Back to Journals » International Journal of Women's Health » Volume 11
Menopausal Acne – Challenges And Solutions
Authors Khunger N , Mehrotra K
Received 15 April 2019
Accepted for publication 22 August 2019
Published 29 October 2019 Volume 2019:11 Pages 555—567
DOI https://doi.org/10.2147/IJWH.S174292
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Everett Magann
Niti Khunger, Krati Mehrotra
Department of Dermatology and STD, Vardhman Mahavir Medical College, Safdarjang Hospital, New Delhi, India
Correspondence: Niti Khunger
Department of Dermatology and STD, Vardhman Mahavir Medical College, Safdarjang Hospital, Ring Road, New Delhi 110029, India
Email [email protected]
Abstract: Although acne is a disease predominant in adolescence, it is being increasingly observed in adult life, including the menopausal period. The etiology of menopausal acne is multifactorial, with hormonal imbalance being the major culprit. There is a relative increase of androgens in the menopausal female that leads to clinical hyperandrogenism manifesting as acne, hirsutism and androgenetic alopecia. Other endocrine disorders including thyroid abnormalities, hyperprolactinemia and insulin resistance also play a role. Genetics, stress, dietary changes, lack of sleep and exercise and other lifestyle changes are implicated as trigger factors. Most menopausal women with isolated few acne lesions have normoandrogenic serum levels and do not require extensive investigations. However, baseline investigations including total testosterone are useful. Patients must also be evaluated for associated comorbidities such as obesity, diabetes, hypertension and dyslipidemia. A detailed history can help to exclude polycystic ovarian syndrome, late-onset congenital adrenal hyperplasia or medications as a cause of acne. The evaluation of menopausal acne and the approach to treatment depend on the severity of acne and associated features. In patients with mild acne without virilization, prolonged topical therapy is the mainstay of treatment. Though combined oral contraceptives are effective, they are relatively contraindicated in the postmenopausal period. Spironolactone is the first choice of therapy in the subset of patients that require oral anti-androgen therapy. Procedural treatment can be useful as it can also help in the treatment of associated acne scars and concomitant skin aging. It is also important to focus on lifestyle changes such as reducing stress, controlling obesity, having a healthy diet, exercise and proper skin care routine to reduce acne. The focus of this article is on the clinical presentation and management challenges of menopausal acne, which represents a special subtype of acne.
Keywords: acne, adult, menopause, hormonal, hyperandrogenism
Introduction
Acne vulgaris is a common chronic inflammatory disease of the pilosebaceous unit that causes polymorphic lesions in the form of open and closed comedones, papules, pustules, and occasionally nodules and cysts, with varying degrees of cutaneous scarring. It is primarily a disease of adolescence and often extends into adulthood.1 Adult acne has conventionally been described as acne presenting in patients beyond 25 years of age.2 Recent studies have shown an increasing incidence in adults as well as in the perimenopausal and postmenopausal age groups.3–6
Menopause is a process that occurs due to a decline in the female reproductive hormones, estrogen and progesterone. Natural menopause is defined as the permanent cessation of menstruation, confirmed after 12 consecutive months of amenorrhea in the absence of any other pathological cause. The transition period that leads up to menopause is known as perimenopause and is defined by WHO as a period of two to eight years leading up to menopause and one year following final menses.7 Studies have reported an average age at menopause of 51 years for Caucasian women in the western world.8 When acne is observed around the perimenopause or menopause, it is termed as menopausal acne, generally in women ≥45 years age.3 It is largely classified as 1) Persistent, 2) New-onset and 3) Recurrent disease.3 Persistent acne starts in adolescence and tends to persist in adults. New-onset acne tends to start in adult life only, while recurrent acne develops in adolescence, clears-out for a sometime and reappears again in adulthood.2,9,10
The prevalence of female adult acne varies in different studies, from 20% to 40%.3–6 Acne has a 50.9% prevalence rate in women aged 20 to 29 years versus 26.3% in women ages 40 to 49 years.1 Persistent acne is more common (75–85%) of cases as compared to late onset acne (20–40%).3 A 2006 survey of Americans reported acne prevalence of 66.8% in women in the teen years, 50.9% in the 20s, 35.2% in the 30s, 26.3% in the 40s and 15.3% in the 50s or older.1 The importance of proper evaluation and management of adult and menopausal acne is that it causes significant morbidity of post acne scarring and greatly impacts the quality of life, causing psychosocial distress in affected patients. It may also be indicative of an underlying hormonal disorder and very rarely an androgen secreting tumor. Management of menopausal acne is challenging and there is a lack of studies focusing on acne in this subset of patients.
Methods
PubMed MEDLINE and Google scholar literature search were conducted using the term menopausal acne, adult acne and menopausal hyperandrogenism from 1995 to 2018. Titles, abstracts and full articles including a systematic review, clinical trial, cohort study, case report or series, or cross‐sectional study were scanned for inclusion and review. A narrative review is presented as there is a paucity of studies focusing primarily on menopausal acne.
Etiopathogenesis
Acne is typically an inflammatory disorder of the pilosebaceous unit and has a multifactorial pathogenesis. The four main interrelated pathogenic factors involved are 1) Excess and altered sebum production, under control of androgens 2) Abnormal follicular hyperkeratinization and differentiation, 3) Follicular canal colonization with Cutibacterium acnes (C. acnes) (formerly Propionibacterium acnes) and 4) Chronic inflammation controlled by complex innate and acquired immunological mechanisms.11
In menopausal women, mechanisms that lead to new onset or recurrence of acne are unclear; however, hormonal factors, specifically androgens, play a major role. In the postmenopausal period, the ovary remains hormonally active and secretes varied amounts of androgens and estrogen.12 Estrogen levels tend to fall sharply after menopause, while androgens decrease gradually.13,14 Androgen secretion depends upon luteinizing hormone (LH) stimulation and a significant increase in gonadotropin levels occurs after menopause. This hormonal imbalance between estrogen and androgen further increases due to decrease in sex hormone-binding globulin (SHBG) levels and is called postmenopausal hyperandrogenism which can lead to acne flare.15 Postmenopausal hyperandrogenism is thus a state of relative excess androgen secretion either of adrenal or ovarian origin. Increased receptor sensitivity to the potent androgens, dihydrotestosterone and dehydroepiandrosterone sulfate (DHEAS) also increases susceptibility to acne.3 It tends to present as hirsutism with increase in terminal hair growth or symptoms/signs of virilization. Thus, there are multiple etiological factors that can cause hyperandrogenism in the menopausal period (Table 1).15–18 In patients with polycystic ovaries, symptoms usually start in adolescence, progress during the reproductive years and then gradually decline in the perimenopausal period. Androgen levels remain elevated during early menopause and decline gradually, persisting till late menopause.15 Rarely hyperandrogenism may have a tumorous origin. A postmenopausal woman presenting with hirsutism, alopecia or acne should always be assessed and looked for any other pre-existing cause of hyperandrogenism.15,19
 |
Table 1 Etiology Of Hyperandrogenism In Postmenopausal Women15 |
Though generally women with menopausal acne have normal androgen levels, the mean androgen levels are reported to exceed that of age-matched controls without acne.20
The sebaceous gland is a neuroendocrine organ under control of androgens as well as neuropeptides, histamine, vitamin D, retinoids and insulin-like growth factor 1 (IGF-1). There is a complex interrelationship between IGF-1, insulin and androgen, which may contribute to the development of menopausal acne. The role of IGF-1 in the development of acne has been underplayed. Cappel et al21 reported that IGF-1 levels and DHEAS levels were higher in adult women with acne than in those without acne and that levels correlated with total acne count, comedones and inflammatory lesion count.
