Back to Journals » International Journal of Nanomedicine » Volume 19
Mesenchymal Stem Cell-Derived Extracellular Vesicles in Bone-Related Diseases: Intercellular Communication Messengers and Therapeutic Engineering Protagonists
Authors Wang Y , Wen J, Lu T, Han W, Jiao K, Li H
Received 9 November 2023
Accepted for publication 23 March 2024
Published 6 April 2024 Volume 2024:19 Pages 3233—3257
DOI https://doi.org/10.2147/IJN.S441467
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Lijie Grace Zhang
Yanyi Wang,1,2,* Juan Wen,1– 3,* Tong Lu,1,2 Wei Han,2,4 Kai Jiao,5 Huang Li1,2
1Department of Orthodontics, Nanjing Stomatological Hospital, Affiliated Hospital of Medical School, Research Institute of Stomatology, Nanjing University, Nanjing, People’s Republic of China; 2Medical School of Nanjing University, Nanjing, People’s Republic of China; 3Centre for Orofacial Regeneration, Reconstruction and Rehabilitation (COR3), School of Dentistry, The University of Queensland, Brisbane, Queensland, 4006, Australia; 4Department of Oral and Maxillofacial Surgery, Nanjing Stomatological Hospital, Affiliated Hospital of Medical School, Research Institute of Stomatology, Nanjing University, Nanjing, People’s Republic of China; 5Department of Stomatology, Tangdu Hospital & State Key Laboratory of Oral and Maxillofacial Reconstruction and Regeneration & National Clinical Research Center for Oral Diseases & Shaanxi Key Laboratory of Stomatology, School of Stomatology, The Fourth Military Medical University, Xi’an, Shaanxi, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Wei Han, Department of Oral and Maxillofacial Surgery, Nanjing Stomatological Hospital, Affiliated Hospital of Medical School, Research Institute of Stomatology, Nanjing University, 30 Central Road, Nanjing, 210008, People’s Republic of China, Email [email protected] Huang Li, Department of Orthodontics, Nanjing Stomatological Hospital, Affiliated Hospital of Medical School, Research Institute of Stomatology, Nanjing University, 30 Central Road, Nanjing, 210008, People’s Republic of China, Tel +86-25-83620173, Fax +86-25-83620200, Email [email protected]
Abstract: Extracellular vesicles (EVs) can deliver various bioactive molecules among cells, making them promising diagnostic and therapeutic alternatives in diseases. Mesenchymal stem cell-derived EVs (MSC-EVs) have shown therapeutic potential similar to MSCs but with drawbacks such as lower yield, reduced biological activities, off-target effects, and shorter half-lives. Improving strategies utilizing biotechniques to pretreat MSCs and enhance the properties of released EVs, as well as modifying MSC-EVs to enhance targeting abilities and achieve controlled release, shows potential for overcoming application limitations and enhancing therapeutic effects in treating bone-related diseases. This review focuses on recent advances in functionalizing MSC-EVs to treat bone-related diseases. Firstly, we underscore the significance of MSC-EVs in facilitating crosstalk between cells within the skeletal environment. Secondly, we highlight strategies of functional-modified EVs for treating bone-related diseases. We explore the pretreatment of stem cells using various biotechniques to enhance the properties of resulting EVs, as well as diverse approaches to modify MSC-EVs for targeted delivery and controlled release. Finally, we address the challenges and opportunities for further research on MSC-EVs in bone-related diseases.
Keywords: extracellular vesicles, mesenchymal stem cells, functional modification, bone-related diseases
Introduction
The skeletal system undergoes a continuous process of remodeling, which involves both formation and degradation of bone tissues.1 Multiple types of cells contribute to the maintenance of bone homeostasis. When the balance between bone synthesis and absorption is disrupted by intrinsic or extrinsic stimuli, it can lead to the development and progression of various bone diseases and defects, including metabolic disorders, inflammation, degeneration, neoplasms, and others.2,3 Various therapeutic strategies have been used in addressing different bone diseases, including physical therapy, medications, and surgical interventions.4–6 Among these, pharmacological treatments have evolved significantly owing to advances in bone biology research. For conditions like osteoporosis and osteoarthritis, drugs such as glucosamine, estrogen replacements, and chondroitin sulfate are commonly prescribed.4,7 However, delivering these drugs specifically to the affected bone tissue poses challenges when administered orally or via intravenous injection, largely due to bodily excretion processes and the complex nature of the diseased skeletal tissues.8 Additionally, the potential toxic effects on other organs and adverse systemic reactions remain concerns. Due to the intrinsic regenerative and anti-inflammatory capacities, MSCs-based cell therapy has also been extensively studied for bone related diseases. However, this strategy is faced with risks associated with tumorigenesis, potential immune reactions and long-term adverse effects.9–11 Therefore, research should prioritize the development of targeted, sustained-release, and safe drug delivery systems to address these issues effectively.
Extracellular vesicles (EVs) are nanoscale cell-derived vesicles. The discovery that EVs can deliver various bioactive molecules among cells makes them promising diagnostic and therapeutic alternatives in many fields.12–14 For example, the dual roles that EVs play in the pathogenesis and treatment of osteoarthritis (OA) have been proved.3,15,16 EVs derived from MSCs (MSC-EVs) possess therapeutic potential identical to that of MSCs, which encompasses various effects such as inducing angiogenesis, promoting cell proliferation and migration, preventing apoptosis, and exerting anti-inflammatory effects.17–19 Moreover, recent breakthroughs in nanotechnology have also prompted the rapid development in EV-based treatment. MSC-EVs possess inherent biocompatibility, biodegradability, and non-immunogenicity, making them promising nanomaterials for drug delivery applications.20 These characteristics position MSC-EVs as a promising modality in various EV-based treatments for bone-related diseases. Despite the promising possibilities, two main hurdles exist when we translate MSC-EVs into clinical applications in the field of bone-related diseases. Firstly, previous reviews have comprehensively summarized the types of MSCs from different sources including bone marrow, adipose tissue, synovium, amnion, and umbilical cord, etc. and analyzed the pros and cons among them, especially for OA treatment.16,17,21 However, the limited expansion capability of MSCs and the lack of standard methodology for EV isolation and purification may lead to low yield and reduced biological activities of MSC-EVs.22,23 Secondly, despite the favorable features such as low immunogenicity and protection for loaded cargo, several limitations arising from the off-target effect and low half-lives of EVs in free form may restrict the application. Accordingly, optimizing EV-based strategies through biotechniques to pretreat MSCs for enhancing the properties of excreted EVs, as well as to modify MSC-EVs to improve targeting ability and achieve controlled release, holds promise in overcoming these application limitations and enhancing therapeutic effects for the treatment of bone-related diseases.
In the present review, communication between different cell types via EVs will be comprehensively presented since EVs can function as biological mediators and play an important role in the both pathogenesis and treatment of diseases. It is followed by a discussion of strategies for that MSC-EVs can be potentially modified for a broader range of bone disease therapies, as illustrated in Figure 1.
Characteristics of MSC-EVs
EV is generally considered as particles delimited by a lipid bilayer, which can be naturally released by almost all cell types. According to biogenesis and size, EVs fall into two major subtypes: exosomes and ectosomes.24–26 Exosomes are produced originally inside multivesicular endosomes (MVEs) or multivesicular bodies (MVBs) and are secreted as very small vesicles with a size range of 30 to 150 nm when MVEs or MVBs fuse with the plasma membranes.12,25,27 Ectosomes are generated in the process of direct budding from the plasma membrane, which display a broader range of sizes (50 to 1000 nm in diameter) and are generally known in the literature as microvesicles (MVs) or microparticles (MPs). Although the findings of EVs heterogeneity are continuously evolving, currently limited purification protocols and analytical procedures make distinguishing the EV heterogeneity still challenging as these subtypes show overlap in size and constituents. Therefore, some studies may reflect the function of exosomes mixed with other EVs. In this review, EV refers to exosomes in general.
As a cellular product, MSC-EVs are released by MSCs from various tissues with a size range of 50–200 nm. Specifically, MSC-EVs can be characterized by surface antigens (CD73, CD90, and CD105) and the absence of non-MSC markers (CD45, CD34, CD14 or CD11b, CD79alpha or CD19, and HLA-DR), as well as CD31, CD41, CD42, CD9, CD81, and P-selectin derived from serum or platelet lysate.28,29 Additionally, MSC-EVs can be distinguished by analyzing the ratio of specific membrane lipids to proteins and the ratio of sphingomyelin to phosphatidylcholine.30,31 Meanwhile, the cargo encapsulated in MSC-EVs reflects the status of their parental cells. Different cargo-sorting mechanisms contribute to the content heterogeneity of MSC-EVs.26,32 To ensure the biological effects of MSC-EVs, it is crucial to preserve their biological integrity. We can measure the enzyme activity of CD73, known as a key MSC surface marker, to reflect the integrity of the cargo.30,31
Although the precise itinerary that MSC-EVs travel from donor cells to acceptor cells remains unclear, previous studies have depicted several significant aspects. The surface molecules of EVs tend to guide them to recipient cells and protect the internal contents from degradation simultaneously. Once attached to a target cell, EVs can enter cells through various pathways, including receptor-ligand interaction, internalization by endocytosis and/or phagocytosis, or direct fusion with the plasma membrane.12,26,27,32 The surface protein CD47 of EVs allow them to escape from endosomal clearance, however, they may exhibit contradictory results attributed to different cell sources, isolation procedures or specific surface profiles.33 Nevertheless, EVs possess the ability to exert their influence on recipient cells through direct delivery of specific cargo into the cytosol.
Cross-Talk Functions of MSC-EVs Involved in the Skeletal Microenvironment
Communication between cells is an important hallmark of multicellular organisms and can be modulated through direct intercellular contact or transfer of bioactive molecules. In the last decades, studies have focused on the role of EVs which can exchange information between cells and organs related to the skeletal system.34,35
Maintaining the Homeostasis of Bone Metabolism
In bone-related diseases, the destructive process involving the resorption of bone by osteoclasts is usually coupled with a productive process in which osteoblasts synthesize bone. Homeostasis in bone metabolism is determined by the delicate balance between bone resorption and bone formation. Osteoclasts, originating from hematopoietic stem cells (HSCs), exert a unique function in the resorption of bone matrices.36 Conversely, osteoblasts and their constituent progenitor cells are derived from MSCs.37 To maintain bone remodeling, the coordinated regulation and communication between osteoblasts and osteoclasts is tightly controlled by the secretion of regulatory factors, such as Cardiotrophin-1, Semaphorin 4D, Ephrin B2, and Ephrin B4.38 In addition, studies have also reported that MSC-EVs could affect the activity of osteoblasts and osteoclasts directly or indirectly, and function as cell-cell communicators between osteoblasts and osteoclast.39 Exosomal cargo, especially some miRNAs and mRNAs, undergoes differential expression when bone marrow MSCs differentiate into osteoblasts. MSC-EVs can be internalized by primary osteoblasts directly, which may potentiate osteogenic differentiation by promoting osteoblast proliferation, upregulating osteogenic signaling pathways or binding directly to extracellular matrix proteins to improve matrix mineralization.35,40 Their pro-osteogenic properties are primarily from exosomal miRNAs (miR-196a, miR-27a, and miR-206) derived from bone marrow mesenchymal stem cells (BMSCs). Additionally, the Wnt-3a protein content was found to be elevated in the EVs derived from adipose tissue-derived MSCs primed with tumor necrosis factor-alpha (TNF-α), thereby enhancing the efficacy of bone regeneration.34,41,42 Therefore, MSC-EVs may exert their capacity through a systematic route rather than a single component. EVs extracted from MSCs have also been shown to influence osteoclast formation. A study about alveolar bone deterioration elucidated that EVs from BMSCs of osteoporosis rats after tooth extraction could accelerate osteoclastogenic differentiation of osteoclast precursor cells.43 These studies highlight the diverse possibilities of communication between osteoblasts and osteoclasts via MSC-EVs.
