Back to Journals » Cancer Management and Research » Volume 11
Meta-analysis of comparing part-solid and pure-solid tumors in patients with clinical stage IA non-small-cell lung cancer in the eighth edition TNM classification
Authors Jiang T, Li M , Lin M, Zhao M, Zhan C , Feng M
Received 30 November 2018
Accepted for publication 25 February 2019
Published 10 April 2019 Volume 2019:11 Pages 2951—2961
DOI https://doi.org/10.2147/CMAR.S196613
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Harikrishna Nakshatri
Tian Jiang, Ming Li, Miao Lin, Mengnan Zhao, Cheng Zhan, Mingxiang Feng
Department of Thoracic Surgery, Zhongshan Hospital, Fudan University, Shanghai 200032, People’s Republic of China
Objective: The aim of the study was to compare the prognoses between part-solid and pure-solid tumors for clinical stage IA non-small-cell lung cancer (NSCLC) patients in the eighth edition TNM classification.
Methods: We searched the literature in PubMed and Web of Science for all eligible articles published before November 31, 2018. The pooled data included overall survival (OS), disease-free survival (DFS) and recurrence-free survival (RFS). The hazard ratio (HR) of OS (pure-solid/part-solid) was used as the measure of differential effects. Pure-solid or part-solid tumors in all studies included were matched according to the solid component size or according to the eighth edition TNM classification.
Results: Seven studies including 2,037 patients with c-stage IA NSCLC were pooled in the meta-analysis. Patients with pure-solid tumors had significantly poorer OS (HR 1.69, 95% CI 1.21‒2.35, P=0.002), DFS (HR 1.27, 95% CI 1.07‒1.51, P=0.006) and RFS (HR 1.74, 95% CI 1.08‒2.80, P=0.020). In subgroup analyses, when the meta-analysis was limited to T1a-1b (≤2 cm) lung cancer, the prognosis for pure-solid tumors was inferior to that for part-solid tumors regarding both OS and RFS. In adenocarcinoma subgroup, there was no difference between the two groups in terms of OS and RFS, but we detected a meaningful difference in DFS.
Conclusion: Part-solid tumors may have a better prognosis than pure-solid tumors in clinical stage IA patients according to the eighth edition TNM classification, and similar results were found for the T1a-1b (≤2 cm) subgroup. There were no substantial differences in OS and RFS between two groups in lung adenocarcinoma. However, we detected a meaningful difference in DFS, which might also suggest a superior prognosis for part-solid tumors. We propose that the part-solid and pure-solid tumors in the same T component category be considered separately.
Keywords: part-solid, pure-solid, stage IA, adenocarcinoma, lung cancer, meta-analysis
Introduction
Traditionally, pure-solid tumors are considered more malignant than part-solid tumors. Pure-solid tumors, even those radiologically found to be small, have a high probability of nodal involvement or lymphatic invasion.1,2 However, only approximately 4% of part-solid tumors have positive nodal involvement.3 Moreover, several studies have indicated that the size of the solid component is a more accurate measurement in predicting the prognosis of patients with stage IA lung non-small-cell lung cancer (NSCLC).4–10
In contrast, for part-solid tumors, the diameter of the solid component is not a prognostic factor. Hattori2 has revealed that the maximum tumor size can be applied only for solid tumors. A ground-glass opacity (GGO) component is a more favorable prognostic indicator,8 and even a small GGO component indicates a better survival in patients with lung adenocarcinoma.11 Pathologically, most of the part-solid cases are minimally invasive.12 Hattori13 has also reported that the survival outcomes significantly differ between subsolid tumors and pure-solid tumors in each clinical T categories in the seventh edition TNM classification.
The eighth edition TNM classification14 recommends that T categories are described according to the tumor size. The American Joint Committee on Cancer15 recommends that only the size of the solid component on CT, or the size of the invasive component in pathological results, be considered when defining the T category. Tumors with the same solid component size can be further divided into two groups: part-solid tumors and pure-solid tumors. Therefore, according to the eighth edition, for the same T stage, whether the prognosis is similar for patients with part-solid tumors compared with pure-solid tumors, and whether they should be treated similarly remains unclear.