Other hormones such as melanocortins and corticotropin-releasing hormone (CRH) also play a role in acne.22 CRH which increases during stress, causes increased expression of 3β-hydroxy-steroid dehydrogenase mRNA, an enzyme responsible for the conversion of dehydroepiandrosterone (DHEA) to testosterone.23 This is one of the mechanisms by which stress aggravates acne.
Recent reports point that lesions of acne develop due to activation of the innate immunity and that this process can occur even before follicular hyperkeratinization, which is responsible for micrcomedone formation. C. acnes induces inflammation through toll-like receptors (TLRs) present in the keratinocytes, sebocytes and dendritic cells. This triggers signalizing cascades that activate transcriptional factors, such as nuclear factor kappa B and phosphokinases. This activation in the monocytes results in the release of two interleukins (ILs), IL-12 and IL-8.22 Of these, IL-12 is the major inflammatory cytokine responsible for the immune response against the invasion of Gram-positive bacteria in the acne lesions.24
C. acnes is now thought to play a secondary role in the development of acne lesions, as patients with acne do not have more C. acnes in sebaceous follicles than normal individuals. Acne might be triggered by the selection of a subset of C. acnes strains, phylotype IA1 due to increased seborrhea and disequilibrium with the skin microbiome, particularly S. epidermidis, which is known to inhibit C. acnes growth.25 In addition, the presence of biofilms further can lead to persistence of pathogenic strains.
Trigger Factors Of Menopausal Acne
Several factors that have been postulated to trigger or aggravate adult acne also play a role in menopausal acne, such as cosmetics, dietary factors, obesity, smoking, ultraviolet radiation, drugs, sleep deprivation and stress.23
Defective barrier function of the skin followed by increase in transepidermal water loss may also be responsible for initiating the inflammatory cascade in acne, which is seen not only in lesional skin but also in perilesional skin in acne.26,27 In a study on 280 patients of adult acne, exposure to sunlight was responsible for aggravation in 93 (33.2%) patients, cosmetics in 40 (14.3%) patients and stress in 72 (25.7%) patients. Associated conditions such as obesity were observed in 6.4%, hirsutism in 5.7% and alopecia in 1.8%, though raised testosterone levels were seen in only 7 out of 230 female patients (3.04%).2
The relationship between obesity, hyperinsulinemia and hyperandrogenism in postmenopausal women is complex. A state of relative functional hyperandrogenism appears to be associated with abdominal obesity in women.28 Obesity also leads to insulin resistance (IR) and hyperinsulinemia, which increases androgen levels, with insulin acting as a co-gonadotropin15,29 In addition, hyperinsulinemia directly reduces serum SHBG levels, further aggravating hyperandrogenism.30 Genetic factors also play a role as a positive family history was found in 38.6%2 in one study and 56.8% in another study.6
Clinical Features
The clinical presentation of adult acne has been classically described as the presence of inflammatory papulopustular lesions in the mandibular and chin area (Figure 1).2,5,11 However, recent reports have challenged this and a multicentric observational study reported that adult acne presented with few mixed comedones and inflammatory papules across all facial zones, similar to adolescents in 90% of the patients, whereas lesions localized specifically to the mandibular area were seen in only 11.2%. Additionally, truncal lesions were common, seen in up to 50% of the patients.6 Another variant involving perimenopausal women has been described as the presence of deep-seated inflammatory papules or nodules with a predominant perioral distribution.9 These lesions tend to produce post-inflammatory persistent erythema, pigmentation and scarring, besides being resistant to treatment.9 A comedonal variant presents as multiple macrocomedones, with a flare of tender inflammatory lesions pre-menstrually. This variant with predominance of comedones and macrocomedones in the frontal and side regions of the face is usually seen in patients beyond 40 years and in smokers (Figure 2).19 Another variant described in menopausal acne is the presence of multiple closed comedones visible on stretching the skin, along with enlarged pores in the nasal and malar areas.31 In elderly patients (>60 years), acne has a predominant truncal distribution and is often resistant to therapy. The scalp, upper arms and gluteal region may also be involved.32 The difference between adult and post-menopausal acne is shown in Table 2. Women with menopausal acne also have increased sensitivity of the skin, hence have a higher frequency of post-inflammatory erythema, hyper and/or hypopigmentation and scarring. These cosmetically disfiguring changes often lead to depression and have a negative influence on the quality of life.33
 |
Table 2 Comparison Of Clinical Features Of Adult And Postmenopausal Acne2 |
 |
Table 3 Procedural Treatment In Active Acne |
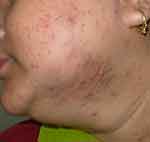 |
Figure 1 Inflammatory papules, deep-seated nodules and comedones on the lower cheek, mandibular area, extending to the neck in an obese perimenopausal woman. |
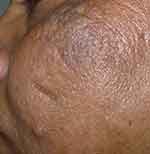 |
Figure 2 Multiple comedones on the upper cheek and a solitary inflammatory papule. |
Signs of hyperandrogenism such as hirsutism, and androgenetic alopecia and acanthosis nigricans should also be examined. Dreno et al6 reported that signs of androgenic abnormalities in adult acne were present in 10.8% of the subjects, whereas in a study from India, obesity was observed in 6.4%, hirsutism in 5.7% and alopecia in 1.8% and raised levels of testosterone were seen only in 3.04%.2
Differential Diagnosis
The differential diagnosis of acneiform and pustular lesions in this age group includes drug-induced acneiform eruptions, rosacea, Favre-Racouchot syndrome and gram-negative folliculitis. A careful drug history should be sought particularly for corticosteroids, androgens, hormone replacement therapy, anabolic steroids, antidepressants, lithium, Vitamin B1, B6, B12 and anti-tuberculous drugs.34 Rosacea presents with inflammatory papules and pustules on an erythematous background, with telangiectasia. A key differentiating factor between acne and papulopustular rosacea is the absence of comedonal lesions in rosacea. Gram-negative folliculitis presents as eruptive monomorphic pustules in the perioral, mandibular and neck distribution. It is more commonly seen in patients on prolonged oral tetracycline use and is caused by gram-negative microbes such as Klebsiella and Serratia. Treatment recommended is isotretinoin.35 Topical steroid abuse on the face is a common cause for acneiform eruptions and aggravation of pre-existing acne, presenting as multiple and deep-seated comedones, nodules and cysts. It is seen in countries where steroids are freely available over the counter and are misused as skin-lightening agents. In a study from India, which included 200 patients, 53 patients were in the age group of 41–50 years who were misusing potent topical steroids on the face, resulting in adverse effects, including acne.36 Steroid abuse on the face is accompanied by telangiectasia and hypertrichosis, which is readily seen with the dermoscope.