Regulating Immune Responses
The close relationship between the immune and skeletal systems has long been appreciated. In the bone marrow, cells are functionally divided into cells engaged in bone metabolism and hematopoietic cells involved in immune responses. Bone cells and hematopoietic cells reside in the same microenvironment and display mutual dependency with tremendous shared molecules such as chemokines, cytokines and etc.44–47 They interact with each other and carry out the functional activities of the bone system.48 Exosomes are excellent intercellular messengers among hematopoietic, mesenchymal, and bone cells in bone marrow.48,49 The forthcoming section will delve into the intricate role of exosomes in facilitating communication between immune cells, with a particular focus on macrophages, and MSCs. This communication plays a crucial role in the regulation of immune responses associated with bone health within the bone marrow microenvironment.
Accumulating evidence has shown that EVs secreted by MSCs could regulate inflammatory and immune responses in bone-related diseases.50–54 For instance, BMSC-EVs could inhibit macrophage activation and exert chondroprotective effects in osteoarthritis.55 MSCs could de-sensitize macrophages to mitochondrial transfer and enhance macrophages bioenergetics via transferring exosomal microRNAs that inhibited Toll-like receptor signalling.56 Macrophages (M0) could be divided into classical activation subtype (M1 phenotype) and alternative activation subtype (M2 phenotype). M2 macrophage polarization upregulates the expression of arginase1 (Arg-1) and CD206, which is conducive to tissue repair.57,58 A recent study has revealed that exosomal CD73 could directly regulate M2 macrophage polarization by activating AKT/ERK signaling pathway through specific adenosine receptors.59 TNF-α preconditioned MSC-EVs could induce macrophage M2 polarization and elevate respiratory markers which positively repaired calvarial tissues.60 Enhanced macrophage polarization to M2 was also observed by MSC-EVs pretreated with interleukin-1β.61 The above studies have demonstrated that MSC-derived EVs could function as immunomodulatory mediators aimed at modulating the phenotypes and properties of macrophages. It has also been demonstrated that M2 macrophage polarization has a positive impact on facilitating MSC-mediated osteogenesis.62 For example, M2-type macrophages derived from mouse bone marrow macrophages enriched miR-5106. M2 macrophage-derived exosomes (M2-exosomes) containing miR-5106 can promote osteogenic differentiation of BMSCs and accelerate fracture healing in vivo by targeting the expression of osteogenic-related genes SIK2 and SIK3.63 Recent study has revealed that M2-exosomes could serve as an immunomodulator to transform M1 into M2 macrophages via the phosphatidylinositide 3-kinase/protein Kinase B (PI3K/AKT) pathway and aid in the enhancement of osteogenic differentiation in BMSCs.64 In addition, after induction into M2 macrophages, murine bone marrow-derived macrophages can secrete miR-21, interfere with the normal signal of the PTEN/PI3K/AKT signaling pathway, and thus regulate the biological behavior of a variety of tumors, including osteosarcoma.65,66 Unexpectedly, compared to exosomes from M0 and M2, exosomes from M1 have a stronger stimulating effect on the proliferation, osteogenesis, and adipogenic differentiation of BMSCs and all three types of exosomes harmed the chondrogenic differentiation of BMSCs.67 The above findings indicate that the exact mechanisms of the macrophage-derived EVs still need to be further investigated and could be used as an effective therapeutic strategy for the bone-related disease.
Accommodating Vascularized Osteogenesis
Previous evidence has revealed that angiogenesis in bone is coupled with osteogenesis, indicating the existence of molecular crosstalk between endothelial and osteoblastic cells.68,69 Exosomes may function as one of the underlying molecular regulators. Several studies suggested that exosomes from MSCs exert a critical effect on the neo-osteogenesis through simultaneous osteogenesis and angiogenesis.70,71 These MSC-exosomes could be internalized by the host endogenous progenitors such as osteoblast precursor cells or vascular endothelial cells followed by the up-regulation of osteogenesis- or angiogenesis-related genes, which is mainly caused by the exosomal signaling. For example, the exosomes derived from BMSCs exhibited the ability to expedite both the proliferation and migration of human umbilical vein endothelial cells (HUVECs) and mouse embryo osteoblast precursor cells and contributed to bone fracture healing eventually.71 The transplantation of exosomes extracted from the human umbilical cord under hypoxia (hypo-exos) effectively enhanced the healing process of bone fracture. Subsequent investigation revealed that this phenomenon occurs as a result of hypo-exos facilitating the transfer of exosomal miR-126, thereby promoting proliferation, migration, and tube formation in recipient HUVECs.72 Similarly, exosomes secreted by endothelial progenitor cells could enhance the angiogenic potential of endothelial cells in a manner depended on exosomal miR-126 and ensure the acceleration of distraction osteogenesis.73 Furthermore, the miR-126 has been found to be associated with the SPRED1/Ras/Erk signaling pathway.72,73 In addition, the functional role of long noncoding RNAs (lncRNAs) through exosome cargo in regulating angiogenesis and osteogenesis has also been elucidated in the CBS+/- mice model, a mouse model of skeletal loss associated with mutation in the Cystathionine β-synthase (CBS) enzyme. The exosome derived from BMSCs in CBS+/- mice model is abundant in lncRNA-19 and facilitates communication between BMSCs and endothelial cells in maintaining bone homeostasis through lnc-H19-Angpt1/Tie2 signaling pathway.74 Therefore, the role of exosomes involved in affecting osteogenic differentiation and angiogenesis has provided new insight into the basic mechanisms of bone reconstruction and homeostasis.75
Modulating Bone Innervation
Bidirectional communication exists between nerves and bones. Within the bone tissue, nerve terminals play a crucial regulatory role in the processes of bone development, turnover, maintenance and regeneration.76,77 Nerves possess the ability to deliver signals and exert an effect on bone homeostasis via neurotransmitters and neuropeptides which can be utilized by bone in turn to regulate neuronal differentiation and nerve growth.78–80 Cells residing in the bone are known to influence sensory neurons, either by promoting or inhibiting axonal growth.81,82 Over the past decade, emerging findings have revealed that EVs may play an crucial role in regulating the bone innervation. A recent study found that under non-pathological conditions, the osteoclast-induced axonal outgrowth of sensory neurons was modulated by the osteoclast-derived EVs rather than netrin-1, one kind of the neurotrophins, that osteoclasts utilized to induce nerve outgrowth during an inflammatory state. The osteoclast-derived EVs then activated the epidermal growth factor signaling. This novel finding provided a new way to intervene and mitigate the adverse effects of uncontrolled bone innervation.83 Exosomes derived from BMSCs (BMSC-exosomes) prolonged the survival of retinal ganglion cells and facilitated axons regeneration. Therefore, it exhibited the potential for the treatment of traumatic and degenerative ocular diseases.84 The therapeutic potential to reduce aberrant nerve invasion of the BMSC-exosomes was also demonstrated in a mice model of Lumbar facet joint osteoarthritis (LFJ OA). BMSC-exosomes inhibited CGRP-positive nerve invasion and abnormal angiogenesis in the subchondral bone area, thus mitigating chronic pain caused by LFJ OA effectively. Furthermore, BMSC-exosomes were found to suppress osteoclastogenesis by inhibiting the RANKL-RANK-TRAF6 signaling pathway, thus attenuating cartilage degeneration and promoting subchondral bone remodeling during OA progression.85 In addition, Schwann cell‑derived exosomes show the ability to induce BMSCs to differentiate into Schwann cells in vitro and participate in the neural regeneration further. This discovery may present a novel therapeutic target for the advancement of nerve regeneration after injury.86 Taken together, it provides insights into the involvement of EVs in the dynamic communication between skeletal tissues and nerves, especially for the future exploration and exploitation of EV-based strategies to treat patients suffering from sustaining pain, although the precise mechanism requires further investigation.
Strategies of Functional-Modified MSC-EVs for Bone-Related Disease Treatment
The application of MSC-EVs in complex and diverse clinical scenarios is faced with several challenges, including the need for demanding separation and purification processes, and suboptimal sizes.87,88 Furthermore, natural MSC-EVs are prone to undergo rapid clearance after systemic administration and may be found mainly in the liver and spleen rather than the targeted bone or cartilage.87,89,90 Besides, rehabilitation of bone defects can be time-consuming, thus constant release instead of robust release is more suitable for tissue regeneration.91,92 Recent efforts have focused on enhancing the therapeutic impact of EVs through their modification and integration with other emerging modalities. This section summarizes the modalities for obtaining functionalized MSC-EVs into two main categories: pretreatment of stem cells using various techniques to confer improved properties to the resulting EVs, and utilization of the available strategies for targeted drug delivery to manipulate endogenous EV biodistribution and controlled EV release.93,94 Table 1 summarizes therapeutic applications of functional-modified MSC-EVs for bone-related diseases.
 |
Table 1 Summary of Therapeutic Applications of Functionalized MSC-EVs for Bone-Related Diseases |
EVs Derived from MSCs Pretreated with Different Techniques
Small Molecules
Small molecules are non-peptide compounds generated from either natural or synthetic ways with a low molecular weight smaller than 1 kDa, which enables them to efficiently penetrate cellular membranes and modulate intercellular signaling pathways.136,137 Recent studies have shown that small molecules have the potential to facilitate EV shedding and enhance production possibly via a cell-specific process while preserving the innate composition and function of EVs at the same time. The yield of MSC-exosomes is significantly increased threefold in response to N-methyldopamine and norepinephrine without altering their pro-angiogenic, anti-inflammatory, and anti-fibrosis bioactivity. This enhancement can be attributed to several enriched exosomal proteins, including COL15A1, COL11A1, LOXL2, AGRN, NID2, HSPG2 and COMP.88 A small heterocyclic compound Kartogenin has been recommended for regenerative medicine.138 EVs derived from Kartogenin-reconditioned MSCs were discovered to possess chondrogenesis-inducing potential via enriched miR-381-3p and demonstrate pronounced regeneration in cartilage repair compared with those EVs secreted from untreated MSCs.133,134 It is also proved that curcumin-treated MSCs could yield EVs to modulate the expression of miR-143 and miR-124 and attenuate osteoarthritis.95
Inflammatory Cytokines
Inflammatory cytokines can trigger MSCs to respond and exert their regulatory functions within the existing microenvironment. Since EVs can inherit the properties from their parental cells, moderate stimulation of stem cells with inflammatory cytokines can enhance the immunomodulatory abilities of EVs.18,139–142 TNF-α preconditioning enabled human gingival tissue-derived MSCs (hGMSCs) to secret EVs with enhanced CD73 expression and enriched exosomal miR-1206b, and theses EVs facilitated M2 macrophage polarization and suppressed periodontal bone loss.102 When exposed to TNF-α (20 ng/mL) and IFN-γ (20 ng/mL), EVs derived from human BMSCs (hBMSCs) can reduce the secretion of pro‐inflammatory cytokines and attenuate inflammation.143 Similarly, small EVs derived from adipose-derived MSCs (ADSCs) under the same inflammatory environment, as referred to in Figure 2, are found to transfer more growth factors and chemokines, such as bone morphogenetic protein 2 (BMP2) and transforming growth factor beta (TGF-β). Researchers also demonstrated that the inflammation-stimulated ADSC-EVs exploit miR-27b-3p to target macrophage colony-stimulating factor-1 to promote M2 macrophage polarization.132 In contrast, the EVs derived from BMSCs coculturing with TGF-β3 manifested better chondroprotective and anti-inflammatory function in a mice OA model.55
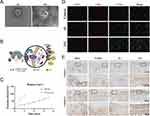 |
Figure 2 EVs stimulated by cytokines may regulate macrophages phenotype. (A) Transmission Electron Microscope (TEM) images of inflammation-stimulated ADSC- derived sEV (IAE) and normal ADSC-derived sEV (AE). Scale bar = 100 nm. (B) Schematic diagram revealed that sEVs were connected to the scaffolds through TGase: glutamine residues (purple), lysine residues (gray), TGase (green), and sEVs (yellow). (C) The release curves of sEVs showed that scaffolds with TGase exhibited complete release up to day 7. (D) Immunofluorescence staining showed that the proportion of M2 macrophages in the entire macrophage population was significantly upregulated by IAE; M1 marker (CD86, red), M2 marker (CD206, green), and nuclei (DAPI, blue). Scale bar = 100 µm. (E) Immunohistochemical staining of collagen I and II of temporomandibular joint sections and magnified defect sites at week 8 posttreatment indicated a good regenerative effect in IAE group. Black boxes indicate magnified microscopic fields of view. Upper panel scale bar, 1 mm; lower panel scale bar, 200 µm. Reprinted from Liu Y, Zhang Z, Wang B, et al. Inflammation-stimulated MSC-derived small extracellular vesicle miR-27b-3p regulates macrophages by targeting CSF-1 to promote temporomandibular joint condylar regeneration. Small. 2022;18(16):1. © 2022 Wiley-VCH GmbH.132 |
Hypoxia
The bone marrow is considered a hypoxic microenvironment compared to other tissues, and the biological fate of MSCs and HSCs is closely linked to the oxygen levels within this niche.144–146 It is well documented that hypoxia, which refers to ~1% oxygen generally compared to normoxia (21% oxygen), renders enhancement of EVs release and function, especially in MSCs.147–150 Gao et al elucidated that hypoxia-treated stem cells from human exfoliated deciduous teeth (SHEDs) enhanced the production of their secreted exosomes, thus exerting improved angiogenic and osteogenic effects.108 Deng et al discovered that hypoxic extracellular vesicles (hypo-EVs) derived from BMSCs can target the PI3K/AKT signaling pathway to enhance the osteogenic capacity of rat calvarial osteoblasts. The bone repair effect induced by MSC-EVs is primarily attributed to their functional cargo, particularly the differentially expressed proteins contained within EVs. They highlighted the importance of biglycan, a crucial protein in the ECM, in promoting osteoblast differentiation and mineralization.109 However, Liu et al demonstrated that exosomes derived from hypoxia-elicited human umbilical cord MSCs (hUMSCs) facilitated bone fracture healing by improved production of exosomal miR-126.72 In a study by Zhang et al, they identified four microRNAs (hsa-miR-181c-5p, hsa-miR-18a-3p, hsa-miR-376a-5p, and hsa-miR-337-5p) of hypo-EVs that mediated cartilage repair in rat osteoarthritis model.97 These reports suggest a general mechanism involving the interaction between distinct exosomal cargo and hypoxia-inducible factor 1 (HIF-1).23,72,151 In general, hypoxia preconditioning is a typical and effective approach to optimizing the therapeutics of MSC-derived EVs for bone-related diseases with an emphasis on conditions such as osteoarthritis and bone defect.
