Back to Journals » OncoTargets and Therapy » Volume 11
Mesenteric fibromatosis misdiagnosed with lymph node metastasis after successful laparoscopic right hemicolectomy: a report of two cases with review of literature
Authors Wang P, Zhou H, Zhou Z, Liang J
Received 26 December 2017
Accepted for publication 17 May 2018
Published 13 August 2018 Volume 2018:11 Pages 4811—4816
DOI https://doi.org/10.2147/OTT.S160844
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr XuYu Yang
Peng Wang, Haitao Zhou, Zhixiang Zhou, Jianwei Liang
Department of Colorectal Surgery, National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100021, People’s Republic of China
Abstract: Fibromatosis is a rare type of tumor derived from the mesenchymal tissue. This is a benign tumor with infiltrating growth but may invade locally and recur following excision. As one type of fibromatosis, mesenteric fibromatosis (MF) accounts for a mere 8% of cases. Although studies have revealed that the etiology of MF is related to trauma, surgery, hormones, and heredity, the specific etiology of fibromatosis remains unclear. With such low incidence, MF has been rarely reported and tends to be misdiagnosed due to insufficient recognition. In this paper, we describe the cases of two patients with MF who were misdiagnosed with lymph node metastasis and who had previously undergone successful laparoscopic right hemicolectomy. We provide this information in order to broaden the clinical understanding of MF.
Keywords: mesenteric fibromatosis, laparoscopic right hemicolectomy, misdiagnosis, lymph node metastasis, colon cancer and Gardner’s syndrome
Introduction
Fibromatosis, also referred to as desmoid fibromatosis, was first described by Muller in 1838.1 This is a rare type of tumor derived from mesenchymal tissue with an annual incidence of only two to four cases per million, and accounts for 3% of all soft tissue tumors.2 Mesenteric fibromatosis (MF), a type of intra-abdominal fibromatosis, has a low morbidity rate.3 While previous studies have revealed that the etiology of MF is related to trauma, surgery, hormones, and heredity,1,3–5 the specific etiology of fibromatosis remains unclear.
Following radical surgery of malignant tumors, a growing abdominal mass is generally considered as evidence of recurrence and metastasis, while other diseases are rarely considered. The diagnosis of these diseases is very difficult, and error therapy caused by error diagnosis could have a significant impact upon patients. Herein, we report the cases of two patients with MF who were misdiagnosed with lymph node metastasis in early-stage colon cancer. These patients had previously undergone successful laparoscopic right hemicolectomy. Based on the rarity and the difficulties in diagnosing this condition, our intention here is to help provide clinicians with a deeper understanding of MF.
Case reports
Case 1
A 54-year-old man was admitted to National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College in August 2011 with a two-month history of positive fecal occult blood test. Pathology results confirmed adenocarcinoma of the ascending colon by endoscopic biopsy. There were no polyps on histology, and there was no family history of familial adenomatous polyposis (FAP)/colorectal cancer; in addition, there was a rise in carcinoembryonic antigen (CEA). Abdomino-pelvic computed tomography (CT) did not show any metastasis to the lymph nodes or distant organs. Other preoperative auxiliary examinations, including chest radiography, electrocardiogram, and blood tests, did not show any obvious contraindications for surgery. Thus, the patient underwent successful laparoscopic right hemicolectomy and postoperative recovery was uneventful. Postoperative pathology showed that a moderately differentiated adenocarcinoma had invaded the superficial muscularis. No lymph node metastasis was found (0/22), so the pathological staging was pT2N0M0. After hospital discharge, the patient was asked to visit the doctors every three months for follow-up.
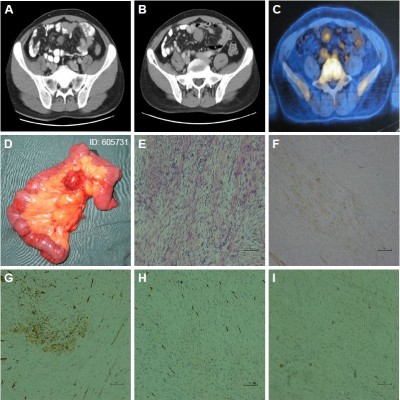
At 12-month follow-up, a CT scan revealed new multiple masses in abdominal cavity: the largest was 1.5 cm in short diameter (Figure 1A). Two months later, another CT scan showed that the masses had grown and that the largest one was 2.3 cm in short diameter (Figure 1B). Ultrasonography (USG) of the whole abdomen showed that the largest mass was ~3 cm and located on the left side of umbilicus. Positron emission tomography-computed tomography (PET-CT) showed multiple nodules located in abdominal cavity, and that the largest one was 2.6 cm in short diameter (SUVmax=1.9) (Figure 1C). Following a multidisciplinary team (MDT) discussion, we considered that excisional biopsy surgery was the best treatment option. The patient was thus readmitted to our institution in October 2012 with a two-month history of newly detected abdominal masses.