Evaluation
The approach to laboratory investigations depends on the clinical examination and associated features (Figure 3).18 If the patient has associated signs of hyperandrogenism such as hirsutism and female pattern hair loss, then a hormonal evaluation is warranted (Figure 3). If there are no obvious signs of hyperandrogenism, it may be due to increased hypersensitivity or increase in number of androgen receptors in the sebocyte and keratinocytes. Here again a baseline hormonal evaluation is useful, though not mandatory. A thorough medical history including details of medications and supplement use, tobacco and illicit drug use must be actively sought and a physical examination, with complete review of systems should be conducted to seek symptoms of hyperandrogenism including acne, hirsutism, seborrhea, androgenetic alopecia, amenorrhea, oligomenorrhea and signs of virilization, such as clitoromegaly, increased muscle mass, decreased breast size, infertility and polycystic ovaries or other endocrinology disorders.34 A menstrual history must include age of menarche, regularity of menses, history of infertility or signs suggestive of hypothyroidism.37
 |
Figure 3 Laboratory evaluation in menopausal acne.15 |
The majority of patients with isolated menopausal acne show no other signs of clinical hyperandrogenism and are therefore considered normoandrogenic. Signs of virilization suggest severe insulin resistance, androgen-secreting tumors or androgen substance abuse. A laboratory test panel including free and total testosterone, dehydroepiandrosterone sulfate, androstenedione, luteinizing hormone and follicle-stimulating hormone, transvaginal and abdominal ultrasound should be done in such patients. Thyroid disease, raised prolactin and nonclassical congenital adrenal hyperplasia should also be investigated.35 Evaluation for metabolic syndrome should be done especially in obese patients and those with acanthosis nigricans, to look for insulin resistance. Microbiologic evaluation is not indicated unless gram-negative folliculitis or staphylococcus aureus folliculitis is considered in the differential diagnosis.35 In the presence of resistant acne lesions, a microbiologic evaluation is worthwhile to rule out resistant C. acnes. If there are nodulocystic lesions, baseline investigations for isotretinoin treatment should be done. It is also important to investigate for co-morbidities in this age group such as diabetes, hypothyroidism and hyperlipidemia.
Approach To Treatment
The treatment of menopausal acne can be challenging as the presence of acne in this age group causes psychosocial impairment and affects the quality of life. There is a tendency to relapse, hence maintenance treatment is essential. The choice of therapy depends on the etiology and severity of acne, response to previous treatment, skin type, dry and sensitive or thick and oily, and associated features. Topical therapies should be chosen with care as they can cause dryness and irritation in older women, who already have dry sensitive skin. Many patients have post acne scars due to previous bouts of acne and these become more prominent as the skin sags due to age. The aim of therapy is to improve acne and its complications like hyperpigmentation and scarring, without causing irritation to mature skin.
Topical Therapy
Topical therapies are the mainstay of acne treatment and judicious use can lead to adherence to treatment, better outcomes and greater patient satisfaction.38 An added advantage of topical therapy is that it can also improve concomitant signs of photoaging that is common in the menopausal age group.
Topical Retinoids
They are the mainstay of treatment and have the added advantage of improving signs of aging and photoaging in older patients. Adapalene (0.3%) is effective and tolerable in adult acne.39 It not only leads to reduction in comedones but also has anti-inflammatory action, which helps in reducing the inflammatory papules, common in this age group. The challenge here is to reduce the irritant potential by gradual retinization of the skin. This is done by first applying it for short duration of 2–4 hrs every other day and gradually increasing the duration till tolerability. In a study of adult acne in women aged 18–41, adapalene 0.3% showed significant reduction of the inflammatory lesions (−61%) and non-inflammatory lesions (−51%) at the 12th week when compared to the vehicle. The side effects were discomfort and dryness.39 Low strength adapalene 0.1% is also efficacious with better tolerability.40 Tretinoin is effective in acne and photoaging but has greater irritant potential. This can be minimized by using the microencapsulated form (0.04%, 0.1%) which releases tretinoin gradually.41 In patients who have sensitive skin, retinol or retinaldehyde may show better tolerability. There have been several studies demonstrating the tolerability of retinol in photoaging skin. Kang et al demonstrated that application of all-trans-retinol on normal human skin enhanced the expression of CRABP II and CRBP mRNAs and proteins similar to tretinoin with only minimal signs of erythema and irritation as compared to tretinoin. However, efficacy was less.42
The most frequent and common adverse effect of topical retinoids is “retinoid reaction”, which is characterized by burning, erythema, pruritus and skin peeling. It is more common with tretinoin and tazarotene as compared to isotretinoin, adapalene, retinol and retinaldehyde.43 The retinoid reaction is due to the presence of free carboxylic acid in the polar end of the retinoid, which is generally seen within the first few weeks of treatment. It is thought to get initiated by release of proinflammatory cytokines such as IL-1, TNF-α, IL-6 and IL-8.44 Retinoids also cause photosensitization, which normally occurs at the beginning of the therapy and returns to normal after few months. Hence, sun protection should be advised at the start of therapy.