In recent years, some studies have reported no differences in the prognosis between the two groups according to the eighth edition,10,16,17 whereas others have found a better prognosis in part-solid tumors.4,9,18 Hence, the objective of our study was to compare the outcomes for pure-solid tumors versus part-solid tumors with the same solid component size in c-stage IA patients, and to explore the justifications for the newly proposed T descriptors in the latest edition.
Methods
Literature search strategy
The analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines19 (Figure 1). The primary procedures were as follows. We searched the PubMed and Web of Science databases for all eligible articles on part-solid and pure-solid tumors in patients with c-stage IA lung adenocarcinoma, published before November 31, 2018. The strategy of keyword search was as follows: (NSCLC OR non-small cell lung cancer OR adenocarcinoma) AND ((part-solid AND solid) OR (mixed AND solid) OR (solid AND subsolid)). No language restrictions were imposed, but only human studies were included. All searched articles were then further assessed manually according to eligibility.
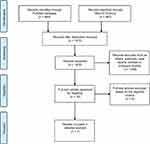 | Figure 1 Flow diagram of the meta-analysis study selection process. |
Eligibility criteria
Two authors (Tian Jiang and Ming Li) assessed each identified study independently and listed all qualified studies if the studies satisfied the following eligibility criteria: 1) study of patients with clinical stage IA lung adenocarcinoma; 2) comparison of part-solid and pure-solid tumors on the basis of the solid component size or the eighth edition TNM classification; 3) comparison of overall survival (OS), disease-free survival (DFS) or recurrence-free survival (RFS) between part-solid and pure-solid tumors; 4) availability of full article text. In addition, we included only the most updated or complete publications when overlapped or insufficient data were present. Records such as letters, editorials, case reports, reviews, irrelevant studies or non-English publications were excluded.
Data extraction and analysis
The following information collected by two authors (Tian J. and Ming Li.) independently: author name, year of publication, author institution, study design, study period, number of enrolled part-solid and pure-solid tumors, age, median follow-up, hazard ratio, 95% confidence interval (CI) and prognosis outcomes. The primary outcomes were OS, DFS and RFS. We preferred survival data after the propensity score matching, for minimizing the selection bias and confounding and matching the effects among the character variables. OS was defined as the time from the date of surgery to the date of death from any cause or to the date of the last follow-up. DFS was considered as the time from the date of surgery to the first event (relapse, progression or death from any cause) or last follow-up. RFS was defined as the time from the date of surgery until the date of recurrence of cancer. Data were extracted from article texts, tables and figures. Any discrepancies between the independently extracted data sets were resolved by discussion until a consensus was reached. The non-randomized controlled studies were assessed with the Newcastle-Ottawa scale on the basis of four main aspects: selection, comparability, exposure and outcomes.
Statistical analysis
We used Review Manager software (Version 5.3) to perform the meta-analysis. Continuous data and dichotomous data were analyzed by using weighted mean differences with 95% CI and odds ratio (OR) with 95% CI, respectively. The method reported by Tierney20 was used to extract the survival data from article figures, and conditional logistic regression was used to calculate the HRs of survival outcome for pure-solid tumors compared with part-solid tumors. Heterogeneity was assessed with P-values and the I2 index. A P-value≤0.05 was considered statistically significant. I2<25% was regarded as low heterogeneity, 25‒75% as moderate and >75% as high.21 Random effect or fixed effect was chosen according to the significance of heterogeneity. The included studies were excluded one by one to analyze the sensitivity. Publication bias was evaluated with funnel plots.