Magnetic Nanoparticles (MNPs) and Static Magnetic Field (SMF)
As exposure to biophysical stimuli, such as electrical field, magnetic field, light irradiation, or mechanical forces, can promote MSCs and other bone progenitors’ cellular behavior, researchers have treated cells with different environmental-mimicking preconditioning to improve EVs secretion and function.152–154 Several studies have proposed the combination of MNPs and moderate (1mT to 1T) SMF in skin wound healing or bone defect repair.112,153,155–157 Here, we emphasized the magnetic stimuli because magnetotherapy has been officially approved by the United State Food and Drug Administration (FDA) in the treatment of orthopedic applications.158 Figure 3 shows that Wu et al fabricated BMSC-EVs by culturing BMSCs in a medium containing 50 µg/mL Fe3O4 nanoparticles. After being exposed to a 100mT SMF, these EVs displayed a robust enhancement to osteogenesis and angiogenesis, which was attributed to the enriched exosomal miR-1260a. They have also demonstrated that exosomal miR-1260a suppressed the expression of HDAC7 and COL4A2, thereby facilitating bone regeneration.112
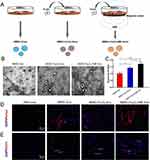 |
Figure 3 Engineered exosomes derived from BMSCs preconditioned with magnetic stimulation. (A) Scheme of the fabrication of three types of exosomes: BMSC-Exos, BMSC-Fe3O4-Exos and BMSC-Fe3O4-SMF-Exos. BMSC-Fe3O4-SMF-Exos were obtained via magnetic stimulation using Fe3O4 nanoparticles and a static magnetic field (SMF). (B) Morphology of exosomes was observed by TEM; the red arrows indicate exosomes. (C) Fe3O4 nanoparticles and SMF increased the production of exosomes in BMSCs. *p<0.05, ***p<0.001. Representative fluorescent images of the osteogenic marker Runx2 (D) and the angiogenic marker CD31 (E) revealed that magnetic stimulation potentiated osteogenesis and angiogenesis in calvarial defects 12 weeks post-treatment; white arrows mark the newly formed vessel. Reprinted from Wu D, Chang X, Tian J, et al. Bone mesenchymal stem cells stimulation by magnetic nanoparticles and a static magnetic field: release of exosomal miR-1260a improves osteogenesis and angiogenesis. J Nanobiotechnology. 2021;19(1):209. Creative Commons.112 |
Exogenous Nucleic Acids
Based on the observation that lipid bilayer encapsulation endows EVs with the advantage of protecting the cargo inside from degradation, EVs can serve as delivery carriers for exogenous nucleic acids. Exogenous microRNA (miRNA) and small interfering RNA (siRNA) can be enclosed and delivered to targeted cells due to their small size.159–162 Several methods, including gene transfection and electroporation, can load these cargo into cells and generate specific cargo-loaded EVs. For instance, the siRNA targeted Shn3 gene (siShn3) was loaded into human induced pluripotent stem cells (iPSCs) through electroporation and these engineered iPSC-EVs exploited desirable anti-osteoporosis properties.99 Tao et al transfected synovial mesenchymal stem cells (SMSCs) with miR-140-5p and obtained the following exosomes overexpressing miR-140-5p, named SMSC-140-Exos. They found that although SMSC-derived exosomes could enhance the proliferation and migration of articular chondrocytes, the side effects lay in the simultaneous harming of ECM secretion. SMSC-140-Exos could overcome the limitation, thus displaying a protective effect in a rat knee OA model.96 BMSCs transfected through liposome/pGFP-BMP2 plasmid or lentiviral particles yielded EVs with overexpression of BMP2 that both displayed exciting therapeutic potency in bone defect models due to the synergy of essential MSC-EV function and up-regulated BMP2 expression.110,123 Engineered MSC-EVs with overexpressed miR-375 or miR-181b were endowed with similar osteogenic capacity.111,124 Previous study has found that MSC-EVs loaded with chemotherapeutic drug doxorubicin could exert antitumor activity and inhibit tumor growth.105 Exogenous nucleic acids transported by EVs can also modulate intracellular gene expression and finally satisfy therapeutic needs. BMSC-derived EVs with overexpressed miR-206 enhanced toxicity against osteosarcoma by down-regulating the expression of transformer 2β (TRA2B) while MSC-EVs with overexpressed miR-150 suppressed migration and invasion of osteosarcoma cells via targeting insulin-like growth factor 2 mRNA binding protein 1 (IGF2BP1).106,107 In a study by Li et al, adenovirus carrying triple point-mutations in the HIF-1α coding sequence was transfected into BMSCs and the as-developed BMSC carried mutant HIF-1α. EVs isolated from such kind of BMSCs inherited mutant HIF-1α and became a beneficial tool for the treatment of steroid-induced avascular necrosis of femoral head.135 For large nucleic acids like clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein 9 (Cas9) system, an additional design to encapsulate them into EVs was needed. Some researchers have realized it. Lin et al developed hybrid exosomes by incubating the original exosomes with liposomes for 12 hours so that the CRISPR/Cas9 system could be loaded into the hybrid exosomes and successfully delivered. They provided a promising exosome-based platform for editing gene in vivo.163
MSC-EVs Designed to Increase Targeted Delivery Efficiency and Sustained Release
Delivery of MSC-EVs to bone is a complicated process due to the sophisticated microenvironment and structure of natural bone tissue.164 Similar to nanomedicine delivery systems, there are two novel strategies for administering MSC-EV to bone tissue. The first approach involves modifying the surface of MSC-EV for systemic or local injection in preclinical models of bone pathologies, with an emphasis on conditions such as osteoporosis, osteoarthritis, and rheumatoid arthritis. The second approach entails integrating MSC-EV with biomaterials for implantable bone regeneration therapy to address bone defects.165–167
Surface Modifications
The primary approach involves incubating MSC-EVs with bone-targeting agents, which are a class of small molecules (such as oligopeptides and bisphosphonates) known for their high affinity to bone tissue. These bone-seeking moieties selectively deposited in bone for extended periods of time and exhibited minimal off-target effects.164 Modification of the MSC-EV with a bone-targeting peptide (DSPE-PEG-Mal-Cys-SDSSD) via hydrophobic interaction endowed the EV complex an ability to recognize osteoblasts specifically and deliver siShn3 successfully, hence exerting excellent anti-osteoporosis effects in an ovariectomy (OVX)-induced osteoporosis mouse model.99 Peptide can also fuse with Lamp 2b, a membrane protein of EVs from cells transfected with specific plasmids, and hold promise for the construction of cell-specific drug delivery.168,169 Along with these, aptamer-based surface modification techniques are being studied. BMSC-specific aptamer-functionalized MSC-EVs have shown improved internalization into BMSCs and therapeutic potential for managing osteoporosis and fracture.100 However, these non-covalent modifications pose challenges in maintaining stability between ligands and MSC-EVs within the complex in vivo environment.101 The covalent binding method, facilitated by biochemical techniques, enables EV imaging and tracking in vivo, while simultaneously enhancing the cell-targeting efficiency of MSC-EVs. Copper-free click chemistry, an ideal bioorthogonal reaction between an azide and a strained cyclooctyne such as the dibenzocyclooctyne group (DBCO) without copper catalysis, has been widely employed.170 The DBCO group can be added onto MSC-EVs by using a heterobifunctional crosslinker to link DBCO and amine-containing molecules on EVs while the azide groups can be introduced to the side chain of lysine via 5-azidopentanoic acid. This process can consequently give rise to MSC-EVs modified with peptides previous conjugated to DBCO or azide groups.171 Wang et al have coupled the bone-targeting alendronate with an azide group to the DBCO-EVs derived from mouse MSCs (mMSCs) and achieved better anti-osteoporosis effect.101 DBCO groups can also be integrated to the biogenesis of MSC-EVs via metabolic glycoengineering mediated click chemistry. As seen in Figure 4, You et al utilized azido-modified monosaccharides as endogenous metabolic substitutes for ADSCs and the DBCO-conjugated dextran sulfate was then introduced to enable the following production of ADSC-EVs decorated with dextran sulfate. The as-developed ADSC-EVs was endowed with the capacity to target the scavenger receptor class A accumulated in rheumatic inflammatory joints, which improved their biodistribution half-lives and regulated macrophage polarization.104 The diversity of bioconjugation methods makes copper-free click chemistry expandable tools for EV modification to optimize therapeutic efficiency.
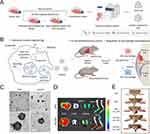 |
Figure 4 Surface modification of EVs via micelle-mediated physical modification and chemical modification. (A) Schematic illustration of DS-EXOs as a cell-free therapeutic system for rheumatoid arthritis (RA). (B) Mechanism by which the DS-EXOs reprogram macrophages. The DS-EXOs reach joints inflamed by RA owing to their ability to target activated macrophages after systemic administration. (C) TEM images of EXOs and DS-EXOs. (D) Ex vivo organ distribution images revealed that the number of Cy5.5-labeled DS-EXOs at the inflamed sites was significantly higher than that of Cy5.5-labeled bare EXOs. (E) Representative images of the collagen-induced arthritis (CIA) mice treated with PBS, bare EXOs and DS-EXOs of different concentrations. Reprinted from You DG, Lim GT, Kwon S, et al. Metabolically engineered stem cell-derived exosomes to regulate macrophage heterogeneity in rheumatoid arthritis. Sci Adv. 2021;7(23):eabe0083. Creative Commons.104 |
Combined with Biomaterials
Biomaterials could support EVs as attachment harbors or combine with EVs as release platforms. Feasible condition and fabricating techniques are required when synthesizing EV-contained biomaterials. The selection of suitable biomaterials compensates for the shortcomings of plain EVs while synergizing in implantable therapeutic forms of damage repair due to the versatility of various biomaterials. Several reviews have elucidated the use of biomaterial-assisted exosomes as a new strategy for bone tissue regeneration.165–167,172 The most commonly used biomaterials can be categorized as metallic materials, bioactive ceramics and polymers.