Benzoyl Peroxide
Benzoyl peroxide is a comedolytic and antimicrobial topical agent for acne with no known resistance.45 However, it should be used with caution in older women as it can cause irritation and dryness, depending on the strength and vehicle. Low strength of 2.5% in a cream base is preferred.
Azelaic Acid
Azelaic acid (20% cream, 15% gel) has anti-inflammatory, comedolytic and antimicrobial properties and is useful as monotherapy in mild acne. It is also a hypopigmenting agent and is thus useful in patients having post-inflammatory hyperpigmentation.46 It is generally well tolerated and a study showed that twice-daily application of azelaic acid 20% cream was moderately effective in mild-to-moderate acne, with a 53.9% decrease in total lesion count at 12 weeks.47
Dapsone
Dapsone 5% gel has antimicrobial and anti-inflammatory properties and is efficacious and well tolerated when used twice daily as monotherapy in adult females. In a study of 68 adult patients with acne, significant reductions in mean total lesions (52% decrease), inflammatory lesions (65%) and comedone counts (41%; all P<0.001) were observed at 12 weeks, with no treatment-related adverse events.48 It has the advantage of having no risk for bacterial resistance and can be used alone as maintenance therapy for long periods.49
Combination Therapies
Combination of benzoyl peroxide with adapalene is more efficacious with a quick onset of action, however with increased irritant potential.38 Hence, it should be used cautiously. Combination of tretinoin and clindamycin,50 benzoyl peroxide and clindamycin51 is better tolerated and may be used in inflammatory papulopustular acne. Monotherapy with clindamycin is not recommended. Addition of non-comedogenic moisturizers with topical anti-acne treatment helps to reduce adverse events and hence increase compliance.
Systemic Therapy
Antiandrogens, hormonal therapy, isotretinoin and systemic antibiotics are indicated for moderate-to-severe acne or resistant or recurrent acne in older women. Antiandrogens such as spironolactone are the treatment of choice, particularly when there are associated symptoms of hyperandrogenism such as hirsutism and androgenetic alopecia. Combined oral contraceptives, though effective, are relatively contraindicated as the risk of adverse events is greater in postmenopausal women.
Spironolactone
Spironolactone has antiandrogenic effects by blocking 5α-reductase activity and androgen receptor in peripheral tissues. Isvy et al52 retrospectively analyzed 70 adult patients with acne, with age ranging from 20 to 52 years, mean age 31.3 ± 8.4 treated with low-dose spironolactone (less than 150 mg/day). Majority of the women (71%) responded to spironolactone on the face as well as the back within a median treatment duration of six months, with decrease in the severity of seborrhea. In their study, the clinical response to spironolactone was correlated with the number of inflammatory lesions and decreased with the concomitant use of oral first or second-generation contraceptives containing progesterone. However, the concomitant use of third and fourth-generation oral contraception had a favorable response. Contraindications to therapy include significant renal impairment, hyperkalemia or medications known to increase serum potassium level such as trimethoprim or angiotensin-converting enzyme inhibitors. Spironolactone is given at doses of 25–200 mg per day, typically starting at 50–100 mg/d. Side effects are dose-dependent and include fatigue, increased diuresis, headache, dizziness, menstrual irregularity, breast pain and hyperpotassemia. Regular monitoring of potassium is not indicated in patients on low doses and without nephropathies.53 A recent review reported that 200 mg/day of spironolactone effectively reduces inflamed lesion counts, though good quality evidence is lacking for lower doses.54 However, Grandhi et al55 reported 86% of 400 patients reported improvement, with only 4% experiencing any side effects.
Flutamide
Flutamide is a non-steroidal androgen antagonist which acts by competitive inhibition of androgen receptors, especially the ones that bind DHT. It can be effective for the treatment of acne, hirsutism and alopecia; however, hepatic toxicity limits the use of flutamide for acne as cases of fatal hepatitis have been reported.56
Hormonal Therapy
Oral contraceptives (OCPs) containing low-dose ethinyl estradiol combined with progestins with antiandrogenic activity (desogestrel, gestodene, levonorgestrel or norgestimate) or cyproterone acetate or drospirenone is recommended for the treatment of mild-to-moderate adult acne. OCPs reduce ovarian androgen production, decrease sebum production and also increase hepatic synthesis of sex hormone–binding globulin, leading to a decrease in circulating free testosterone. However, use of estrogen in older women has safety issues such as increased cardiovascular disease, hypercoagulability and an increased risk of endometrial and breast cancers. Therefore, in menopausal women with acne before prescribing OCPs, contraindications to hormonal therapy must be investigated to prevent side effects (Box 1). These include history of deep venous thrombosis and thromboembolic events, smoking after the age of 35 years, active liver disease, migraine, breast cancer, hypertension, diabetes mellitus with vascular changes,and long-term immobilization.57
There is a three times increased risk of venous thrombosis that depends on the dose of ethinylestradiol and the progestogen used.58 The risk is similar for combined oral contraceptives with 30–35 μg ethinylestradiol and cyproterone acetate, drospirenone, gestodene and desogestrel, and about 50–80% higher than with levonorgestrel.58 The greatest risk is in the first year of use. Common side effects of OCPs include nausea, headache, breast tenderness and bloating due to the estrogen component, whereas weight gain, fatigue, irritability, increased low-density lipoprotein level (LDL), decreased libido and breast tenderness are attributed to the progestin component.59 OCPs carry a risk of developing hypertension and hence blood pressure should be monitored during follow-up visits.
To summarize the use of hormonal therapy in menopausal acne is an effective option which improves the quality of life, but it should be initiated only after proper evaluation and exclusion of contraindications. Though it is relatively safe, possible serious complications can occur and should be discussed with the patient.60 Patients on hormonal therapy must also be monitored regularly to detect adverse effects.