Results
Study and patient characteristics
In total, we screened 1,531 abstracts through searching PubMed and Web Of Science, of which 16 records were assessed for eligibility after exclusion of duplicates, letters, editorials, case reports or other irrelevant studies. Seven studies were deemed to meet the inclusion criteria described above for further meta-analysis. All seven studies were observational reports. Notably, a study from Hwang16 published in 2016 was excluded, owing to overlapping data with the other study from the same author.9 A review of study characteristics is presented in Table 1, and the detailed staging of part-solid and pure-solid tumors is presented in Table 2. Part-solid or pure-solid tumors in all studies included for further meta-analysis were matched according to the size of the solid component on the basis of the eighth edition.
 | Table 1 Main characteristics of the included studies |
 | Table 2 A summary of the staging of lung cancer in comparative studies for part-solid tumors versus pure-solid tumors |
Survival outcomes between part-solid and pure-solid tumors
In total, five studies including eight subgroups compared part-solid tumors to solid tumors to assess the OS. Three studies presented evidence of a better survival outcome associated with part-solid tumors compared with pure-solid tumors, whereas two studies demonstrated no difference in OS between the two groups. Comparative data indicated that part-solid tumors were associated with a better OS than pure-solid tumors (HR 1.69, 95% CI 1.21‒2.35, P=0.002; Figure 2A). The pooled data of four studies indicated that part-solid tumors have a significant advantage in DFS, as compared with pure-solid tumors (HR 1.27, 95% CI 1.07‒1.51, P=0.006; Figure 2B). Moreover, three studies including six subgroups provided available data on RFS. The HR comparing pure-solid and part-solid tumors was 1.74 (95% CI 1.08‒2.80, P=0.020; Figure 2C). The pooled data showed that pure-solid tumors had a significantly shorter RFS than part-solid tumors. The included studies were excluded one by one to analyze the sensitivity. Heterogeneity tests showed a high heterogeneity for OS (Chi2=40.36, df=6, P<0.001, I2=85%) and RFS (Chi2=40.95, df=5, P<0.001, I2=88%), but a low heterogeneity for DFS (Chi2=4.02, df=3, P=0.260, I2=25%). The funnel plots for OS, DFS and RFS were symmetrical (Figure 3).
Survival outcomes between part-solid and pure-solid tumors in T1a-1b (≤2 cm) lung cancer
We then performed a subgroup analysis based on tumor size. When the meta‐analysis was limited to three and two studies presenting data on T1a-1b (≤2 cm) lung cancer, the pure-solid tumors might be inferior to part-solid tumors regarding both OS and RFS. The combined HR was 1.91 (95% CI 1.22‒2.99, P=0.005; Figure 4A) and 2.33 (95% CI 1.62‒3.35, P<0.001; Figure 4B), respectively. However, the pooled data demonstrated no difference in DFS between the two groups (HR 1.73, 95% CI 0.82‒3.68, P<0.001; Figure 4C). The heterogeneity for outcomes in T1a-1b lung cancer was moderate; the I2 statistic for heterogeneity for OS was 74% (Chi2=11.56, df=3, P=0.009), for RFS 55% (Chi2=4.46, df=2, P=0.110) and for DFS 67% (Chi2=3.04, df=1, P=0.080). The included studies were excluded one by one to analyze the sensitivity. The funnel plots for OS, DFS and RFS were symmetrical (Figure 5).
Survival outcomes between part-solid and pure-solid tumors in lung adenocarcinoma
When we stratified trials based on the pathologic findings and compared the prognosis for pure-solid and part-solid tumors in lung adenocarcinoma, we detected no significant trends in OS (HR 1.22, 95% CI 0.57‒2.59, P=0.610; Figure 6A) and RFS (HR 1.43, 95% CI 0.91‒2.26, P=0.130; Figure 6B). However, we detected a significant interaction in DFS, with a significant risk increase in pure-solid tumors compared with part-solid tumors (HR 1.30, 95% CI 1.07‒1.58, P=0.009; Figure 6C). The heterogeneity for OS in lung adenocarcinoma was significantly high (Chi2=8.60, df=2, P=0.010, I2=77%), that for DFS was moderate (Chi2=3.86, df=2, P=0.150, I2=48%), and that for RFS was low (Chi2=0.49, df=2, P=0.780, I2=0%). The included studies were excluded one by one to analyze the sensitivity. The funnel plots for OS, DFS and RFS were symmetrical (Figure 7).