Exploratory laparotomy was performed with resection of the middle ileum segment, and end-to-end anastomosis between the proximal ileum segment and the distal ileum was performed. The surgical specimen consisted of an angulated segment of the small intestine, which measured ~25 cm in length, and in the mesentery, showed several masses of varying sizes, the largest of which measured 3.5×3×3 cm (Figure 1D). Microscopic examination revealed that these masses consisted of well-differentiated fibroblasts and that the ileum muscularis had been invaded by the homogeneous proliferation of mesenchymal cells (Figure 1E). The mitotic activity of these cells was <5/50 high power field. No lymph node metastasis was found. The immunohistochemistry results revealed that the tumor was positive for α-SMA, β-catenin (Figure 1F), and WT-1 (Figure 1G) and negative for CD117, CD34 (Figure 1H), S-100, desmin, DOG1, AE1/AE3 (Figure 1I), CK5/6, MC, and EMA. No recurrence was noted within a period of more than five years of follow-up.
Case 2
In September 2013, a 52-year-old man was admitted to our institution with a one-month history of right lower quadrant discomfort. Colonoscopy revealed a mass of 4×3×3 cm at the ileocecal junction, and pathology led to a diagnosis of adenocarcinoma. There were no polyps on histology, and there was no family history of FAP/colorectal cancer; in addition, CEA was normal. As with case 1, we performed successful laparoscopic right hemicolectomy. Postoperative recovery was uneventful. Postoperative pathology showed that a moderately differentiated adenocarcinoma had invaded the deep muscularis. Lymphovascular tumor embolus and nerve invasion were not observed. There was no evidence of lymph node metastasis (0/38); consequently, the pathological staging was pT2N0M0. After hospital discharge, the patient was asked to visit the doctors every three months for follow-up.
At 12-month follow-up, a CT scan showed that a mass of 1.1 cm in short diameter located in the mesentery, indicating that lymph node metastasis should be considered (Figure 2A). Five more CT scans, taken between January 2015 and December 2016, revealed that the mass was growing larger and larger. In December 2016, a CT scan revealed that the mass with a short diameter of 1.8 cm was closely associated with the left ureter and the left hydronephrosis. In February 2017, PET-CT showed that the left ureter was compressed by a mass which was ~2.8×1.8 cm in size with increased metabolism (SUVmax=4.1), located beside the left common iliac vessel (Figure 2B). Above the mass, left ureterectasia and hydronephrosis were evident (Figure 2C). In February 2017, and following MDT discussion, the patient was readmitted to our institution with more than a two-year history of a newly formed retroperitoneal mass.
Exploratory laparotomy was performed with resection of the mass only. During surgery, we found that the mass was located at the mesocolon of the sigmoid colon and was adhered to the adjacent mesenteriolum and left ureter. Macrography showed that the shaggy mass was 4.5×2.5×3.5 cm in size (Figure 2D) with a cyst-solid cross section. The colors of the cross section were brown red and grayish white, and the boundary was unclear. Microscopic examination revealed that the mass consisted of spindle cells; cells were relatively sparse and clearly normal, and mitotic activity was rare (Figure 2E). The immunohistochemistry results revealed that the tumor was positive for β-catenin, S-100 (Figure 2F), and desmin (Figure 2G), and negative for α-SMA, CD117 (Figure 2H), CD34, DOG1 (Figure 2I), and AE1/AE3. Despite essential close follow-up, the patient remains in good condition at present.
Consent
Written informed consent was provided by the patients to have the case details and any accompanying images published.
Discussion
While fibromatosis is histologically benign, tumors with infiltrating growth may invade locally and recur following excision, although these seldom metastasize.5,6 MF is a rare type of fibromatosis, which accounts for ~8% of fibromatosis.2,3 Most cases of MF are sporadic, and may be connected with trauma, surgery, and endocrine factors.1,3–5 MF secondary to the urological operation and resection of a gastrointestinal stromal tumor (GIST) has also been reported.7,8 Several papers have also reported that MF could occur in association with FAP and as a component of Gardner’s syndrome,9–11 the proportions of which have been reported as 10%–15% and 4%–29%, respectively; thus, heredity may represent a significant causative factor.