The desired mechanical strength and load-bearing capacity of metallic materials make them suitable for orthopedic implants, with titanium alloys being particularly favored for their excellent biocompatibility, high porosity, corrosion resistance, and other properties.173 3D printed titanium scaffolds with available porosity facilitated MSC-EV attachment and enhanced osteogenesis. EV-contained titanium nanotube may become another osteoimmunomodulatory candidate for targeted bone differentiation.174
Bioactive ceramics, such as hydroxyapatite (HAP), β-tricalcium phosphate (β-TCP) and mesoporous bioactive glass (MBG), are ideal alternatives because they can optimally imitate the properties of bone.175 Liang et al selected a traditional porous HAP scaffold as carriers for EVs derived from BMSCs stimulated by dimethyloxaloylglycine. The functionalized HAP scaffold activated the AKT/mTOR pathway and demonstrated superior osteogenesis in rats with cranial defects.113 β-TCP are remarkable for their degradability and osteoconductivity.176 Several studies have confirmed that MSC-EVs from different sources can be integrated with β-TCP scaffolds by co-incubation, dropwise, freeze-drying or other methods. These complexes contributed to bone regeneration in models such as critical-sized cranial defects and alveolar bone defects in periodontitis.53,114,115,127 For MBG, a lyophilized hierarchical scaffold combined with BMSC-EVs in its micron-scale porosities was proved to achieve a more significant osteogenic efficiency.116 In these combinations, MSC-EVs may confer improved osteoinductive properties to scaffolds while the scaffolds provide excellent shelters for MSC-EVs to maintain their bioactivity and realize sustained release in return, which is a win-win combination of physical structure and biochemical clues.
Polymers can be divided into natural or synthetic categories. Natural polymers, composed of chitosan (CS), hyaluronic acid (HA), silk, collagen, gelatine, or alginate, etc., show prominent biocompatibility and bioactivity owing to the tissue source. They usually share similar density to soft tissue. For example, Liu et al developed a loose, porous, and tunable scaffold composed of silk that mimicked the cartilage structure. Transglutaminase (TGase) was added to the scaffold to catalyze interaction between the side chains of lysine (ε-amine) and glutamine (g-acyl), which are both contained in the membrane of ADSC-derived sEVs and silk fibroin. Figure 2 shows that this functionalized scaffold prolonged the release of EVs in vitro and obtained better therapeutic effects for temporomandibular joint (TMJ) osteochondral defects in vivo. However, the degradation property of the scaffold needed further optimization as half of the samples tested became flocculent precipitates while the others remained stable over an eight-week period.132 By contrast, synthetic polymers, compounded with polycaprolactone (PCL), polylactic acid (PLA), polylactide-co-glycolide (PLGA), polyetheretherketone (PEEK) and so on, are more controllable in physicochemical properties and biodegradation rate while lack of innate bioactivity. Their combination with bioactive MSC-EVs however can fill this gap to a certain extent. PCL and PLA are FDA-approved polyester polymers. PCL scaffolds modified with BMSC-EVs and silver nanoparticles (Ag) exerted synergistic immunomodulatory effect and boosted the osteogenic differentiation of BMSCs.131 Diomede et al utilized polyethyleneimine (PEI) to bridge EVs derived from human gingival MSCs and 3D printed PLA scaffolds. It enhanced the adhesion between PLA scaffolds and MSC-EVs and favored bone healing of calvaria defect.117 Usually, direct attachment of EVs to polymer scaffolds may result in inefficient attachment, so it is necessary to modify the synthetic polymer scaffold and EV surfaces to enhance their adhesion.172 For instance, PEEK is a typical aromatic polymer material with high biocompatibility and stability that may yet emerge as an alternative to metals. Tannic acid-modified PEEK implants immobilized BMSC-EVs via reversible hydrogen bond and allowed for the sustained release of BMSC-EVs for up to 14 days. In vitro rat femoral drilling model revealed that the EV-coated PEEK possessed the ability to facilitate M2 polarization and achieve desired osseointegration.125 Polydopamine (PDA) is another capable helper to anchor both synthetic and natural substances onto a variety of substrates. Li et al constructed a mussel-inspired immobilization by combining ADSC-derived EVs with PLGA scaffolds coated with PDA. The PDA-mediated surface on the scaffold provided a platform for the controlled release of EVs and therefore enhanced optimal EV-based bone repair in mice calvarial defects.118 This bioinspired PDA coating was also seen on an injectable PLGA microspheres (PMS-PDA microspheres) and demonstrated prolonged release of MSC-EVs for 21 days, hence improving vascularized bone regeneration in rat calvarial defects.108 Above studies have revealed that synthetic polymers have shown good potential as carriers for MSC-EVs and scaffolds for bone tissue engineering. It may be possible to meet competing demands more effectively by combining synthetic and natural polymers.177
Hydrogels, as hydrophilic gels with crosslinked networks, have the ability to mimic the structure of the natural ECM. They are usually composed of natural polymers, synthetic polymers, or hybrid polymers as their basic units. These fabricated biomaterials serve as effective carriers for MSC-EVs.175 Hydrogels are crosslinked through different interactions such as covalent or non-covalent interactions, while MSC-EVs can be loaded into the hydrogels via different strategies.178 For example, EVs from dental pulp stem cells (DPSCs) encapsulated in CS hydrogels effectively ameliorated periodontal lesion in periodontitis mice.103 Another study developed PLGA-PEG-PLGA triblock copolymer microspheres to deliver EVs from DPSCs, which provided pro-mineralization cues and accelerated bone healing at mouse calvarial defects.119 Similarly, Tao et al utilized poly(D,l-lactide)-b-poly(ethylene glycol)-b-poly(D,l-lactide) (PDLLA-PEG-PDLLA) triblock copolymer gels as carriers for SMSC-EVs. The triblock gels can be applied in intra-articular injection and have been validated their potential to delay the progression of OA.98
It is also feasible to process and customize hydrogels to produce EV-loaded hydrogels with enhanced properties. Xing et al combined azido-modified MSC-EVs (Az-EVs) with DBCO-conjugated collagen hydrogel. As shown in Figure 5, Az-EVs were prepared via metabolic labeling while the conjugation reaction between DBCO and the primary amines of collagen (type I) was mediated via the DBCO-N-Hydroxysuccinimide ester. An increased EV dose (ten times higher than natural EVs incorporated) was chemoselectively immobilized within the EV-biomaterial platform via click chemistry. Meanwhile, the platform exhibited comparable pro-angiogenic properties and immunoregulatory effects.130 The as-developed hydrogels will be beneficial to vascularized osteogenesis. Another biopolymer hydrogel was composed of small intestinal submucosa (SIS). Ma et al managed to improve its mechanical properties by integrating 3-(3,4-dihydroxyphenyl) propionic acid (CA) into SIS hydrogel. Furthermore, they designed novel fusion peptides to combine SIS hydrogel and BMSC-EVs, which prolonged the retention of BMSC-EVs and showed superior performance for skull defect repair.120 Guan et al introduced aldehyde-functionalized chondroitin sulfate into gelatin methacryloyl by dynamic Schiff base reaction and loaded BMSCs-EVs to form a kind of ECM-mimic hydrogel (GMOCS). The GMOCS hydrogel maintained the release of EVs for 14 days, regulated immune microenvironment and facilitated cartilage regeneration.128
 |
Figure 5 Immobilizing exosomes within collagen hydrogels for spatially vascularization and host integration. (A) Scheme of the overall strategy for engineering Az-EVs via metabolic glycan labeling and the following Az-EV immobilization within DBCO-collagen. (B) TEM image showed the morphology of EVs. (C) Assessment of immobilization and chronic release of radiolabeled Az-EVs and control non-Az-EVs in DBCO-collagen and unmodified collagen gels (n = 3). (D) Gross appearance of collagen gel explants 7 days post-implantation. (E) Histological analysis of host vascular ingrowth via endothelial CD31 staining (n = 7). (F) Histological analysis of M1 macrophage staining (CCR7) (n = 7). Adapted from Xing Y, Yerneni SS, Wang W, Taylor RE, Campbell PG, Ren X. Engineering pro-angiogenic biomaterials via chemoselective extracellular vesicle immobilization. Biomaterials. 2022;281:121357. Creative Commons.130 |
Besides, the incorporation of inorganic substances or bioactive ceramics with mechanical strength into conventional hydrogels can partially compensate for their relatively poor mechanical properties. The biohybrid hydrogels can fill irregular bone defects and release EVs locally, thereby promoting in situ bone tissue regeneration. For example, Zhang et al cocultured rat BMSCs (rBMSCs) with polyethylene glycol maleate citrate (PEGMC) hydrogels and different concentrations of β-TCP to form a composite hydrogel (PG/TCP). The injectable PG/TCP obtained an optimal combination of desirable mechanical, osteogenic and angiogenic properties when the TCP content was 20%(w/v).129 Inspired by the design of biohybrid hydrogels, Zhang et al utilized 3D printing to fabricate a customized porous nanohydroxyapatite/poly-ε-caprolactone (nHP) scaffold. The nHP scaffold was then immersed in a hydrogel solution containing methacrylic anhydride-functionalized HA, umbilical MSC-derived EVs (hUMSC-EVS) and photoinitiator Irgacure-29 to construct EXOs/Gel/nHP composites for cranial defect repair after ultraviolet exposure. The hydrogel system was conducive to the durable release of hUMSC-EVs and robust bone regeneration at the site of rat cranial defects.121 The aforementioned studies have illustrated the prevailing use of hydrogels as carriers for MSC-EVs owning to their excellent biocompatibility and porous structural properties. However, the fabricated hydrogel should be prepared and processed to achieve comprehensive structural, physical and biological properties tailored to therapeutic requirements. This approach ensures that MSC-EVs can exert their maximal function while maintaining an appropriate degradation rate.
Recently, the potential of organic-inorganic composites for exosome loading has also been explored. Metal-organic frameworks (MOFs) are organic-inorganic hybrid materials constructed from functional organic ligands and metal ion/cluster nodes via coordination reaction.172 They have become a hot spot for drug delivery with the advantage of structural and functional diversity, porosity and large specific surface area.179 Kang et al synthesized PLGA/Mg-gallate MOF composite scaffolds and immobilized MSC-EVs onto the surface of MOF. These coating EVs exhibited a sustained release from the scaffold for up to 10 days, along with the continuous release of Mg ions and gallic acid. The EVs-functionalized MOF composite actualized the improvement of osteogenesis, angiogenesis and anti-inflammation.122
Perspectives and Future Directions
With the deepening cognition of intercellular communication, researchers have raised concern about the role of EVs spanning from the maintenance of physiological states to the pathogenesis of diseases. As a kind of cell derivative, EVs are born with plentiful appealing features as an effective tool to deliver functional cargo and convey specific biological information between cells. Over the past decade, there has been a remarkable advancement in the utilization of EV-based therapies for bone-related diseases. However, despite the substantial progress, there still exists a considerable amount to be uncovered and understood regarding this intricate subject matter.