Isotretinoin
Isotretinoin is indicated for the treatment of severe recalcitrant nodulocystic acne, but it can also be used to treat patients with moderate inflammatory acne that is either resistant to conventional treatment, or relapses quickly after discontinuation of oral therapy or causes significant acne scarring or psychosocial distress.35 Isotretinoin is usually initiated at a starting dose of 0.5 mg/kg/day for the first month and then increased to 1 mg/kg/day as tolerated and is given with meals for better absorption. In severe cases, lower initial doses along with corticosteroid may be needed to prevent an isotretinoin induced flare. Low-dose isotretinoin (0.25–0.4 mg/kg/day), intermittent regimes and lower cumulative dose regimens have been used to minimize the side effects. However, these are not as effective as the standard 120–150 mg cumulative dose in preventing relapse.61 In addition to its potential teratogenicity, side effects of isotretinoin include cheilitis, xerosis, xerophthalmia, decreased night vision, headache, hypertriglyceridemia, cardiovascular risk factors, bone demineralization, hepatotoxicity and mood changes. A possible link to depression, anxiety, suicidal tendencies and inflammatory bowel disease has been reported though not conclusively proved. With standard dosing, most side effects resolve after discontinuation of therapy but require constant monitoring particularly in menopausal women.35
Systemic Antibiotics
There has been a sea change in the use of systemic antibiotics for acne due to antibiotic resistance, as compared to previous long-term use. It is now recommended to be used in inflammatory papulopustular acne when topical treatment fails and for the shortest possible duration.35 The tetracyclines doxycycline and minocycline and macrolides like azithromycin and erythromycin are used for acne. The use of penicillins and cephalosporins should be limited to patients with treatment failure. Doxycycline is used in doses of 50–100 mg/day. Sub-antimicrobial doses of 40 mg/day have also been used for their anti-inflammatory effect.62 Doxycycline is more photosensitizing as compared to minocycline; hence, sun protection should be advised to patients. To reduce gastrointestinal side effects, it should be taken with meals and at least one hour before bedtime. It should not be taken simultaneously with iron and calcium as they interfere with its absorption. It is metabolized by the liver but can be given to patients with renal derangement.35 Minocycline at a dose of 50–100 mg/day is used for acne but has not shown to be superior to doxycycline. It has less gastrointestinal side effects, but the incidence of immune-mediated serious adverse events such as drug-induced lupus, drug reaction with eosinophilia (DRESS) and hypersensitivity syndromes is higher.34 Other adverse events include tinnitus, dizziness and minocycline-induced pigmentation, which can be persistent.
Macrolides including erythromycin and azithromycin are now not considered as first-line systemic antibiotics for acne.35 They also have anti-inflammatory properties, but the mechanism of action is unclear. Azithromycin has been used in varying pulsed doses ranging from 500 mg three times a week to 4 consecutive days a month.34 Side effects are mainly gastrointestinal distress. Penicillins, cephalosporins and trimethoprim-sulfamethoxazole should be reserved for patients who are intolerant to tetracyclines or macrolides.35
Older patients often have higher rates of treatment failure and approximately 80% of the women fail multiple courses of systemic antibiotics and approximately 30% to 40% fail after a course of isotretinoin.63,64 If acne recurs immediately after a course of isotretinoin, an underlying endocrine abnormality should be suspected.65
Procedural Therapy
Procedural therapy in active acne is indicated when there is predominant comedonal acne, recalcitrant acne, nodulocystic acne when isotretinoin leads to a flare of acne or when it is contraindicated or needs to be avoided. It is also useful when there is a need for quick response for psychological and social reasons. The basic aim is prevention of scarring by reducing inflammation faster leading to quicker resolution of lesions. If done correctly, they can prevent, reduce the incidence and severity of permanent post acne scars (Table 3). Comedone extraction causes quick clearance of comedones with faster esthetic improvement. Chemical peels are resurfacing procedures and useful adjuncts in the treatment of acne. They have comedolytic, keratolytic and anti-inflammatory properties targeting several pathogenetic features of acne.66 Chemical peeling agents useful in active acne include salicylic acid, mandelic acid, glycolic acid and retinol peels. Combination peels like salicylic-mandelic acid in a gel base or lactic acid peels are particularly useful in sensitive mature skins. Glycolic and retinol peels have the added advantage of treating pigmented scars and photoaging. Laser and light systems can be an adjunct to traditional topical and oral medications, without significant adverse events. They appear to target the pathogenetic factors of acne by reducing inflammatory acne lesions, decreasing sebaceous gland activity and inhibiting C. acnes. More recently, various lasers such as the 585 and 595 nm pulsed dye lasers, 532 KTP laser, 1450 nm diode laser, 1540 nm erbium glass laser, intense pulsed light and radiofrequency devices have been used for the treatment of inflammatory acne vulgaris, but are not very effective for comedonal lesions.67–69 They also have the advantage of improving post acne scars. Low intensity visible blue, red or green light targets the endogenous porphyrins and in the presence of oxygen generate reactive singlet oxygen species that damage the cell membranes of the bacteria and reduce acne.
Long-term improvements up to 1 year have been documented with laser treatments and they need to be explored further in menopausal acne, which is predominantly inflammatory, as they cause minimal side effects.70 However, a recent Cochrane review on light therapies for acne concluded that there is a need for larger studies of better quality to provide evidence of the utility and long-term benefits in acne, particularly considering the cost of laser therapies.71
Skin Care, Cosmetics And Cosmeceuticals
Skin care routine should be carefully selected to avoid irritation and dryness due to topical medications. The face should be washed once or twice daily with a mild soap or syndet or a benzoyl peroxide face wash. Frequent washing should be avoided as it can dry the skin, impair barrier function and cause further irritation. In patients with thick oily skin salicylic acid or glycolic acid-based face washes can be used. The moisturizer or sunscreen should be noncomedogenic, nonacnegenic and hypoallergenic. They should be applied initially before other topical agents. In a review of moisturizers for acne, dimethicone, glycerin, hyaluronic acid and sodium pyrrolidone carboxylic acid are the most suitable for acne.72 Scrubs should be avoided in acne patients as they may irritate the skin. Patients should be advised not to pick or pop the acne as it can prolong inflammation, lead to infection and cause scarring. Tanning should be avoided as it can cause persistent hyperpigmentation in the inflammatory acne lesions. All old cosmetics should be replaced and oil-based foundations and cosmetics should be avoided. Water-based cosmetics should be applied and all makeup should be washed off before going to bed. Cosmetic camouflage of acne lesions can be advised to reduce stress.