Discussion
The eighth edition classification suggests that the T component category is described on the basis of the solid component size.22 According to the context of the new edition classification, it was difficult for us to differentiate the radiological part-solid from pure-solid tumors because both were in the same T category if they had the same solid component size. Moreover, previous studies have indicated a significant prognostic difference between part-solid and pure-solid tumors.11–13 Therefore, it is essential to determine whether part-solid tumors and pure-solid tumors have similar prognoses, and whether the two groups in the same T component category should be considered separately.
A retrospective study from Hattori13 previously demonstrated the importance of the GGO component in the T category. Hattori has revealed that the solid component size and the presence of the GGO component are considered as independent prognostic factors for OS. The results of the study also suggested that even in the same T category, both pathological results and survival outcomes differed significantly between part-solid tumors and pure-solid tumors. A study from Shin18 has indicated that although the maximum tumor size of part-solid tumors is larger than that of pure-solid tumors, the prognosis of part-solid tumors is still better. In a report by Tsutani,4 after matching of the solid component size, the pure-solid tumors, compared with their part-solid counterparts, were found to be more malignant and to have a poorer DFS. However, the results of the two most recent retrospective studies,17,23 in which the distinction between part-solid and pure-solid might not be considered as important, are inconsistent with the findings from the studies mentioned above.
In our meta-analysis, part-solid tumors were associated with a better prognosis than pure-solid tumors (OS: HR 1.69, 95% CI 1.21‒2.35, P=0.002; DFS: HR 1.27, 95% CI 1.07‒1.51, P=0.006; RFS: HR 1.74, 95% CI 1.08‒2.80, P=0.020). Beyond tumor size, some other prognostic predictors might result in the discrepancy in prognosis between the two groups. SUVmax, for example, has been reported as a significant predictor of malignant behavior,24,25 and pure-solid tumors are associated with a high SUVmax value and thus have greater malignant potential. Tsutani4 has conducted a matched analysis to compare the survival outcomes of pure-solid tumors and part-solid tumors with the same solid component. The results showed that the pure-solid tumors were more frequently associated with a high SUVmax value and a poorer prognosis. However, after matching of both solid component size and SUVmax value, the prognostic difference in two groups disappeared. Moreover, the predominant subtype remained different between pure-solid and part-solid lung adenocarcinoma, thus potentially also leading to the discrepancy in prognosis. The lepidic subtype was associated with a better prognosis, whereas the solid and micropapillary subtypes were associated with a poorer prognosis.26 The solid and micropapillary subtypes are frequently observed in pure-solid lung tumors.18 In contrast, even with the same solid component size and a larger maximum tumor size, part-solid adenocarcinomas have a higher proportion of the lepidic subtype. Collectively, our meta-analysis results indicating that part-solid tumors are associated with a favorable prognosis may challenge the justification for the eighth edition of TNM classification of lung cancer.
To eliminate the bias of different staging systems included in the literature, we stratified trials by tumor size. The subgroup analysis from Hwang’s study9 revealed that only part-solid tumors with a solid component size ≤2 cm had a better prognosis (DFS and OS) than pure-solid tumors, whereas part-solid tumors with >2 cm solid component size did not. Thus, it seems that as the solid component size increases, the prognosis of part-solid tumors dramatically worsens and approaches that of pure-solid tumors. However, Su10 has found that part-solid tumors with a solid component size neither ≤2 cm nor 2–3 cm show significant longer RFS than pure-solid tumors. Owing to a lack of evidence, whether the discrepancy in the prognosis between pure-solid and part-solid tumors differs as the solid component size increases remains unclear. In stratified analyses, similar results were obtained for stage T1a-1b (≤2 cm) lung cancer. The results revealed that part-solid tumors are associated with a better OS and RFS than pure-solid tumors. Interestingly, the HRs of OS, RFS and DFS were all higher in the T1a-1b subgroup. Hattori27 has revealed that even small (<2 cm) pure-solid tumors have a frequent locoregional recurrence after segmentectomy. On the basis of previous studies and our results, sublobar resection might be applied with great caution for pure-solid tumors, even small tumors, and might be appropriate for part-solid tumors as long as the solid component size meets the criteria.
