Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Mesotherapy-Induced Cutaneous Foreign Body-Type Granulomatous Reaction in the Face Treated with Minocycline: Case Report and Literature Review
Authors Zhang Q , Yang L , Yang F , Liu L , Jiang X
Received 2 February 2023
Accepted for publication 23 March 2023
Published 3 April 2023 Volume 2023:16 Pages 861—867
DOI https://doi.org/10.2147/CCID.S403601
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Anne-Claire Fougerousse
Qian Zhang,1,* Lihua Yang,1,* Fengjuan Yang,1 Lian Liu,1 Xian Jiang1,2
1Department of Dermatology, West China Hospital, Sichuan University, Chengdu, People’s Republic of China; 2Laboratory of Dermatology, Clinical Institute of Inflammation and Immunology, Frontiers Science Center for Disease-Related Molecular Network, West China Hospital, Sichuan University, Chengdu, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xian Jiang, West China Hospital, Sichuan University, #37 Guoxue Alley, Wuhou District, Chengdu, Sichuan, 610041, People’s Republic of China, Tel +86-028-85423315, Fax +86-028-85422560, Email [email protected]
Abstract: Mesotherapy is a popular treatment that delivers substances deep into the skin but induces foreign body-type granulomatous reactions. However, such reactions caused by unauthorized use of topical tranexamic acid products in mesotherapy have never been reported before. We herein demonstrated a case of a 33-year-old woman with multiple mesotherapy-induced foreign body-type granulomas disseminated across her face. The patient was diagnosed with echo-color Doppler (ECD) and successfully treated with medications including minocycline hydrochloride. Then, we reviewed the differential diagnosis and current treatment for foreign body-type granulomatous reaction.
Keywords: mesotherapy, foreign body-type granulomatous reaction, tranexamic acid, minocycline, face
Introduction
Mesotherapy is a widely appreciated invasive technique in which microinjections of medications are delivered to dermis or deeper skin layers. Ease of operation and better transdermal permeation ability than topical application contributed to the popularity of the mesotherapy.1 Numerous adverse effects on account of mesotherapy have been reported, such as granulomatous reactions caused by products including phosphatidylcholine, deoxycholate, buflomedil, silica, carnitine, and many others.2 Tranexamic acid (TA) is a plasmin inhibitor which is normally used for preventing excessive bleeding, while it is also described as a safe and promising treatment for melasma.3 However, foreign body-type granulomatous reaction caused by intradermally injecting topical TA products has never been reported before. Currently, there is no standardized treatment for foreign body-type granulomatous reaction, intralesional injections of corticosteroids and surgery are often used but not always effective.4
Case Report
A 33-year-old woman presented to our hospital complaining of facial swelling, subcutaneous nodules spread over her face, accompanied by foreign body sensation for 28 days. The patient stated that after the last mesotherapy with a mixture of hyaluronic acid (HA), vitamin C, and TA solution, she experienced unexpected redness, swelling, and pain in the intervention sites. She had safely received mesotherapy with the same HA and vitamin C from this institution before to treat melasma. The TA solution was intended for topical use, but clinicians erroneously used it in mesotherapy. Approximately 10 days after the mesotherapy, the patient found several bean-size subcutaneous nodules that increased over time scattered across her face, with only swelling persisted. The patient went to clinics and hospitals for remedies and received fluid infusion and oral medications. Three-day fluid infusion [compound glycyrrhizin (CG), 10mL/d, dexamethasone, 2mg/d, clindamycin phosphate, 0.9g/d] relieved her swelling without affecting the nodules, but swelling recurred 2 days afterwards. Then, she reported to our hospital for treatment of the nodules and prevention of the reoccurrence of the facial swelling (Figure 1).
Physical examination revealed several nodules on both sides of her cheek, forehead and chin, the nodules had distinct borders and hard texture without causing itch or pain. Most of the nodules were deep in the skin and were only visible when pressure was applied from the interior of the mouth with the tongue. The largest nodule, however, was around bean size, which was palpated about 1cm under the inner corner of the left eye, protruding from the skin. Irregular brown macules were symmetrically scattered over the bilateral eyelids and malar aspects of the face. No other abnormality was noticed during the rest of the clinical examination. Since the patients declined biopsy, an echo-color Doppler (ECD) was applied approximately 4 months after the culprit mesotherapy. ECD detected several inhomogeneous hypoechoic nodules exhibiting vascular supply in the face and forehead. The biggest nodule was on the left side of the face (5*7*6mm), with distinct borders and an irregular shape. The enhanced echogenicity and increased blood flow signal were detected in the granulomatous peripheral fat layers (Figure 2a-d).