Acne cosmeceuticals target the various etiopathogenic factors, such as reduction of sebum, and have antibacterial, anti-inflammatory properties. They can reduce dryness, irritation, photosensitivity and improve barrier repair and play an important role in the management of acne in the older patient. They may also enhance the penetration or reduce side effects of prescribed topical medications.73 Nicotinamide 4% decreases the severity of mild-to-moderate acne in comparison to clindamycin.74 It also has skin-lightening, sebostatic and anti-inflammatory properties. Topical comedolytics, such as retinol, retinaldehyde, alpha-hydroxy acids like glycolic acid, beta-hydroxy acids like salicylic acid, and polyhydroxy acids such as gluconolactone and lactobionic acid are present in a wide variety of over the counter formulations for acne and also have skin-lightening effects. Botanical agents like plant extracts of Echinacea purpurea, Garcinia mangostana and many others have antiacne effect by inhibiting proliferation of P. acnes, reversing bacterial-induced inflammation and also normalized elevated cytokine levels including IL-6 and IL-8.75 The use of cosmeceuticals for acne by patients is common and may be useful for mild-to-moderate acne in older patients, but they do not demonstrate superiority to prescription medications.76
Lifestyle Changes And Co-Morbidities
The rising incidence of adult and menopausal acne has been linked to the modern lifestyle.22 Societal pressures, demands of balancing work, career and family life and keeping up with social media contribute to reduced and irregular sleeping time. This sleep deprivation is an internal stressor leading to rise in stress hormones like CRH and P substance that directly influence acne.
Several studies conducted on humans demonstrated that weight loss, exercise, getting adequate sleep, reducing stress, cessation of smoking, healthy diet with a low glycemic load, avoiding milk can all help to reduce acne. Bovine milk, whey protein supplements and high glycemic diets play a potential role in the pathogenesis of acne by increasing insulin and IGF-1 levels.77
Conclusion
There is a plethora of skin changes in the menopausal period and now surprisingly acne is one of them, which was once considered as a disease of adolescence. The majority of adult and menopausal women with acne have persistent or relapsing acne from adolescence. Hormonal imbalance with relative hyperandrogenism is the major factor, while stress, genetic factors, cosmetics, dietary and lifestyle changes all play a role. Treatment is a challenge in clinical practice as most anti-acne products are designed for adolescent oily skins, with fewer products for sensitive mature skins in this age group. Prolonged topical therapy with judicious use of systemic therapy is the treatment of choice as acne tends to be more resistant, deep seated and prolonged in this subset. The use of a topical antibiotic as monotherapy should be avoided and combination therapy with benzoyl peroxide, topical retinoids, azelaic acid or salicylic acid is recommended as first-line treatment according to the patient’s skin and tolerability, including an oral agent if necessary. Spironolactone is the treatment of choice for patients requiring systemic antiandrogen therapy. The subset of patients who will require combined contraceptives should be selected with care to reduce the incidence of side effects. Procedural treatment can play a bigger role as it can also help in the treatment of acne scars and concomitant skin aging. However, detailed studies specifically evaluating prevalence, etiopathogenesis, presentation and management of this subset of women with menopausal acne are required to increase the evidence on which future treatment recommendations can be based.
 |
Box 1 Contraindications To Combined Oral Contraceptives In Menopausal Women |
Disclosure
The authors report no conflicts of interest in this work.
References
1. Collier CN, Harper JC, Cafardi JA, et al. The prevalence of acne in adults 20 years and older. J Am Acad Dermatol. 2008;58:56–59. doi:10.1016/j.jaad.2007.06.045
2. Khunger N, Kumar C. A clinic-epidemiological study of adult acne: is it different from adolescent acne? Indian J Dermatol Venereol Leprol. 2012;78:335–341. doi:10.4103/0378-6323.95450
3. Zeichner JA, Baldwin HE, Cook-Bolden FE, Eichenfield LF, Fallon-Friedlander S, Rodriguez DA. Emerging issues in adult female acne. J Clin Aesthet Dermatol. 2017;10:37–46.
4. Goulden V, Stables GI, Cunliffe WJ. The prevalence of facial acne in adults. J Am Acad Dermatol. 1999;41:577–580.
5. Poli F, Dreno B, Verschoore M. An epidemiological study of acne in female adults: results of a survey conducted in France. J Eur Acad Dermatol Venereol. 2001;15:541–545. doi:10.1046/j.1468-3083.2001.00357.x
6. Dreno B, Thiboutot D, Layton A, et al. Large-scale international study enhances understanding of an emerging acne population: adult females. J Eur Acad Dermatol Venereol. 2015;29(6):1096–1106. doi:10.1111/jdv.12669
7. WHO. Scientific group on research on the menopause in the 1990s. WHO Technical Report Series Geneva, Switzerland, WHO. 1996.
8. Contestabile E, Derzko C. Canadian consensus on menopause and perimenopause. J Obstet Gynaecol Can. 2001;23:836–841.
9. Williams C, Layton AM. Persistent acne in women: implications for the patient and for therapy. Am J Clin Dermatol. 2006;7:281–290. doi:10.2165/00128071-200607050-00002
10. Dumont-Wallon G, Dreno B. Specificity of acne in women older than 25 years. Presse Med. 2008;37:585–591. doi:10.1016/j.lpm.2007.07.014
11. Zaenglein AL. Acne Vulgaris. N Engl J Med. 2019;10(380):199–200.
12. Fogle RH, Stanczyk FZ, Zhang X, Paulson RJ. Ovarian androgen production in postmenopausal women. J Clin Endocrinol Metab. 2007;92:3040–3043. doi:10.1210/jc.2007-0581
13. Sluijmer AV, Heineman MJ, De Jong FH, Evers JL. Endocrine activity of the postmenopausal ovary: the effects of pituitary down-regulation and oophorectomy. J Clin Endocrinol Metab. 1995;80:2163–2167. doi:10.1210/jcem.80.7.7608272
14. Adashi EY. The climacteric ovary as a functional gonadotropin-driven androgen-producing gland. Fertil Steril. 1994;62:20–27. doi:10.1016/S0015-0282(16)56810-1
15. Markopoulos MC, Kassi E, Alexandraki KI, Mastorakos G, Kaltsas G. Hyperandrogenism after menopause. Eur J Endocrinol. 2015;172:R79–R91. doi:10.1530/EJE-14-0468
16. Markopoulos MC, Rizos D, Valsamakis G, Deligeoroglou E, Grigoriou O, Chrousos GP. Hyperandrogenism in women with polycystic ovary syndrome persists after menopause. J Clin Endocrinol Metab. 2011;96(3):623–631. doi:10.1210/jc.2010-0130
17. Davison SL, Bell R, Donath S, Montalto JG, Davis SR. Androgen levels in adult females: changes with age, menopause, and oophorectomy. J Clin Endocrinol Metab. 2005;90(7):3847. doi:10.1210/jc.2005-0212