Firstly, given the diverse origins, culture and preconditioning methods and isolation techniques of MSC-EVs, there may exist inherent heterogeneity. Standardized purification methods and characterization criteria should be established. Meanwhile, there is a need to optimize methods for large-scale production of MSCs without compromising their structural and functional integrity.27,180 Secondly, the mechanisms of intercellular and interorgan crosstalk mediated by MSC-EVs remain to be elucidated by further studies. MSC-EVs exert therapeutic effects in skeletal disorders through the functional cargo inside, including diverse proteins, miRNA and others. The cargo can regulate crucial molecules and signaling pathways involved in bone-related diseases. However, current available researches mainly focus on miRNAs when illustrating the mechanism of the MSC-EV cargo. The biological function of miRNA requires sufficient concentration, appropriate structure, and binding with accessory proteins such as RNA-induced silencing complexes. In contrast, protein function only requires adequate concentration and typically at lower levels compared to miRNA under equivalent conditions.35,181,182 Accordingly, there is still much to uncover regarding the functional components of MSC-EVs beyond miRNAs. Thirdly, originated as a natural acellular factor and nanovesicle, MSC-EVs are promising candidates for tissue engineering and drug delivery. Various fabricated procedures, modification and loading strategies have been explored to functionalized MSC-EVs. These can broaden EV application and improve EV therapeutic efficiency. However, at present, these strategies still need optimization to ensure the efficient delivery of MSC-EVs while preserving their inherent bioactivity. For example, additional procedures such as ultracentrifugation and size exclusion chromatography are typically necessary for purifying MSC-EVs with chemical modifications, which may affect EV functionality. In contrast, tangential flow filtration enables the one-step acquisition of modified EVs without the need for additional purification.104 Besides, traditional freeze-drying processes may compromise the phospholipid membrane structure of EVs, thereby impairing their biological activity. Loading EVs onto scaffolds with high surface area and porosity can offer excellent protection, preserving EV integrity during freeze-drying and ensuring their bioactivity.116 In addition, interest in EV-based biomaterials is shifting towards a unique permutation of biochemical and biophysical cues to achieve improved properties and therapeutic goals. However, the challenge lies in coordinating these cues to maximize their synergies. Considering the physicochemical parameters of biomaterials, such as pore size and hardness, is crucial for obtaining optimal osteogenic efficacy.183,184 It is important to keep orderliness and coordination of the final construct in mind when we combine different components as a whole. Lastly, the combination of different scaffolds, delivery routes, and treatment frequencies may impact the application dosage of MSC-EVs while there is a lack of systematic studies evaluating the extent of these variables’ influences. Therefore, it is necessary to discuss and optimize these aspects concerning specific MSC-EVs and bone defects/disease types. Further investigations into these key points would help us have a comprehensive understanding of MSC-EVs and pave way for the application of functionalized MSC-EVs either alone or combined with other modalities in the bone-related disease from bench to bedside.
Author Contributions
Professor Wei Han and Professor Huang Li share co-corresponding authorship. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by grants from the Natural Science Foundation of Jiangsu Province (SBK2021021787), the Major Project of the Health Commission of Jiangsu Province (ZD2022025), and the Key Project of the Nanjing Health Commission (ZKX20048).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Yin X, Zhou C, Li J, et al. Autophagy in bone homeostasis and the onset of osteoporosis. Bone Res. 2019;7:28. doi:10.1038/s41413-019-0058-7
2. Chen D, Shen J, Zhao W, et al. Osteoarthritis: toward a comprehensive understanding of pathological mechanism. Bone Res. 2017;5:16044. doi:10.1038/boneres.2016.44
3. Ni Z, Zhou S, Li S, et al. Exosomes: roles and therapeutic potential in osteoarthritis. Bone Res. 2020;8:25. doi:10.1038/s41413-020-0100-9
4. Khosla S, Hopbauer LC. Osteoporosis treatment: recent developments and ongoing challenges. Lancet Diabetes Endocrinol. 2017;5(11):898–907. doi:10.1016/S2213-8587(17)30188-2
5. Berenbaum F, Griffin TM, Liu-Bryan R. Review: metabolic regulation of inflammation in osteoarthritis. Arthritis Rheumatol. 2017;69(1):9–21. doi:10.1002/art.39842
6. Nandi SK, Bandyopadhyay S, Das P, et al. Understanding osteomyelitis and its treatment through local drug delivery system. Biotechnol Adv. 2016;34(8):1305–1317. doi:10.1016/j.biotechadv.2016.09.005
7. Hunter DJ. Pharmacologic therapy for osteoarthritis--the era of disease modification. Nat Rev Rheumatol. 2011;7(1):13–22. doi:10.1038/nrrheum.2010.178
8. Hirabayashi H, Fujisaki J. Bone-specific drug delivery systems: approaches via chemical modification of bone-seeking agents. Clin Pharmacokinet. 2003;42(15):1319–1330. doi:10.2165/00003088-200342150-00002
9. von Bahr L, Batsis I, Moll G, et al. Analysis of tissues following mesenchymal stromal cell therapy in humans indicates limited long-term engraftment and no ectopic tissue formation. Stem Cells. 2012;30(7):1575–1578. doi:10.1002/stem.1118
10. Hoogduijn MJ, Roemeling-van Rhijn M, Korevaar SS, Engela AU, Weimar W, Baan CC. Immunological aspects of allogeneic and autologous mesenchymal stem cell therapies. Hum Gene Ther. 2011;22(12):1587–1591. doi:10.1089/hum.2011.039
11. Sissung TM, Figg WD. Stem cell clinics: risk of proliferation. Lancet Oncol. 2020;21(2):205–206. doi:10.1016/S1470-2045(19)30787-9
12. Mathieu M, Martin-Jaular L, Lavieu G, Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21(1):9–17. doi:10.1038/s41556-018-0250-9
13. Zhang X, Zhang H, Gu J, et al. Engineered extracellular vesicles for cancer therapy. Adv Mater. 2021;33(14):1. doi:10.1002/adma.202005709
14. Muraca M, Cappariello A. The role of Extracellular Vesicles (EVs) in the epigenetic regulation of bone metabolism and osteoporosis. Int J Mol Sci. 2020;21(22). doi:10.3390/ijms21228682
15. Lin J, Wang L, Lin J, Liu Q. The role of extracellular vesicles in the pathogenesis, diagnosis, and treatment of osteoarthritis. Molecules. 2021;26(16):4987. doi:10.3390/molecules26164987
16. Liu Z, Zhuang Y, Fang L, Yuan C, Wang X, Lin K. Breakthrough of extracellular vesicles in pathogenesis, diagnosis and treatment of osteoarthritis. Bioact Mater. 2023;22:423–452. doi:10.1016/j.bioactmat.2022.10.012
17. Varderidou-Minasian S, Lorenowicz MJ. Mesenchymal stromal/stem cell-derived extracellular vesicles in tissue repair: challenges and opportunities. Theranostics. 2020;10(13):5979–5997. doi:10.7150/thno.40122
18. Phinney DG, Pittenger MF. Concise review: MSC-derived exosomes for cell-free therapy. Stem Cells. 2017;35(4):851–858. doi:10.1002/stem.2575
19. Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol. 2014;14(3):195–208. doi:10.1038/nri3622
20. Herrmann IK, Wood MJA, Fuhrmann G. Extracellular vesicles as a next-generation drug delivery platform. Nat Nanotechnol. 2021;16(7):748–759. doi:10.1038/s41565-021-00931-2
21. Fuloria S, Subramaniyan V, Dahiya R, et al. Mesenchymal stem cell-derived extracellular vesicles: regenerative potential and challenges. Biology. 2021;10(3):172. doi:10.3390/biology10030172
22. Chen TS, Arslan F, Yin Y, et al. Enabling a robust scalable manufacturing process for therapeutic exosomes through oncogenic immortalization of human ESC-derived MSCs. J Transl Med. 2011;9:47. doi:10.1186/1479-5876-9-47
23. Zhu J, Lu K, Zhang N, et al. Myocardial reparative functions of exosomes from mesenchymal stem cells are enhanced by hypoxia treatment of the cells via transferring microRNA-210 in an nSMase2-dependent way. Artif Cells Nanomed Biotechnol. 2018;46(8):1659–1670. doi:10.1080/21691401.2017.1388249
24. Witwer KW, Théry C. Extracellular vesicles or exosomes? On primacy, precision, and popularity influencing a choice of nomenclature. J Extracell Vesicles. 2019;8(1):1648167. doi:10.1080/20013078.2019.1648167
25. Théry C, Witwer KW, Aikawa E, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the international society for extracellular vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7(1):1535750. doi:10.1080/20013078.2018.1535750
26. van Niel G, Carter DRF, Clayton A, Lambert DW, Raposo G, Vader P. Challenges and directions in studying cell-cell communication by extracellular vesicles. Nat Rev Mol Cell Biol. 2022;23(5):369–382. doi:10.1038/s41580-022-00460-3
27. Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478). doi:10.1126/science.aau6977
28. Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy. 2006;8(4):315–317. doi:10.1080/14653240600855905
29. van Balkom BWM, Gremmels H, Giebel B, Lim SK. Proteomic signature of mesenchymal stromal cell-derived small extracellular vesicles. Proteomics. 2019;19(1–2):e1800163. doi:10.1002/pmic.201800163
30. Witwer KW, Van Balkom BWM, Bruno S, et al. Defining mesenchymal stromal cell (MSC)-derived small extracellular vesicles for therapeutic applications. J Extracell Vesicles. 2019;8(1):1609206. doi:10.1080/20013078.2019.1609206
31. Gimona M, Brizzi MF, Choo ABH, et al. Critical considerations for the development of potency tests for therapeutic applications of mesenchymal stromal cell-derived small extracellular vesicles. Cytotherapy. 2021;23(5):373–380. doi:10.1016/j.jcyt.2021.01.001
32. Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200(4):373–383. doi:10.1083/jcb.201211138
33. Elsharkasy OM, Nordin JZ, Hagey DW, et al. Extracellular vesicles as drug delivery systems: why and how? Adv Drug Deliv Rev. 2020;159:332–343. doi:10.1016/j.addr.2020.04.004
34. Huber J, Griffin MF, Longaker MT, Quarto N. Exosomes: a tool for bone tissue engineering. Tissue Eng Part B Rev. 2022;28(1):101–113. doi:10.1089/ten.teb.2020.0246
35. Masaoutis C, Theocharis S. The role of exosomes in bone remodeling: implications for bone physiology and disease. Dis Markers. 2019;2019:9417914. doi:10.1155/2019/9417914