A diagnosis of foreign body granuloma was established, and the patient received treatment with minocycline hydrochloride (50mg/night), 0.03% tacrolimus cream, CG (40mL/d infusion), vitamin C (10mL/d infusion) and local microwave physiotherapy for 17 days during her stay in the hospital. The swelling improved substantially, and nodules became much softer and smaller but still palpable (Figure 3). Minocycline hydrochloride (100mg/night) and 0.03% tacrolimus cream (applied when having pruritus sensation) were continued for 4 months after the patient was allowed home. The cosmetic appearance of the patient significantly improved, the sizes of the granulomas decreased significantly, and pruritus and swelling did not reoccur according to a 7-month follow-up (Figure 4).
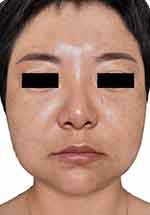 |
Figure 4 Photograph of the patient after 4 months of treatment at home with minocycline (100mg/ night) and 0.03% tacrolimus cream. |
During the treatment, the patient received rigorous photoprotection and was advised not to consume spicy food. Melasma on the face of the patient progressed, while no other discomfort was reported.
Discussion
Various chemicals are used in mesotherapy to exert diverse actions; thus, different injectants may has differing adverse effects.5 Over the years, HA, produced naturally in the body, have been the mainstay injections of mesotherapy for skin rejuvenation, by affecting cell proliferation and enhancing mRNA expression through human fibroblasts. HA rarely incurs adverse reactions according to clinical practices, with overall rates of foreign body-type granulomatous reaction from 0.04% to 0.4%.6,7 Moreover, the patient had safely received mesotherapy with the same HA and vitamin C before without any complications, which helped exclude the possibility of vitamins and HA causing the disease.
Mesotherapy induces infectious and noninfectious complications. Mycobacterium infections are the most frequently published infectious complications, the treatment of which can be difficult due to antibiotic resistance.2 Though the main adverse events reported were infectious, several noninfectious adverse reactions in isolated cases were also described, including foreign body-type granulomatous reaction, nodule formation, abscesses, panniculitis, fat necrosis, cyst formation, ulcer formation and alopecia.7
Correspondingly, intradermal injection of drugs that are not approved for transdermal delivery is dangerous. Invasive administration of topical products via injecting, tattooing, microneedling and electroporation has been reported to induce a granulomatous reaction. The nonfunctional components including metals, silica and so on in topical products might cause problems. Impurity of the topical products can also incur complications.8
TA is a plasmin inhibitor that is used for preventing excessive bleeding. It significantly reduces the Melasma Area and Severity Index (MASI), which is a well-recognized index for quantifying the severity of melasma.3 Due to the limitations on the quantity and quality of the current studies, the advantages in the clinical efficacy and side effects of mesotherapy compared with other administration methods are unclear. Most studies showed TA delivered through mesotherapy induced very minor and transient side effects usually including erythema, irritation, dryness, and scales.9 Two rare cases of hypopigmentation were also reported.10 However, foreign body-type granulomatous reaction as a side effect of TA mesotherapy treatment has not been described before.
Diagnosis of foreign body granuloma primarily depends on its clinical manifestations. It can develop rapidly or slowly after the injection of dermal fillers and is characterized by a noninflammatory granulomatous tissue which increases in size over time, to the size of a pea or bean.7 The granulomatous reaction is the attempt of the body to remove the intrusive materials that macrophages fail to eliminate.11 Accompanying symptoms include persisting or transient swelling, itch, edema, erythema, change in pigmentation and pain in some situations. Despite the self-limited nature of the disease, foreign body granulomas take years to resolve.7 Histologically, foreign body granulomas caused by biological substances such as hyaluronic acid or collagen mainly present with palisaded granulomatous tissue composed of giant cells and macrophages, while lipogranulomas caused by longer lasting artificial substances such as silicone fluids usually show vacuoles of various sizes, surrounded by lymphocytes, plasma cells, eosinophils, and scattered giant cells.8
Echo‑color Doppler (ECD) is extensively used in the dermatological field for visually evaluating lesions. A retrospective study showed promising application value of ECD with 90% sensitivity and 78% accuracy on identifying granulomas and the fillers. On sonography, HA implants are visible as anechoic round structures resembling pseudocysts, while the mixed formulation of HA and lidocaine appears as pseudocysts with inner echoes (debris) and septa. Lipofilling usually appears as an area of hyper-echogenicity with regular margins. In contrast, non-absorbable fillers such as silicone fluids are hyperechoic and show a posterior reverberation or scattering artifact.12 Therefore, ECD should be included in auxiliary examination when patients decline biopsy.