18. Rothman MS, Wierman ME. How should postmenopausal androgen excess be evaluated? Clin Endocrinol (Oxf). 2011;75:160.
19. Capitanio B, Sinagra JL, Bordignon V, Cordiali Fei P, Picardo M, Zouboulis CC. Underestimated clinical features of postadolescent acne. J Am Acad Dermatol. 2010;63(5):782–788. doi:10.1016/j.jaad.2009.10.015
20. Perkins AC, Maglione J, Hillebrand GG, et al. Acne vulgaris in women: prevalence across the life span. J Womens Health (Larchmt). 2012;21:223–230. doi:10.1089/jwh.2011.3305
21. Cappel M, Mauger D, Thiboutot D. Correlation between serum levels of insulin-like growth factor 1, dehydroepiandrosterone sulfate, and dihydrotestosterone and acne lesion counts in adult women. Arch Dermatol. 2005;141(3):333–338. doi:10.1001/archderm.141.3.333
22. Albuquerque RG, Rocha MA, Bagatin E, Tufik S, Andersen ML. Could adult female acne be associated with modern life? Arch Dermatol Res. 2014;306:683–688. doi:10.1007/s00403-014-1482-6
23. Bagatin E, Freitas THP, Machado MCR, Ribeiro BM, Nunes S, Rocha MADD. Adult female acne: a guide to clinical practice. An Bras Dermatol. 2019;94:62–75. doi:10.1590/abd1806-4841.20198203
24. Kim J. Review of the innate immune response in acne vulgaris: activation of toll-like receptor 2 in acne triggers inflammatory cytokine responses. Dermatology. 2005;211(3):193–198. doi:10.1159/000087011
25. Dréno B, Pécastaings S, Corvec S, Veraldi S, Khammari A, Roques C. Cutibacterium acnes (Propionibacterium acnes) and acne vulgaris: a brief look at the latest updates. Eur Acad Dermatol Venereol. 2018;32(Suppl 2):5–14. doi:10.1111/jdv.15043
26. Del Rosso JQ, Harper JC, Graber EM, Thiboutot D, Silverberg NB, Eichenfield LF. Status report from the American Acne & Rosacea Society on medical management of acne in adult women, part 2: topical therapies. Cutis. 2015;96:321–325.
27. Rocha MA, Bagatin E. Skin barrier and microbiome in acne. Arch Dermatol Res. 2018;310:181–185. doi:10.1007/s00403-017-1795-3
28. Pasquali R. Obesity and androgens: facts and perspectives. Fertil Steril. 2006;85:1319–1340. doi:10.1016/j.fertnstert.2005.11.046
29. Al-Ozairi E, Michael E, Quinton R. Insulin resistance causing severe postmenopausal hyperandrogenism. Int J Gynaecol Obstet. 2008;100(3):280–281. doi:10.1016/j.ijgo.2007.08.017
30. Nestler JE, Powers LP, Matt DW, Steingold KA, Plymate SR, Rittmaster RS. A direct effect of hyperinsulinemia on serum sex hormone-binding globulin levels in obese women with the polycystic ovary syndrome. J Clin Endocrinol Metab. 1991;72:83–89. doi:10.1210/jcem-72-1-83
31. Ramos-e-Silva M, Ramos-e-Silva S, Carneiro S. Acne in women. Br J Derm. 2015;172(Suppl. 1):20–26. doi:10.1111/bjd.13638
32. Marks R. Acne and its management beyond the age of 35 years. Am J Clin Dermatol. 2004;5(6):459–462. doi:10.2165/00128071-200405060-00011
33. Rapp DA, Brenes GA, Feldman SR, et al. Anger and acne: implications for quality of life, patient satisfaction and clinical care. Br J Dermatol. 2004;151(1):183–189. doi:10.1111/j.1365-2133.2004.06078.x
34. Tan AU, Schlosser BJ, Paller AS. A review of diagnosis and treatment of acne in adult female patients. Int J Womens Dermatol. 2017;4:56–71. doi:10.1016/j.ijwd.2017.10.006
35. Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74(5):945–973. doi:10.1016/j.jaad.2015.12.037
36. Sharma R, Abrol S, Wani M. Misuse of topical corticosteroids on facial skin a study of 200 patients. J Dermatol Case Rep. 2017;11(1):5–8.
37. Kamangar F, Shinkai K. Acne in the adult female patient: a practical approach. Int J Dermatol. 2012;51(10):1162–1174. doi:10.1111/j.1365-4632.2012.05519.x
38. Gold LS, Baldwin H, Rueda MJ, Kerrouche N, DrÉno B. Adapalene-benzoyl peroxide gel is efficacious and safe in adult female acne, with a profile comparable to that seen in teen-aged females. J Clin Aesthet Dermatol. 2016;9:23–29.
39. Berson D, Alexis A. Adapalene 0.3% for the treatment of acne in women. J Clin Aesthet Dermatol. 2013;6:32–35.
40. Thielitz A, Lux A, Wiede A, Kropf S, Papakonstantinou E, Gollnick H. A randomized investigator-blind parallel-group study to assess efficacy and safety of azelaic acid 15% gel vs. adapalene 0.1% gel in the treatment and maintenance treatment of female adult acne. J Eur Acad Dermatol Venereol. 2015;29:789–796. doi:10.1111/jdv.12823
41. Berger R, Rizer R, Barba A, et al. Tretinoin gel microspheres 0.04% versus 0.1% in adolescents and adults with mild to moderate acne vulgaris: a 12-week, multicenter, randomized, double-blind, parallel-group, phase IV trial. Clin Ther. 2007;29:1086–1097. doi:10.1016/j.clinthera.2007.06.021
42. Kang S, Duell EA, Fisher GJ, et al. Application of retinol to human skin in vivo induces epidermal hyperplasia and cellular retinoid binding proteins characteristic of retinoic acid but without measurable retinoic acid levels or irritation. J Invest Dermatol. 1995;105:549–556. doi:10.1111/1523-1747.ep12323445
43. Mukherjee S, Date A, Patravale V, Korting HC, Roeder A, Weindl G. Retinoids in the treatment of skin aging: an overview of clinical efficacy and safety. Clin Interv Aging. 2006;1(4):327–348.