Clinical symptoms, based upon the mass size, number, and location, vary from vague abdominal discomfort, abdominal distension, and pain, although the most common symptom is an asymptomatic abdominal mass. Physical examination is unlikely to detect such abnormalities, unless they are of a sufficient size. Bowel obstruction, bowel perforation, ureterectasia, ischemia, and hydronephrosis have all been reported as complications of MF.12–14 Furthermore, Kim et al15 reported a rare complication in a young woman who developed secondary hypertension as a result of MF.
CT, magnetic resonance imaging (MRI), and USG can all be used to diagnose and follow up cases of MF. The extent of the masses, and the relationship between the mass and nearby organs, can be clearly shown by CT scans and MRI, along with an assessment of resection. USG is particularly sensitive to the location, number, size, and bloodstream of mass. The only preoperative qualitative method is needle aspiration cytology, guided by either CT or USG. However, this is a difficult and dangerous invasive manipulation for MF. Bleeding, perforation, and spreading may occur as serious complications. Differential diagnoses include GIST, lymphoma, inflammatory fibroid polyp, fibrosarcoma, and carcinoid tumor.16–18 Immunohistochemistry is an effective method to help confirm the diagnosis. In these cases, distinction between GIST and MF should be done based on the results of CD34, β-catenin, DOG1, and KIT.16
Surgery is the most effective method for resectable masses. Studies have shown that the local recurrence rate is 25%–50% at five years after complete resection.19,20 For cases with incomplete resection and recurrent cases, radiotherapy may be effective, although this is rarely used for MF because of the severe adverse events. Khorsand and Karakousis21 reported that radiotherapy reduced the recurrence of MF to 20%–40% compared to 40%–70% reduction achieved with surgery only. Santti et al22 also concluded that radiotherapy was a valuable option for the treatment of desmoid tumors. In addition, chemotherapy (vincristine, cyclophosphamide, and dactinomycin – either alone or in combination), nonsteroidal anti-inflammatory drugs, hormonal manipulation (tamoxifen, aromatase inhibitors, and gonadotropin), and molecular target therapy (imatinib, sorafenib, and sunitinib) may be alternative choices.5,17,19,23,24
In this paper, we reported the cases of two patients with MF who were misdiagnosed with lymph node metastasis and who had previously underwent successful laparoscopic right hemicolectomy. The two middle-aged patients, both with a pathological staging of pT2N0M0, showed a newly detected mesenteric mass one year or so after successful right hemicolectomy. Furthermore, both patients were misdiagnosed with lymph node metastasis at the outset. It is reported that more than 90% of ascending colon cancer cases with stage pT2N0M0 can survive up to five years after successful right hemicolectomy with no local recurrence or metastasis.25 Thus, the probability of lymph node metastasis in early-stage cases following radical surgery is uncommon. Although only regular follow-up is needed for patients with stage pT2N0N0, they need to accept chemotherapy once postoperative lymph node metastasis has been diagnosed. However, further chemotherapy should not be taken blindly and should be based upon definite pathological results by aspiration biopsy. For cases in which aspiration biopsy is difficult, laparoscopic exploration, or exploratory laparotomy, remains a method with diagnostic and therapeutic value. In addition, MDT discussion plays an important role in avoiding misdiagnoses and provides the best mode of diagnosis and therapy for special cases such as these.
Conclusion
MF has a low morbidity rate and tends to be misdiagnosed due to insufficient recognition. Its clinical symptoms and imaging features are nontypical. A growing mass, which is newly detected during the process of postsurgical follow-up, should raise concern and indicates potential recurrence and metastasis. However, differential diagnosis, including MF, should also be considered carefully.
Disclosure
The authors have no conflicts of interest in this work.