In stratified analyses based on pathological findings, we saw no strong differences in OS and RFS between part-solid and pure-solid tumors in lung adenocarcinoma, results consistent with findings from the previous studies,10,18,23 but inconsistent with those from Hwang.9 In Hwang’s study, however, only a small number of recurrences or deaths from part-solid tumors was observed, owing to their rarity. These effects might also have been found because the lung adenocarcinoma has its own specific biological characteristics. Fukui28 and Kawase29 have both reported that patients with stage I adenocarcinoma have significantly better OS than those with stage I squamous cell carcinoma. Therefore, it seemed that the discrepancy in prognosis decreased between part-solid tumors and pure-solid tumors in terms of adenocarcinoma. However, this result needed to be interpreted with caution. Firstly, the articles included for analysis of adenocarcinoma was limited. Secondly, though there was no significant trend in OS and RFS, we detected a significant interaction in DFS, with a significant risk increase in pure-solid tumors than in part-solid tumors (HR 1.30, 95% CI 1.07‒1.58, P=0.009), consistent with Shin’s article.18 Indeed, since the tyrosine kinase inhibitor or the immunotherapy was effective to some relapsed or metastatic adenocarcinoma, the OS might not be an optimal parameter to compare two groups in adenocarcinoma. Thirdly, in Yamanashi’s article,23 they performed the comparison between two groups with propensity score matching, so the discrepancy of HR for RFS might disappear. Thus, we hope that there are more studies to further explore the survival outcomes of pure-solid and part-solid tumors of adenocarcinoma.
Limitations
The main limitation of this meta-analysis is that only seven studies were included, all of which were retrospective. A further limitation was that the studies varied in several aspects, including differences in pathological results, tumor size, tumor evaluation methods, GGO ratios of part-solid tumors and treatment regimens. For example, we noticed the effect of the surgical procedure on the prognosis for part-solid tumors and pure-solid tumors. With regard to the operative modes, sub-lobar lung resection is appropriate for pure ground-glass nodules or radiologically noninvasive part-solid tumors, whereas lobectomy with systematic or selective lymph node sampling is warranted for pure-solid or radiologically invasive part-solid tumors.30–33 However, whether various surgical methods might influence the prognosis for part-solid and pure-solid tumors warrants further investigation. In the current study, we stratified studies by tumor size and tumor type to attenuate bias. However, more detailed analysis of other subgroups is not shown owing to a paucity of data. Further studies with a large sample and randomized controlled trials are needed in the future for a more reliable conclusion.
Conclusion
In conclusion, part-solid tumors have a better prognosis than pure-solid tumors in c-stage IA patients, according to the eighth edition of TNM classification, and similar results were found for the T1a-1b (≤2 cm) subgroup. Though were no substantial differences in OS and RFS between two groups in lung adenocarcinoma, we detected a meaningful difference in DFS, which might also suggest a superior prognosis for part-solid tumors. We propose that the part-solid and pure-solid tumors in the same T component categories be considered separately.
Acknowledgments
This work was supported by the Training Programme for the Talents of Zhongshan Hospital, Fudan University (Grant No. 2015ZSYXGG03), and the National Natural Science Foundation of China (Grant Nos. 81370587, 81500568). We thank International Science Editing for editing this manuscript.