36. Xing L, Schwarz EM, Boyce BF. Osteoclast precursors, RANKL/RANK, and immunology. Immunol Rev. 2005;208:19–29. doi:10.1111/j.0105-2896.2005.00336.x
37. Boyce BF. Advances in the regulation of osteoclasts and osteoclast functions. J Dent Res. 2013;92(10):860–867. doi:10.1177/0022034513500306
38. Sims NA, Walsh NC. Intercellular cross-talk among bone cells: new factors and pathways. Curr Osteoporos Rep. 2012;10(2):109–117. doi:10.1007/s11914-012-0096-1
39. van der Eerden BC. MicroRNAs in the skeleton: cell-restricted or potent intercellular communicators? Arch Biochem Biophys. 2014;561:46–55. doi:10.1016/j.abb.2014.04.016
40. Zhao P, Xiao L, Peng J, Qian YQ, Huang CC. Exosomes derived from bone marrow mesenchymal stem cells improve osteoporosis through promoting osteoblast proliferation via MAPK pathway. Eur Rev Med Pharmacol Sci. 2018;22(12):3962–3970. doi:10.26355/eurrev_201806_15280
41. Qin Y, Wang L, Gao Z, Chen G, Zhang C. Bone marrow stromal/stem cell-derived extracellular vesicles regulate osteoblast activity and differentiation in vitro and promote bone regeneration in vivo. Sci Rep. 2016;6(1):21961. doi:10.1038/srep21961
42. Lu Z, Chen Y, Dunstan C, Roohani-Esfahani S, Zreiqat H. Priming adipose stem cells with tumor necrosis factor-alpha preconditioning potentiates their exosome efficacy for bone regeneration. Tissue Eng Part A. 2017;23(21–22):1212–1220. doi:10.1089/ten.tea.2016.0548
43. Xu S, Wang Z. Bone marrow mesenchymal stem cell-derived exosomes enhance osteoclastogenesis during alveolar bone deterioration in rats. RSC Adv. 2017;7(34):21153–21163. doi:10.1039/C6RA27931G
44. Takayanagi H. Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat Rev Immunol. 2007;7(4):292–304. doi:10.1038/nri2062
45. Tsukasaki M, Takayanagi H. Osteoimmunology: evolving concepts in bone-immune interactions in health and disease. Nat Rev Immunol. 2019;19(10):626–642. doi:10.1038/s41577-019-0178-8
46. Okamoto K, Nakashima T, Shinohara M, et al. Osteoimmunology: the conceptual framework unifying the immune and skeletal systems. Physiol Rev. 2017;97(4):1295–1349. doi:10.1152/physrev.00036.2016
47. Walsh MC, Choi Y. Biology of the RANKL-RANK-OPG system in immunity, bone, and beyond. Front Immunol. 2014;5:511. doi:10.3389/fimmu.2014.00511
48. Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505(7483):327–334. doi:10.1038/nature12984
49. Méndez-Ferrer S, Michurina TV, Ferraro F, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466(7308):829–834. doi:10.1038/nature09262
50. Vonk LA, van Dooremalen SFJ, Liv N, et al. Mesenchymal stromal/stem cell-derived extracellular vesicles promote human cartilage regeneration. Theranostics. 2018;8(4):906–920. doi:10.7150/thno.20746
51. Li S, Liu J, Liu S, Jiao W, Wang X. Mesenchymal stem cell-derived extracellular vesicles prevent the development of osteoarthritis via the circHIPK3/miR-124-3p/MYH9 axis. J Nanobiotechnology. 2021;19(1):194. doi:10.1186/s12951-021-00940-2
52. Wang Y, Zhou X, Wang D. Mesenchymal stem cell-derived extracellular vesicles inhibit osteoporosis via MicroRNA-27a-induced inhibition of DKK2-mediated Wnt/β-catenin pathway. Inflammation. 2022;45(2):780–799. doi:10.1007/s10753-021-01583-z
53. Lei F, Li M, Lin T, Zhou H, Wang F, Su X. Treatment of inflammatory bone loss in periodontitis by stem cell-derived exosomes. Acta Biomater. 2022;141:333–343. doi:10.1016/j.actbio.2021.12.035
54. Xu R, Shen X, Si Y, et al. MicroRNA-31a-5p from aging BMSCs links bone formation and resorption in the aged bone marrow microenvironment. Aging Cell. 2018;17(4):e12794. doi:10.1111/acel.12794
55. Cosenza S, Ruiz M, Toupet K, Jorgensen C, Noel D. Mesenchymal stem cells derived exosomes and microparticles protect cartilage and bone from degradation in osteoarthritis. Sci Rep. 2017;7(1):16214. doi:10.1038/s41598-017-15376-8
56. Phinney DG, Di Giuseppe M, Njah J, et al. Mesenchymal stem cells use extracellular vesicles to outsource mitophagy and shuttle microRNAs. Nat Commun. 2015;6:8472. doi:10.1038/ncomms9472
57. Van Dyken SJ, Locksley RM. Interleukin-4- and interleukin-13-mediated alternatively activated macrophages: roles in homeostasis and disease. Annu Rev Immunol. 2013;31:317–343. doi:10.1146/annurev-immunol-032712-095906
58. Varin A, Gordon S. Alternative activation of macrophages: immune function and cellular biology. Immunobiology. 2009;214(7):630–641. doi:10.1016/j.imbio.2008.11.009
59. Teo KYW, Zhang S, Loh JT, et al. Mesenchymal stromal cell exosomes mediate M2-like macrophage polarization through CD73/Ecto-5’-nucleotidase activity. Pharmaceutics. 2023;15(5):1489. doi:10.3390/pharmaceutics15051489
60. Kang M, Huang CC, Gajendrareddy P, et al. Extracellular vesicles from TNFα preconditioned MSCs: effects on immunomodulation and bone regeneration. Front Immunol. 2022;13:878194. doi:10.3389/fimmu.2022.878194
61. Song Y, Dou H, Li X, et al. Exosomal miR-146a contributes to the enhanced therapeutic efficacy of interleukin-1β-primed mesenchymal stem cells against sepsis. Stem Cells. 2017;35(5):1208–1221. doi:10.1002/stem.2564
62. Gong L, Zhao Y, Zhang Y, Ruan Z. The macrophage polarization regulates msc osteoblast differentiation in vitro. Ann Clin Lab Sci. 2016;46(1):65–71.
63. Xiong Y, Chen L, Yan C, et al. M2 macrophagy-derived exosomal miRNA-5106 induces bone mesenchymal stem cells towards osteoblastic fate by targeting salt-inducible kinase 2 and 3. J Nanobiotechnology. 2020;18(1):66. doi:10.1186/s12951-020-00622-5
64. Wang Y, Lin Q, Zhang H, et al. M2 macrophage-derived exosomes promote diabetic fracture healing by acting as an immunomodulator. Bioact Mater. 2023;28:273–283. doi:10.1016/j.bioactmat.2023.05.018
65. Yang J, Zou Y, Jiang D. Honokiol suppresses proliferation and induces apoptosis via regulation of the miR21/PTEN/PI3K/AKT signaling pathway in human osteosarcoma cells. Int J Mol Med. 2018;41(4):1845–1854. doi:10.3892/ijmm.2018.3433
66. Zheng P, Chen L, Yuan X, et al. Exosomal transfer of tumor-associated macrophage-derived miR-21 confers cisplatin resistance in gastric cancer cells. J Exp Clin Cancer Res. 2017;36(1):53. doi:10.1186/s13046-017-0528-y
67. Xia Y, He XT, Xu XY, Tian BM, An Y, Chen FM. Exosomes derived from M0, M1 and M2 macrophages exert distinct influences on the proliferation and differentiation of mesenchymal stem cells. PeerJ. 2020;8:e8970. doi:10.7717/peerj.8970
68. Kusumbe AP, Ramasamy SK, Adams RH. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature. 2014;507(7492):7492):323–328. doi:10.1038/nature13145
69. Ramasamy SK, Kusumbe AP, Wang L, Adams RH. Endothelial Notch activity promotes angiogenesis and osteogenesis in bone. Nature. 2014;507(7492):376–380. doi:10.1038/nature13146
70. Zhang Y, Hao Z, Wang P, et al. Exosomes from human umbilical cord mesenchymal stem cells enhance fracture healing through HIF-1α-mediated promotion of angiogenesis in a rat model of stabilized fracture. Cell Prolif. 2019;52(2):e12570. doi:10.1111/cpr.12570
71. Zhang L, Jiao G, Ren S, et al. Exosomes from bone marrow mesenchymal stem cells enhance fracture healing through the promotion of osteogenesis and angiogenesis in a rat model of nonunion. Stem Cell Res Ther. 2020;11(1):38. doi:10.1186/s13287-020-1562-9
72. Liu W, Li L, Rong Y, et al. Hypoxic mesenchymal stem cell-derived exosomes promote bone fracture healing by the transfer of miR-126. Acta Biomater. 2020;103:196–212. doi:10.1016/j.actbio.2019.12.020
73. Jia Y, Zhu Y, Qiu S, Xu J, Chai Y. Exosomes secreted by endothelial progenitor cells accelerate bone regeneration during distraction osteogenesis by stimulating angiogenesis. Stem Cell Res Ther. 2019;10(1):12. doi:10.1186/s13287-018-1115-7
74. Behera J, Kumar A, Voor MJ, Tyagi N. Exosomal lncRNA-H19 promotes osteogenesis and angiogenesis through mediating Angpt1/Tie2-NO signaling in CBS-heterozygous mice. Theranostics. 2021;11(16):7715–7734. doi:10.7150/thno.58410
75. Yang W, Zhu W, Yang Y, et al. Exosomal miR-100-5p inhibits osteogenesis of hBMSCs and angiogenesis of HUVECs by suppressing the BMPR2/Smad1/5/9 signalling pathway. Stem Cell Res Ther. 2021;12(1):390. doi:10.1186/s13287-021-02438-y
76. Artico M, Bosco S, Cavallotti C, et al. Noradrenergic and cholinergic innervation of the bone marrow. Int J Mol Med. 2002;10(1):77–80.
77. Hohmann EL, Elde RP, Rysavy JA, Einzig S, Gebhard RL. Innervation of periosteum and bone by sympathetic vasoactive intestinal peptide-containing nerve fibers. Science. 1986;232(4752):868–871. doi:10.1126/science.3518059
78. Jones RE, Salhotra A, Robertson KS, et al. Skeletal stem cell-Schwann cell circuitry in mandibular repair. Cell Rep. 2019;28(11):2757–2766 e5. doi:10.1016/j.celrep.2019.08.021
79. Carr MJ, Toma JS, Johnston APW, et al. Mesenchymal precursor cells in adult nerves contribute to mammalian tissue repair and regeneration. Cell Stem Cell. 2019;24(2):240–256 e9. doi:10.1016/j.stem.2018.10.024
80. Wan -Q-Q, Qin W-P, Shen M-J, et al. Simultaneous regeneration of bone and nerves through materials and architectural design: are we there yet? Adv Funct Mater. 2020;30(48):2003542. doi:10.1002/adfm.202003542
81. Leitao L, Neto E, Conceicao F, et al. Osteoblasts are inherently programmed to repel sensory innervation. Bone Res. 2020;8:20. doi:10.1038/s41413-020-0096-1
82. Zhu S, Zhu J, Zhen G, et al. Subchondral bone osteoclasts induce sensory innervation and osteoarthritis pain. J Clin Invest. 2019;129(3):1076–1093. doi:10.1172/JCI121561
83. Neto E, Leitão L, Mateus JC, et al. Osteoclast-derived extracellular vesicles are implicated in sensory neurons sprouting through the activation of epidermal growth factor signaling. Cell Biosci. 2022;12(1):127. doi:10.1186/s13578-022-00864-w
84. Mead B, Tomarev S. Bone marrow-derived mesenchymal stem cells-derived exosomes promote survival of retinal ganglion cells through miRNA-dependent mechanisms. Stem Cells Transl Med. 2017;6(4):1273–1285. doi:10.1002/sctm.16-0428
85. Li J, Ding Z, Li Y, et al. BMSCs-derived exosomes ameliorate pain via abrogation of aberrant nerve invasion in subchondral bone in lumbar facet joint osteoarthritis. J Orthop Res. 2020;38(3):670–679. doi:10.1002/jor.24497
86. Wang H, Jia Y, Li J, Liu Q. Schwann cell‑derived exosomes induce bone marrow‑derived mesenchymal stem cells to express Schwann cell markers in vitro. Mol Med Rep. 2020;21(3):1640–1646. doi:10.3892/mmr.2020.10960
87. Wiklander OP, Nordin JZ, O’Loughlin A, et al. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J Extracell Vesicles. 2015;4:26316. doi:10.3402/jev.v4.26316