Several diseases are easily confused with foreign body granuloma (Table 1). Nodules are easily mistaken for foreign body granulomas, which usually occur around the mouth and eyes when dermal fillers are injected superficially, commonly resulting from operational errors. In contrast to granulomas, nodules are uniform in size, harder in palpation, whiter in color and do not grow, because they are encapsuled in a fibrous coating with little cellular reaction. In some cases, the histology of nodules is similar to foreign body granulomas, with dense foreign material, macrophages and giant cells, which are hard to differentiate and diagnose.7,8 Surgical removal is the most adapted way to resolve nodules. Besides, delayed hypersensitivity should also be taken into consideration in terms of differential diagnosis. The onset of the reaction (usually 1 month after the filler injections) and a positive skin test are key clinical features of delayed hypersensitivity reactions that are different from foreign body granuloma. However, their histological features are similar. Treatment with orally administered steroids helps the condition.13
 |
Table 1 Differential Diagnosis of Foreign Body Granuloma |
Treatment with minocycline for foreign body-type granulomatous reaction is efficient. Conventionally, granulomas can be treated with intralesional or systemic use of corticosteroids or surgery.4 The patient had received dexamethasone intravenously with temporary satisfying results, but the sudden rebound of swelling implied long-term usage of corticosteroids was needed, which would incur complications. Surgical removal was also precluded for the large number and the fluctuation in size and location of the granulomas. Alternative treatments are needed, such as UV light therapy, antihistamines, tacrolimus, minocycline, and immunosuppressive agents.8 Minocycline is an antibiotic that effectively inhibits granulomatous processes in both infectious and non-infectious granulomatous diseases. Its anti-inflammatory property largely relies on inhibiting neutrophil chemotaxis, and direct anti-granuloma activities depend on the inhibition of protein kinase C, which is critical in foreign body-type granulomatous reaction.14 Minocycline can be used alone or as an adjunct with other drugs. Amongst the treatments, rifampin, ofloxacin, and minocycline hydrochloride combination therapy and minocycline monotherapy were reported to be effective on granuloma annulare and silicone granuloma respectively.15 Additionally, tumor necrosis factor-α blockade was also found to be feasible in treating recalcitrant silicone granulomas resulting from silicone injections on facial skin.16 In this case, good results were drawn from empirical treatment with once-daily regimen of CG, 0.03% tacrolimus cream and minocycline per day for the first 17 days in the hospital; minocycline and 0.03% tacrolimus cream for the rest months at home. CG is also anti-inflammatory, it was discontinued as I.V. was not available at home. Minocycline was used throughout the entire treatment period, and discontinuation led to a return of symptoms; therefore, minocycline is the key medication of this treatment protocol. However, further research is needed on the mechanism of minocycline treating foreign body-type granulomatous reaction.
Conclusion
Foreign body-type granulomatous reaction caused by the unauthorized use of a mixture containing topical TA products in mesotherapy is previously unreported. The case presented in this report demonstrates the potential severity of such a reaction, which can result in subcutaneous nodules and swelling. When patients refuse to undergo a biopsy, ECD should be considered as an additional diagnostic test. This patient was successfully treated using minocycline hydrochloride and other medications. However, there is no standardized treatment for foreign body-type granulomatous reactions, and further research is needed to develop effective treatment protocols. Mesotherapy for skin rejuvenation, which involves the use of HA and vitamins, is generally considered safe. Meanwhile, caution should be exercised when using other injectables, and only authorized products should be used.
Ethics Approval and Consent for Publication
This case report has been performed in accordance with the principles stated in the Declaration of Helsinki. A written informed consent, provided by the Taylor & Francis group® for publication of this case report and including photography and medical information, was signed by the patient. Institutional approval was not required to publish the case details.