Author contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Suzuki K, Kusumoto M, Watanabe S, Tsuchiya R, Asamura H. Radiologic classification of small adenocarcinoma of the lung: radiologic-pathologic correlation and its prognostic impact. Ann Thorac Surg. 2006;81(2):413–419. doi:10.1016/j.athoracsur.2005.07.058
2. Hattori A, Matsunaga T, Takamochi K, Oh S, Suzuki K. Neither maximum tumor size nor solid component size is prognostic in part-solid lung cancer: impact of tumor size should be applied exclusively to solid lung cancer. Ann Thorac Surg. 2016;102(2):407–415. doi:10.1016/j.athoracsur.2016.02.074
3. Matsunaga T, Suzuki K, Takamochi K, Oh S. What is the radiological definition of part-solid tumour in lung cancer ? Dagger. Eur J Cardiothorac Surg. 2017;51(2):242–247. doi:10.1093/ejcts/ezw344
4. Tsutani Y, Miyata Y, Yamanaka T, et al. Solid tumors versus mixed tumors with a ground-glass opacity component in patients with clinical stage IA lung adenocarcinoma: prognostic comparison using high-resolution computed tomography findings. J Thorac Cardiovasc Surg. 2013;146(1):17–23. doi:10.1016/j.jtcvs.2012.11.019
5. Tsutani Y, Miyata Y, Nakayama H, et al. Solid tumor size on high-resolution computed tomography and maximum standardized uptake on positron emission tomography for new clinical T descriptors with T1 lung adenocarcinoma. Ann Oncol. 2013;24(9):2376–2381. doi:10.1093/annonc/mdt230
6. Tsutani Y, Miyata Y, Nakayama H, et al. Prognostic significance of using solid versus whole tumor size on high-resolution computed tomography for predicting pathologic malignant grade of tumors in clinical stage IA lung adenocarcinoma: a multicenter study. J Thorac Cardiovasc Surg. 2012;143(3):607–612. doi:10.1016/j.jtcvs.2011.10.037
7. Hattori A, Matsunaga T, Hayashi T, Takamochi K, Oh S, Suzuki K. Prognostic impact of the findings on thin-section computed tomography in patients with subcentimeter non-small cell lung cancer. J Thorac Oncol. 2017;12(6):954–962. doi:10.1016/j.jtho.2017.02.015
8. Hattori A, Matsunaga T, Takamochi K, Oh S, Suzuki K. Importance of ground glass opacity component in clinical stage IA radiologic invasive lung cancer. Ann Thorac Surg. 2017;104(1):313–320. doi:10.1016/j.athoracsur.2017.01.076
9. Hwang EJ, Park CM, Ryu Y, et al. Pulmonary adenocarcinomas appearing as part-solid ground-glass nodules: is measuring solid component size a better prognostic indicator ? Eur Radiol. 2015;25(2):558–567. doi:10.1007/s00330-014-3441-1
10. Su H, Dai C, She Y, et al. Which T descriptor is more predictive of recurrence after sublobar resection: whole tumour size versus solid component size ? Eur J Cardiothorac Surg. 2018;54:1028–1036. doi:10.1093/ejcts/ezy225
11. Berry MF, Gao R, Kunder CA, et al. Presence of even a small ground-glass component in lung adenocarcinoma predicts better survival. Clin Lung Cancer. 2018;19(1):e47–e51. doi:10.1016/j.cllc.2017.06.020
12. Lee KH, Goo JM, Park SJ, et al. Correlation between the size of the solid component on thin-section CT and the invasive component on pathology in small lung adenocarcinomas manifesting as ground-glass nodules. J Thorac Oncol. 2014;9(1):74–82. doi:10.1097/JTO.0000000000000019
13. Hattori A, Matsunaga T, Takamochi K, Oh S, Suzuki K. Prognostic impact of a ground glass opacity component in the clinical T classification of non-small cell lung cancer. J Thorac Cardiovasc Surg. 2017;154(6):2102–2110. e2101. doi:10.1016/j.jtcvs.2017.08.037
14. Chansky K, Detterbeck FC, Nicholson AG, et al. The IASLC lung cancer staging project: external validation of the revision of the TNM stage groupings in the eighth edition of the TNM classification of lung cancer. J Thorac Oncol. 2017;12(7):1109–1121. doi:10.1016/j.jtho.2017.04.011
15. Rami-Porta R, Asamura H, Travis WD, Rusch VW. Lung cancer – major changes in the American joint committee on cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67(2):138–155. doi:10.3322/caac.21390
16. Hwang EJ, Park CM, Kim YT, Kim H, Goo JM. Microscopic invasions, prognoses, and recurrence patterns of stage I adenocarcinomas manifesting as part-solid ground-glass nodules: comparison with adenocarcinomas appearing as solid nodules after matching their solid parts’ size. Medicine. 2016;95(15):e3419. doi:10.1097/MD.0000000000004864
17. Takenaka T, Yamazaki K, Miura N, Takeo S. Prognostic ability of new T1 descriptors in the tumour, node and metastasis classification of surgically treated non-small-cell lung cancer. Interact Cardiovasc Thorac Surg. 2018;27:714–719. doi:10.1093/icvts/ivy164
18. Shin KW, Cho S, Chung JH, et al. Comparison of prognosis of solid and part-solid node-negative adenocarcinoma with the same invasive component size. Ann Thorac Surg. 2017;103(5):1654–1660. doi:10.1016/j.athoracsur.2016.10.040
19. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Reprint–preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Phys Ther. 2009;89(9):873–880.
20. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi:10.1186/1745-6215-8-16
21. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi:10.1136/bmj.327.7414.557
22. Travis WD, Asamura H, Bankier AA, et al. The IASLC lung cancer staging project: proposals for coding T categories for subsolid nodules and assessment of tumor size in part-solid tumors in the forthcoming eighth edition of the TNM classification of lung cancer. J Thorac Oncol. 2016;11(8):1204–1223. doi:10.1016/j.jtho.2016.03.025
23. Yamanashi K, Okumura N, Yamamoto Y, Takahashi A, Nakashima T, Matsuoka T. Comparing part-solid and pure-solid tumors in the TNM classification of lung cancer (eighth edition). Thorac Cardiovasc Surg. Epub 2018 May 29.
24. Nakayama H, Okumura S, Daisaki H, et al. Value of integrated positron emission tomography revised using a phantom study to evaluate malignancy grade of lung adenocarcinoma: a multicenter study. Cancer. 2010;116(13):3170–3177. doi:10.1002/cncr.25244
25. Okada M, Nakayama H, Okumura S, et al. Multicenter analysis of high-resolution computed tomography and positron emission tomography/computed tomography findings to choose therapeutic strategies for clinical stage IA lung adenocarcinoma. J Thorac Cardiovasc Surg. 2011;141(6):1384–1391. doi:10.1016/j.jtcvs.2011.02.007
26. Sica G, Yoshizawa A, Sima CS, et al. A grading system of lung adenocarcinomas based on histologic pattern is predictive of disease recurrence in stage I tumors. Am J Surg Pathol. 2010;34(8):1155–1162. doi:10.1097/PAS.0b013e3181e4ee32
27. Hattori A, Matsunaga T, Takamochi K, Oh S, Suzuki K. Locoregional recurrence after segmentectomy for clinical-T1aN0M0 radiologically solid non-small-cell lung carcinoma. Eur J Cardiothorac Surg. 2017;51(3):518–525. doi:10.1093/ejcts/ezw336
28. Fukui T, Taniguchi T, Kawaguchi K, et al. Comparisons of the clinicopathological features and survival outcomes between lung cancer patients with adenocarcinoma and squamous cell carcinoma. Gen Thorac Cardiovasc Surg. 2015;63(9):507–513. doi:10.1007/s11748-015-0564-5
29. Kawase A, Yoshida J, Ishii G, et al. Differences between squamous cell carcinoma and adenocarcinoma of the lung: are adenocarcinoma and squamous cell carcinoma prognostically equal ? Jpn J Clin Oncol. 2012;42(3):189–195. doi:10.1093/jjco/hyr188
30. Ginsberg RJ, Rubinstein LV;
31. Nakamura K, Saji H, Nakajima R, et al. A phase III randomized trial of lobectomy versus limited resection for small-sized peripheral non-small cell lung cancer (JCOG0802/WJOG4607L). Jpn J Clin Oncol. 2010;40(3):271–274. doi:10.1093/jjco/hyp156
32. Suzuki K, Koike T, Asakawa T, et al. A prospective radiological study of thin-section computed tomography to predict pathological noninvasiveness in peripheral clinical IA lung cancer (Japan Clinical Oncology Group 0201). J Thorac Oncol. 2011;6(4):751–756. doi:10.1097/JTO.0b013e31821038ab
33. Asamura H, Hishida T, Suzuki K, et al. Radiographically determined noninvasive adenocarcinoma of the lung: survival outcomes of Japan Clinical Oncology Group 0201. J Thorac Cardiovasc Surg. 2013;146(1):24–30. doi:10.1016/j.jtcvs.2012.12.047
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.