88. Wang J, Bonacquisti EE, Brown AD, Nguyen J. Boosting the biogenesis and secretion of mesenchymal stem cell-derived exosomes. Cells. 2020;9(3):1. doi:10.3390/cells9030660
89. Lai CP, Mardini O, Ericsson M, et al. Dynamic biodistribution of extracellular vesicles in vivo using a multimodal imaging reporter. ACS Nano. 2014;8(1):483–494. doi:10.1021/nn404945r
90. Imai T, Takahashi Y, Nishikawa M, et al. Macrophage-dependent clearance of systemically administered B16BL6-derived exosomes from the blood circulation in mice. J Extracell Vesicles. 2015;4:26238. doi:10.3402/jev.v4.26238
91. Vidal L, Kampleitner C, Brennan MA, Hoornaert A, Layrolle P. Reconstruction of large skeletal defects: current clinical therapeutic strategies and future directions using 3D printing. Front Bioeng Biotechnol. 2020;8:61. doi:10.3389/fbioe.2020.00061
92. Bahney CS, Zondervan RL, Allison P, et al. Cellular biology of fracture healing. J Orthop Res. 2019;37(1):35–50. doi:10.1002/jor.24170
93. Brennan MA, Layrolle P, Mooney DJ. Biomaterials functionalized with MSC secreted extracellular vesicles and soluble factors for tissue regeneration. Adv Funct Mater. 2020;30(37):1909125. doi:10.1002/adfm.201909125
94. Luan X, Sansanaphongpricha K, Myers I, Chen H, Yuan H, Sun D. Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacol Sin. 2017;38(6):754–763. doi:10.1038/aps.2017.12
95. Qiu B, Xu X, Yi P, Hao Y. Curcumin reinforces MSC-derived exosomes in attenuating osteoarthritis via modulating the miR-124/NF-kB and miR-143/ROCK1/TLR9 signalling pathways. J Cell Mol Med. 2020;24(18):10855–10865. doi:10.1111/jcmm.15714
96. Tao SC, Yuan T, Zhang YL, Yin WJ, Guo SC, Zhang CQ. Exosomes derived from miR-140-5p-overexpressing human synovial mesenchymal stem cells enhance cartilage tissue regeneration and prevent osteoarthritis of the knee in a rat model. Theranostics. 2017;7(1):180–195. doi:10.7150/thno.17133
97. Zhang B, Tian X, Qu Z, Hao J, Zhang W. Hypoxia-preconditioned extracellular vesicles from mesenchymal stem cells improve cartilage repair in osteoarthritis. Membranes. 2022;12(2):225. doi:10.3390/membranes12020225
98. Tao SC, Huang JY, Gao Y, et al. Small extracellular vesicles in combination with sleep-related circRNA3503: a targeted therapeutic agent with injectable thermosensitive hydrogel to prevent osteoarthritis. Bioact Mater. 2021;6(12):4455–4469. doi:10.1016/j.bioactmat.2021.04.031
99. Cui Y, Guo Y, Kong L, et al. A bone-targeted engineered exosome platform delivering siRNA to treat osteoporosis. Bioact Mater. 2022;10:207–221. doi:10.1016/j.bioactmat.2021.09.015
100. Luo ZW, Li FX, Liu YW, et al. Aptamer-functionalized exosomes from bone marrow stromal cells target bone to promote bone regeneration. Nanoscale. 2019;11(43):20884–20892. doi:10.1039/C9NR02791B
101. Wang Y, Yao J, Cai L, et al. Bone-targeted extracellular vesicles from mesenchymal stem cells for osteoporosis therapy. Int J Nanomed. 2020;15:7967–7977. doi:10.2147/IJN.S263756
102. Nakao Y, Fukuda T, Zhang Q, et al. Exosomes from TNF-α-treated human gingiva-derived MSCs enhance M2 macrophage polarization and inhibit periodontal bone loss. Acta Biomater. 2021;122:306–324. doi:10.1016/j.actbio.2020.12.046
103. Shen Z, Kuang S, Zhang Y, et al. Chitosan hydrogel incorporated with dental pulp stem cell-derived exosomes alleviates periodontitis in mice via a macrophage-dependent mechanism. Bioact Mater. 2020;5(4):1113–1126. doi:10.1016/j.bioactmat.2020.07.002
104. You DG, Lim GT, Kwon S, et al. Metabolically engineered stem cell-derived exosomes to regulate macrophage heterogeneity in rheumatoid arthritis. Sci Adv. 2021;7(23):eabe0083. doi:10.1126/sciadv.abe0083
105. Wei H, Chen F, Chen J, et al. Mesenchymal stem cell derived exosomes as nanodrug carrier of doxorubicin for targeted osteosarcoma therapy via SDF1-CXCR4 axis. Int J Nanomed. 2022;17:3483–3495. doi:10.2147/IJN.S372851
106. Zhang H, Wang J, Ren T, et al. Bone marrow mesenchymal stem cell-derived exosomal miR-206 inhibits osteosarcoma progression by targeting TRA2B. Cancer Lett. 2020;490:54–65. doi:10.1016/j.canlet.2020.07.008
107. Xu Z, Zhou X, Wu J, et al. Mesenchymal stem cell-derived exosomes carrying microRNA-150 suppresses the proliferation and migration of osteosarcoma cells via targeting IGF2BP1. Transl Cancer Res. 2020;9(9):5323–5335. doi:10.21037/tcr-20-83
108. Gao Y, Yuan Z, Yuan X, et al. Bioinspired porous microspheres for sustained hypoxic exosomes release and vascularized bone regeneration. Bioact Mater. 2022;14:377–388. doi:10.1016/j.bioactmat.2022.01.041
109. Deng J, Wang X, Zhang W, et al. Versatile hypoxic extracellular vesicles laden in an injectable and bioactive hydrogel for accelerated bone regeneration. Adv Funct Mater. 2023;33(21):2211664. doi:10.1002/adfm.202211664
110. Huang CC, Kang M, Lu Y, et al. Functionally engineered extracellular vesicles improve bone regeneration. Acta Biomater. 2020;109:182–194. doi:10.1016/j.actbio.2020.04.017
111. Chen S, Tang Y, Liu Y, et al. Exosomes derived from miR-375-overexpressing human adipose mesenchymal stem cells promote bone regeneration. Cell Prolif. 2019;52(5):e12669. doi:10.1111/cpr.12669
112. Wu D, Chang X, Tian J, et al. Bone mesenchymal stem cells stimulation by magnetic nanoparticles and a static magnetic field: release of exosomal miR-1260a improves osteogenesis and angiogenesis. J Nanobiotechnology. 2021;19(1):209. doi:10.1186/s12951-021-00958-6
113. Liang B, Liang JM, Ding JN, Xu J, Xu JG, Chai YM. Dimethyloxaloylglycine-stimulated human bone marrow mesenchymal stem cell-derived exosomes enhance bone regeneration through angiogenesis by targeting the AKT/mTOR pathway. Stem Cell Res Ther. 2019;10(1):335. doi:10.1186/s13287-019-1410-y
114. Zhang J, Liu X, Li H, et al. Exosomes/tricalcium phosphate combination scaffolds can enhance bone regeneration by activating the PI3K/Akt signaling pathway. Stem Cell Res Ther. 2016;7(1):136. doi:10.1186/s13287-016-0391-3
115. Ying C, Wang R, Wang Z, et al. BMSC-exosomes carry mutant HIF-1α for improving angiogenesis and osteogenesis in critical-sized calvarial defects. Front Bioeng Biotechnol. 2020;8:565561. doi:10.3389/fbioe.2020.565561
116. Liu A, Lin D, Zhao H, et al. Optimized BMSC-derived osteoinductive exosomes immobilized in hierarchical scaffold via lyophilization for bone repair through Bmpr2/Acvr2b competitive receptor-activated Smad pathway. Biomaterials. 2021;272:120718. doi:10.1016/j.biomaterials.2021.120718
117. Diomede F, Gugliandolo A, Cardelli P, et al. Three-dimensional printed PLA scaffold and human gingival stem cell-derived extracellular vesicles: a new tool for bone defect repair. Stem Cell Res Ther. 2018;9(1):104. doi:10.1186/s13287-018-0850-0
118. Li W, Liu Y, Zhang P, et al. Tissue-engineered bone immobilized with human adipose stem cells-derived exosomes promotes bone regeneration. ACS Appl Mater Interfaces. 2018;10(6):5240–5254. doi:10.1021/acsami.7b17620
119. Swanson WB, Zhang Z, Xiu K, et al. Scaffolds with controlled release of pro-mineralization exosomes to promote craniofacial bone healing without cell transplantation. Acta Biomater. 2020;118:215–232. doi:10.1016/j.actbio.2020.09.052
120. Ma S, Wu J, Hu H, et al. Novel fusion peptides deliver exosomes to modify injectable thermo-sensitive hydrogels for bone regeneration. Mater Today Bio. 2022;13:100195. doi:10.1016/j.mtbio.2021.100195
121. Zhang Y, Xie Y, Hao Z, et al. Umbilical mesenchymal stem cell-derived exosome-encapsulated hydrogels accelerate bone repair by enhancing angiogenesis. ACS Appl Mater Interfaces. 2021;13(16):18472–18487. doi:10.1021/acsami.0c22671
122. Kang Y, Xu C, Meng L, Dong X, Qi M, Jiang D. Exosome-functionalized magnesium-organic framework-based scaffolds with osteogenic, angiogenic and anti-inflammatory properties for accelerated bone regeneration. Bioact Mater. 2022;18:26–41. doi:10.1016/j.bioactmat.2022.02.012
123. Li F, Wu J, Li D, et al. Engineering stem cells to produce exosomes with enhanced bone regeneration effects: an alternative strategy for gene therapy. J Nanobiotechnology. 2022;20(1):135. doi:10.1186/s12951-022-01347-3
124. Liu W, Yu M, Chen F, et al. A novel delivery nanobiotechnology: engineered miR-181b exosomes improved osteointegration by regulating macrophage polarization. J Nanobiotechnology. 2021;19(1):269. doi:10.1186/s12951-021-01015-y
125. Fan L, Guan P, Xiao C, et al. Exosome-functionalized polyetheretherketone-based implant with immunomodulatory property for enhancing osseointegration. Bioact Mater. 2021;6(9):2754–2766. doi:10.1016/j.bioactmat.2021.02.005
126. Zhai M, Zhu Y, Yang M, Mao C. Human mesenchymal stem cell derived exosomes enhance cell-free bone regeneration by altering their miRNAs profiles. Adv Sci. 2020;7(19):2001334. doi:10.1002/advs.202001334
127. Qi X, Zhang J, Yuan H, et al. Exosomes secreted by human-induced pluripotent stem cell-derived mesenchymal stem cells repair critical-sized bone defects through enhanced angiogenesis and osteogenesis in osteoporotic rats. Int J Biol Sci. 2016;12(7):836–849. doi:10.7150/ijbs.14809
128. Guan P, Liu C, Xie D, et al. Exosome-loaded extracellular matrix-mimic hydrogel with anti-inflammatory property Facilitates/promotes growth plate injury repair. Bioact Mater. 2022;10:145–158. doi:10.1016/j.bioactmat.2021.09.010
129. Zhang B, Huang J, Liu J, Lin F, Ding Z, Xu J. Injectable composite hydrogel promotes osteogenesis and angiogenesis in spinal fusion by optimizing the bone marrow mesenchymal stem cell microenvironment and exosomes secretion. Mater Sci Eng C Mater Biol Appl. 2021;123:111782. doi:10.1016/j.msec.2020.111782
130. Xing Y, Yerneni SS, Wang W, Taylor RE, Campbell PG, Ren X. Engineering pro-angiogenic biomaterials via chemoselective extracellular vesicle immobilization. Biomaterials. 2022;281:121357. doi:10.1016/j.biomaterials.2021.121357
131. Lu H, Zhang Y, Xiong S, et al. Modulatory role of silver nanoparticles and mesenchymal stem cell-derived exosome-modified barrier membrane on macrophages and osteogenesis. Front Chem. 2021;9:699802. doi:10.3389/fchem.2021.699802
132. Liu Y, Zhang Z, Wang B, et al. Inflammation-stimulated MSC-derived small extracellular vesicle miR-27b-3p regulates macrophages by targeting CSF-1 to promote temporomandibular joint condylar regeneration. Small. 2022;18(16):e2107354. doi:10.1002/smll.202107354
133. Jing H, Zhang X, Luo K, et al. miR-381-abundant small extracellular vesicles derived from kartogenin-preconditioned mesenchymal stem cells promote chondrogenesis of MSCs by targeting TAOK1. Biomaterials. 2020;231:119682. doi:10.1016/j.biomaterials.2019.119682