Funding
This study was supported by Clinical Research Innovation Project, West China Hospital, Sichuan University (grant no. 19HXCX010) and the National Natural Science Foundation of China (grant no. 82273559).
Disclosure
The authors have no conflicts of interest to declare for this work.
References
1. Tan J, Rao B. Mesotherapy-induced panniculitis treated with dapsone: case report and review of reported adverse effects of mesotherapy. J Cutan Med Surg. 2006;10(2):92–95. doi:10.2310/7750.2006.00013
2. Plachouri KM, Georgiou S. Mesotherapy: safety profile and management of complications. J Cosmet Dermatol. 2019;18(6):1601–1605. doi:10.1111/jocd.13115
3. Zhang L, Tan WQ, Fang QQ, et al. Tranexamic acid for adults with melasma: a systematic review and meta-analysis. Biomed Res Int. 2018;2018:1683414. doi:10.1155/2018/1683414
4. Lemperle G, Gauthier-Hazan N. Foreign body granulomas after all injectable dermal fillers: Part 2. Treatment options. Plast Reconstr Surg. 2009;123(6):1864–1873. doi:10.1097/PRS.0b013e3181858f4f
5. Sanchis-Bielsa JM, Bagán JV, Poveda R, et al. Foreign body granulomatous reactions to cosmetic fillers: a clinical study of 15 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108(2):237–241. doi:10.1016/j.tripleo.2009.03.032
6. Lee JC, Daniels MA, Roth MZ. Mesotherapy, microneedling, and chemical peels. Clin Plast Surg. 2016;43(3):583–595. doi:10.1016/j.cps.2016.03.004
7. Lemperle G, Gauthier-Hazan N, Wolters M, et al. Foreign body granulomas after all injectable dermal fillers: part 1. Possible causes. Plast Reconstr Surg. 2009;123(6):1842–1863. doi:10.1097/PRS.0b013e31818236d7
8. Lee JM, Kim YJ. Foreign Body Granulomas after the Use of Dermal Fillers: pathophysiology, Clinical Appearance, Histologic Features, and Treatment. Arch Plast Surg. 2015;42(2):232–239. doi:10.5999/aps.2015.42.2.232
9. Kim HJ, Moon SH, Cho SH, et al. Efficacy and Safety of Tranexamic Acid in Melasma: a Meta-analysis and Systematic Review. Acta Derm Venereol. 2017;97(7):776–781. doi:10.2340/00015555-2668
10. Litaiem N, Daadaa N, Karray M, et al. Hypopigmentation as a side effect of melasma treatment with tranexamic acid intradermal microinjections. Dermatol Ther. 2020;33(4):e13503. doi:10.1111/dth.13503
11. Pagán AJ, Ramakrishnan L. The formation and function of granulomas. Annu Rev Immunol. 2018;36:639–665. doi:10.1146/annurev-immunol-032712-100022
12. Scotto Di Santolo M, Massimo C, Tortora G, et al. Clinical value of high-resolution (5-17 MHz) echo-color Doppler (ECD) for identifying filling materials and assessment of damage or complications in aesthetic medicine/surgery. Radiol Med. 2019;124(6):568–574. doi:10.1007/s11547-018-0969-1
13. Soltani-Arabshahi R, Wong JW, Duffy KL, et al. Facial allergic granulomatous reaction and systemic hypersensitivity associated with microneedle therapy for skin rejuvenation. JAMA Dermatol. 2014;150(1):68–72. doi:10.1001/jamadermatol.2013.6955
14. Webster GF, Toso SM, Hegemann L. Inhibition of a model of in vitro granuloma formation by tetracyclines and ciprofloxacin. Involvement of protein kinase C. Arch Dermatol. 1994;130(6):748–752. doi:10.1001/archderm.1994.01690060078008
15. Singh M, Solomon IH, Calderwood MS, et al. Silicone-induced granuloma after buttock augmentation. Plast Reconstr Surg Glob Open. 2016;4(2):e624. doi:10.1097/GOX.0000000000000618
16. Silverberg OM, Cyrenne BM, Croitoru D, et al. A case of recalcitrant silicone granuloma treated with Adalimumab: a case report. SAGE Open Medical Case Reports. 2022;10:2050313X221093444. doi:10.1177/2050313X221093444
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



