021;5(1):2000792. doi:10.1002/smtd.202000792
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.