134. Liu C, Li Y, Yang Z, Zhou Z, Lou Z, Zhang Q. Kartogenin enhances the therapeutic effect of bone marrow mesenchymal stem cells derived exosomes in cartilage repair. Nanomedicine. 2020;15(3):273–288. doi:10.2217/nnm-2019-0208
135. Li H, Liu D, Li C, et al. Exosomes secreted from mutant-HIF-1α-modified bone-marrow-derived mesenchymal stem cells attenuate early steroid-induced avascular necrosis of femoral head in rabbit. Cell Biol Int. 2017;41(12):1379–1390. doi:10.1002/cbin.10869
136. Hankenson KD, Gagne K, Shaughnessy M. Extracellular signaling molecules to promote fracture healing and bone regeneration. Adv Drug Deliv Rev. 2015;94:3–12. doi:10.1016/j.addr.2015.09.008
137. Balmayor ER. Targeted delivery as key for the success of small osteoinductive molecules. Adv Drug Deliv Rev. 2015;94:13–27. doi:10.1016/j.addr.2015.04.022
138. Chen P, Liao X. Kartogenin delivery systems for biomedical therapeutics and regenerative medicine. Drug Deliv. 2023;30(1):2254519. doi:10.1080/10717544.2023.2254519
139. Wang Y, Chen X, Cao W, Shi Y. Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat Immunol. 2014;15(11):1009–1016. doi:10.1038/ni.3002
140. Shi Y, Wang Y, Li Q, et al. Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat Rev Nephrol. 2018;14(8):493–507. doi:10.1038/s41581-018-0023-5
141. Groh ME, Maitra B, Szekely E, Koc ON. Human mesenchymal stem cells require monocyte-mediated activation to suppress alloreactive T cells. Exp Hematol. 2005;33(8):928–934. doi:10.1016/j.exphem.2005.05.002
142. Takeuchi S, Tsuchiya A, Iwasawa T, et al. Small extracellular vesicles derived from interferon-γ pre-conditioned mesenchymal stromal cells effectively treat liver fibrosis. NPJ Regen Med. 2021;6(1):19. doi:10.1038/s41536-021-00132-4
143. Harting MT, Srivastava AK, Zhaorigetu S, et al. Inflammation-stimulated mesenchymal stromal cell-derived extracellular vesicles attenuate inflammation. Stem Cells. 2018;36(1):79–90. doi:10.1002/stem.2730
144. Spencer JA, Ferraro F, Roussakis E, et al. Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature. 2014;508(7495):269–273. doi:10.1038/nature13034
145. Takubo K, Goda N, Yamada W, et al. Regulation of the HIF-1alpha level is essential for hematopoietic stem cells. Cell Stem Cell. 2010;7(3):391–402. doi:10.1016/j.stem.2010.06.020
146. Nombela-Arrieta C, Pivarnik G, Winkel B, et al. Quantitative imaging of haematopoietic stem and progenitor cell localization and hypoxic status in the bone marrow microenvironment. Nat Cell Biol. 2013;15(5):533–543. doi:10.1038/ncb2730
147. King HW, Michael MZ, Gleadle JM. Hypoxic enhancement of exosome release by breast cancer cells. BMC Cancer. 2012;12:421. doi:10.1186/1471-2407-12-421
148. Xi L, Peng M, Liu S, et al. Hypoxia-stimulated ATM activation regulates autophagy-associated exosome release from cancer-associated fibroblasts to promote cancer cell invasion. J Extracell Vesicles. 2021;10(11):e12146. doi:10.1002/jev2.12146
149. Hu X, Wu R, Shehadeh LA, et al. Severe hypoxia exerts parallel and cell-specific regulation of gene expression and alternative splicing in human mesenchymal stem cells. BMC Genomics. 2014;15:303. doi:10.1186/1471-2164-15-303
150. Hu X, Xu Y, Zhong Z, et al. A large-scale investigation of hypoxia-preconditioned allogeneic mesenchymal stem cells for myocardial repair in nonhuman primates: paracrine activity without remuscularization. Circ Res. 2016;118(6):970–983. doi:10.1161/CIRCRESAHA.115.307516
151. Zhu LP, Tian T, Wang JY, et al. Hypoxia-elicited mesenchymal stem cell-derived exosomes facilitates cardiac repair through miR-125b-mediated prevention of cell death in myocardial infarction. Theranostics. 2018;8(22):6163–6177. doi:10.7150/thno.28021
152. Ross CL, Siriwardane M, Almeida-Porada G, et al. The effect of low-frequency electromagnetic field on human bone marrow stem/progenitor cell differentiation. Stem Cell Res. 2015;15(1):96–108. doi:10.1016/j.scr.2015.04.009
153. Yun HM, Ahn SJ, Park KR, et al. Magnetic nanocomposite scaffolds combined with static magnetic field in the stimulation of osteoblastic differentiation and bone formation. Biomaterials. 2016;85:88–98. doi:10.1016/j.biomaterials.2016.01.035
154. Luo L, Wu Z, Wang Y, Li H. Regulating the production and biological function of small extracellular vesicles: current strategies, applications and prospects. J Nanobiotechnology. 2021;19(1):422. doi:10.1186/s12951-021-01171-1
155. Chen G, Zhuo Y, Tao B, et al. Moderate SMFs attenuate bone loss in mice by promoting directional osteogenic differentiation of BMSCs. Stem Cell Res Ther. 2020;11(1):487. doi:10.1186/s13287-020-02004-y
156. Wu D, Kang L, Tian J, et al. Exosomes derived from bone mesenchymal stem cells with the stimulation of Fe(3)O(4) nanoparticles and static magnetic field enhance wound healing through upregulated miR-21-5p. Int J Nanomed. 2020;15:7979–7993. doi:10.2147/IJN.S275650
157. Kim EC, Leesungbok R, Lee SW, et al. Effects of moderate intensity static magnetic fields on human bone marrow-derived mesenchymal stem cells. Bioelectromagnetics. 2015;36(4):267–276. doi:10.1002/bem.21903
158. Markov MS. Expanding use of pulsed electromagnetic field therapies. Electromagn Biol Med. 2007;26(3):257–274. doi:10.1080/15368370701580806
159. Wei JW, Huang K, Yang C, Kang CS. Non-coding RNAs as regulators in epigenetics (Review). Oncol Rep. 2017;37(1):3–9. doi:10.3892/or.2016.5236
160. Kamerkar S, LeBleu VS, Sugimoto H, et al. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature. 2017;546(7659):498–503. doi:10.1038/nature22341
161. Gonzalez-King H, García NA, Ontoria-Oviedo I, Ciria M, Montero JA, Sepúlveda P. Hypoxia inducible factor-1α potentiates jagged 1-mediated angiogenesis by mesenchymal stem cell-derived exosomes. Stem Cells. 2017;35(7):1747–1759. doi:10.1002/stem.2618
162. Liu Y, Lin L, Zou R, Wen C, Wang Z, Lin F. MSC-derived exosomes promote proliferation and inhibit apoptosis of chondrocytes via lncRNA-KLF3-AS1/miR-206/GIT1 axis in osteoarthritis. Cell Cycle. 2018;17(21–22):2411–2422. doi:10.1080/15384101.2018.1526603
163. Lin Y, Wu J, Gu W, et al. Exosome-Liposome Hybrid Nanoparticles Deliver CRISPR/Cas9 System in MSCs. Adv Sci. 2018;5(4):1700611. doi:10.1002/advs.201700611
164. Ordikhani F, Zandi N, Mazaheri M, et al. Targeted nanomedicines for the treatment of bone disease and regeneration. Med Res Rev. 2021;41(3):1221–1254. doi:10.1002/med.21759
165. Lu Y, Mai Z, Cui L, Zhao X. Engineering exosomes and biomaterial-assisted exosomes as therapeutic carriers for bone regeneration. Stem Cell Res Ther. 2023;14(1):55. doi:10.1186/s13287-023-03275-x
166. Ma S, Zhang Y, Li S, Li A, Li Y, Pei D. Engineering exosomes for bone defect repair. Front Bioeng Biotechnol. 2022;10:1091360. doi:10.3389/fbioe.2022.1091360
167. Zhang M, Li Y, Feng T, et al. Bone engineering scaffolds with exosomes: a promising strategy for bone defects repair. Front Bioeng Biotechnol. 2022;10:920378. doi:10.3389/fbioe.2022.920378
168. Liang Y, Xu X, Li X, et al. Chondrocyte-targeted MicroRNA delivery by engineered exosomes toward a cell-free osteoarthritis therapy. ACS Appl Mater Interfaces. 2020;12(33):36938–36947. doi:10.1021/acsami.0c10458
169. Xu X, Liang Y, Li X, et al. Exosome-mediated delivery of kartogenin for chondrogenesis of synovial fluid-derived mesenchymal stem cells and cartilage regeneration. Biomaterials. 2021;269:120539. doi:10.1016/j.biomaterials.2020.120539
170. Hong V, Presolski SI, Ma C, Finn MG. Analysis and optimization of copper-catalyzed azide-alkyne cycloaddition for bioconjugation. Angew Chem Int Ed Engl. 2009;48(52):9879–9883. doi:10.1002/anie.200905087
171. Tian T, Zhang H-X, C-p H, et al. Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy. Biomaterials. 2018;150:137–149. doi:10.1016/j.biomaterials.2017.10.012
172. Wang W, Liang X, Zheng K, et al. Horizon of exosome-mediated bone tissue regeneration: the all-rounder role in biomaterial engineering. Mater Today Bio. 2022;16:100355. doi:10.1016/j.mtbio.2022.100355
173. Glenske K, Donkiewicz P, Köwitsch A, et al. Applications of metals for bone regeneration. Int J Mol Sci. 2018;19(3):826. doi:10.3390/ijms19030826
174. Wei F, Li M, Crawford R, Zhou Y, Xiao Y. Exosome-integrated titanium oxide nanotubes for targeted bone regeneration. Acta Biomater. 2019;86:480–492. doi:10.1016/j.actbio.2019.01.006
175. Dec P, Modrzejewski A, Pawlik A. Existing and novel biomaterials for bone tissue engineering. Int J Mol Sci. 2022;24(1):529. doi:10.3390/ijms24010529
176. Bohner M, Santoni BLG, Döbelin N. β-tricalcium phosphate for bone substitution: synthesis and properties. Acta Biomater. 2020;113:23–41. doi:10.1016/j.actbio.2020.06.022
177. Wang T, Zhou Y, Zhang W, et al. Exosomes and exosome composite scaffolds in periodontal tissue engineering. Front Bioeng Biotechnol. 2023;11:1287714. doi:10.3389/fbioe.2023.1287714
178. Ju Y, Hu Y, Yang P, Xie X, Fang B. Extracellular vesicle-loaded hydrogels for tissue repair and regeneration. Mater Today Bio. 2023;18:100522. doi:10.1016/j.mtbio.2022.100522
179. Liu Y, Zhu Z, Pei X, et al. ZIF-8-modified multifunctional bone-adhesive hydrogels promoting angiogenesis and osteogenesis for bone regeneration. ACS Appl Mater Interfaces. 2020;12(33):36978–36995. doi:10.1021/acsami.0c12090
180. Davies OG. Extracellular vesicles: from bone development to regenerative orthopedics. Mol Ther. 2023;31(5):1251–1274. doi:10.1016/j.ymthe.2023.02.021
181. Toh WS, Lai RC, Zhang B, Lim SK. MSC exosome works through a protein-based mechanism of action. Biochem Soc Trans. 2018;46(4):843–853. doi:10.1042/BST20180079
182. Liu S, Xu X, Liang S, et al. The application of MSCs-derived extracellular vesicles in bone disorders: novel cell-free therapeutic strategy. Front Cell Dev Biol. 2020;8:619. doi:10.3389/fcell.2020.00619
183. Dai W, Zhang L, Yu Y, et al. 3D bioprinting of heterogeneous constructs providing tissue‐specific microenvironment based on host–guest modulated dynamic hydrogel bioink for osteochondral regeneration. Adv Funct Mater. 2022;32:2200710. doi:10.1002/adfm.202200710
184. Ding X, Gao J, Yu X, et al. 3D-printed porous scaffolds of hydrogels modified with TGF-beta1 binding peptides to promote in vivo cartilage regeneration and animal gait restoration. ACS Appl Mater Interfaces. 2022;14(14):15982–15995. doi:10.1021/acsami.2c00761
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.