References
Polat C, Aktepe F, Turel S, Yazicioglu B, Ozkececi T, Arikan Y. A giant mesenteric fibromatosis case presenting with mechanical intestinal obstruction and successfully resected with partial duodeno-jejunectomy and right hemicolectomy. Clinics (Sao Paulo). 2010;65(1):110–113. | ||
Sakorafas GH, Nissotakis C, Peros G. Abdominal desmoid tumors. Surg Oncol. 2007;16(2):131–142. | ||
Batori M, Chatelou E, Mariotta G, et al. Giant mesenteric fibromatosis. Eur Rev Med Pharmacol Sci. 2005;9(4):223–225. | ||
Sinukumar S, Gomes RM, Kumar RK, Desouza A, Saklani A. Sporadic giant mesenteric fibromatosis. Indian J Surg Oncol. 2014;5(3):242–245. | ||
Chaudhary P. Mesenteric fibromatosis. Int J Colorectal Dis. 2014;29(12):1445–1451. | ||
Anandaravi BN, Jagadish Kumar CD, Sreejith PS, Mayur M, Roopa URS. Giant aggressive mesenteric fibromatosis – a case report. J Clin Diagn Res. 2015;9(2):PD07–PD08. | ||
Zilberman DE, Mor Y, Fridman E, Ramon J. Mesenteric fibromatosis mimicking tumor recurrence following radical cystectomy and bladder replacement. Urol Case Rep. 2015;3(2):40–41. | ||
Chu Y, Guo Q, Wu D. Mesenteric fibromatosis after resection for gastrointestinal stromal tumor of stomach: a case report. Medicine (Baltimore). 2017;96(48):e8792. | ||
Galletto P, Leoz ML, Castells A, Balaguer F. Tumores desmoides intraabdominales en la poliposis adenomatosa familiar. [Intraabdominal desmoid tumors in familial adenomatous polyposis]. Gastroenterol Hepatol. 2013;36(9):580–586. Spanish [with English abstract]. | ||
Chika N, Kumamoto K, Suzuki O, et al. [A case of a desmoid tumor the developed around ileostomy in a patient with FAP]. Gan To Kagaku Ryoho. 2015;42(12):1947–1949. Japanese [with English abstract]. | ||
Park JH, Yagerman S, Feng H, Kim RH, Meehan SA, Lewin J. Gardner-Diamond syndrome. Dermatol Online J. 2016;22(12):48–50. | ||
Isik A, Soyturk M, Süleyman S, et al. Correlation of bowel wall thickening seen using computerized tomography with colonoscopies: a preliminary study. Surg Laparosc Endosc Percutan Tech. 2017;27(3):154–157. | ||
Isik A, Gursul C, Peker K, Aydın M, Fırat D, Yılmaz İ. Metalloproteinases and their inhibitors in patients with inguinal hernia. World J Surg. 2017;41(5):1259–1266. | ||
Yoshida T, Ogawa T, Fujinaga T. Mesenteric fibromatosis with hydronephrosis: a case report. Hinyokika Kiyo. 1994;40(3):245–247. | ||
Kim MS, Noh JH, Jung MC, Kim YB. Mesenteric desmoid-type fibromatosis causing secondary hypertension in a young woman. Obstet Gynecol Sci. 2014;57(5):412–414. | ||
Karbeet Radhakrishna G, Bhat PR, Shenoy RK, Pai S, Singh H. Primary mesenteric fibromatosis: a case report with brief review of literature. Indian J Surg. 2013;75(Suppl 1):131–133. | ||
Cruz RP, Guerra EE, Cambruzzi E, Pêgas KL. Mesenteric fibromatosis affecting duodenum and jejunum. Int J Colorectal Dis. 2016;31(3):715. | ||
Nicolas G, Kfoury T, Shimlati R, Tohmeh M, Wakim R. Incidental finding and management of mesenteric fibromatosis. Am J Case Rep. 2016;17:389–394. | ||
Grignol VP, Pollock R, Howard JH. Management of desmoids. Surg Clin North Am. 2016;96(5):1015–1030. | ||
Williams AD, Heightchew K, Siripirapu V. Diagnostic and therapeutic dilemmas in intra-abdominal desmoid tumors: a case report and literature review. Int J Surg Case Rep. 2016;26:150–153. | ||
Khorsand J, Karakousis CP. Desmoid tumors and their management. Ann J Surg. 1985;149(2):215–218. | ||
Santti K, Beule A, Tuomikoski L, et al. Radiotherapy in desmoid tumors: treatment response, local control, and analysis of local failures. Strahlenther Onkol. 2017;193(4):269–275. | ||
Penel N, Le Cesne A, Bui BN, et al. Imatinib for progressive and recurrent aggressive fibromatosis (desmoid tumors): an FNCLCC/French Sarcoma Group phase II trial with a long-term follow-up. Ann Oncol. 2011;22(2):452–457. | ||
Tonelli F, Ficari F, Valanzano R, Brandi ML. Treatment of desmoids and mesenteric fibromatosis in familial adenomatous polyposis with raloxifene. Tumori. 2003;89(4):391–396. | ||
Moamer S, Baghestani A, Pourhoseingholi MA, Hajizadeh N, Ahmadi F, Norouzinia M. Evaluation of prognostic factors effect on survival time in patients with colorectal cancer, based on Weibull Competing-Risks Model. Gastroenterol Hepatol Bed Bench. 2017;10(1):54–59. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.