44. Kim BH, Lee YS, Kang KS. The mechanism of retinol-induced irritation and its application to anti-irritant development. Toxicol Lett. 2003;146:65–73. doi:10.1016/j.toxlet.2003.09.001
45. Canavan TN, Chen E, Eleweski BE. Optimizing non-antibiotic treatments for patients with acne: a review. Dermatol Ther (Heidelb). 2016;6:555–578. doi:10.1007/s13555-016-0138-1
46. Kircik LH. Efficacy and safety of azelaic acid (AzA) gel 15% in the treatment of post-inflammatory hyperpigmentation and acne: a 16-week, baseline-controlled study. J Drugs Dermatol. 2011;10:586–590.
47. Schaller M, Sebastian M, Ress C, Seidel D, Hennig M. A multicentre, randomized, single-blind, parallel-group study comparing the efficacy and tolerability of benzoyl peroxide 3%/clindamycin 1% with azelaic acid 20% in the topical treatment of mild-to-moderate acne vulgaris. J Eur Acad Dermatol Venereol. 2016;30(6):966–973. doi:10.1111/jdv.13652
48. Alexis AF, Burgess C, Callender VD, et al. The efficacy and safety of topical dapsone gel, 5% for the treatment of acne vulgaris in adult females with skin of color. J Drugs Dermatol. 2016;15:197–204.
49. Kircik LH. Use of dapsone 5% gel as maintenance treatment of acne vulgaris following completion of oral doxycycline and dapsone 5% gel combination treatment. J Drugs Dermatol. 2016;15:191–195.
50. Dréno B, Bettoli V, Ochsendorf F, et al. Efficacy and safety of clindamycin phosphate 1.2%/tretinoin 0.025% formulation for the treatment of acne vulgaris: pooled analysis of data from three randomised, double-blind, parallel-group, phase III studies. Eur J Dermatol. 2014;24:201–209. doi:10.1684/ejd.2014.2293
51. Kircik LH. Fixed combination of clindamycin phosphate 1.2% and benzoyl peroxide 3.75% aqueous gel: long-term use in adult females with moderate acne vulgaris. J Drugs Dermatol. 2017;16:543–546.
52. Isvy-Joubert A, Nguyen JM, Gaultier A, et al. Adult female acne treated with spironolactone: a retrospective data review of 70 cases. Eur J Dermatol. 2017;1(27):393–398.
53. Plovanich M, Weng QY, Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151:941–944. doi:10.1001/jamadermatol.2015.34
54. Layton AM, Eady EA, Whitehouse H, Del Rosso JQ, Fedorowicz Z, van Zuuren EJ. Oral spironolactone for acne vulgaris in adult females: a hybrid systematic review. Am J Clin Dermatol. 2017;18(2):169–191. doi:10.1007/s40257-016-0245-x
55. Grandhi R, Alikhan A. Spironolactone for the treatment of acne: a 4-year retrospective study. Dermatol Basel Switz. 2017;233(2–3):141–144. doi:10.1159/000471799
56. Thiboutot D. Acne: hormonal concepts and therapy. Clin Dermatol. 2004;22:419–428. doi:10.1016/j.clindermatol.2004.03.010
57. De Leo V, Musacchio MC, Cappelli V, et al. Hormonal contraceptives: pharmacology tailored to women’s health. Hum Reprod Update. 2016;22:634–646. doi:10.1093/humupd/dmw016
58. de Bastos M, Stegeman BH, Rosendaal FR, et al. Combined oral contraceptives: venous thrombosis. Cochrane Database Syst Rev. 2014;CD010813.
59. Salvaggio HL, Zaenglein AL. Examining the use of oral contraceptives in the management of acne. Int J Womens Health. 2010;2:69–76.
60. Słopień R, Milewska E, Rynio P, Męczekalski B. Use of oral contraceptives for management of acne vulgaris and hirsutism in women of reproductive and late reproductive age. Menopause Rev. 2018;17(1):1–4. doi:10.5114/pm.2018.74895
61. Agarwal US, Besarwal RK, Bhola K. Oral isotretinoin in different dose regimens for acne vulgaris: A randomized comparative trial. Indian J Dermatol Venereol Leprol. 2011;77(6):688–694. doi:10.4103/0378-6323.86482
62. Moore A, Ling M, Bucko A, Manna V, Rueda MJ. Efficacy and safety of subantimicrobial dose, modified-release doxycycline 40 mg versus doxycycline 100 mg versus placebo for the treatment of inflammatory lesions in moderate and severe acne: a randomized, double-blinded, controlled study.J. Drugs Dermatol. 2015;14(6):581–586.
63. Blasiak RC, Stamey CR, Burkhart CN, Lugo-Somolinos A, Morrell DS. High-dose isotretinoin treatment and the rate of retrial, relapse, and adverse effects in patients with acne vulgaris. JAMA Dermatol. 2013;149(12):1392–1398. doi:10.1001/jamadermatol.2013.2723
64. Rademaker M. Making sense of the effects of the cumulative dose of isotretinoin in acne vulgaris. Int J Dermatol. 2016;55(5):518–523. doi:10.1111/ijd.13145
65. Lowenstein EJ. Diagnosis and management of the dermatologic manifestations of the polycystic ovary syndrome. Dermatol Ther. 2006;19:210–223. doi:10.1111/dth.2006.19.issue-4
66. Castillo DE, Keri JE. Chemical peels in the treatment of acne: patient selection and perspectives. Clin Cosmet Investig Dermatol. 2018;11:365–372. doi:10.2147/CCID.S137788
67. Gold MH. Therapeutic and aesthetic uses of photodynamic therapy part two of a five-part series: lasers and light treatments for acne vulgaris promising therapies. J Clin Aesthet Dermatol. 2008;1:28–34.
68. Haedersdal M, Togsverd-Bo K, Wulf HC. Evidence-based review of lasers, light sources and photodynamic therapy in the treatment of acne vulgaris. J Eur Acad Dermatol Venereol. 2008;22:267–278. doi:10.1111/j.1468-3083.2007.02503.x
69. Patidar MV, Deshmukh AR, Khedkar MY. Efficacy of intense pulsed light therapy in the treatment of facial acne vulgaris: comparison of two different fluences. Indian J Dermatol. 2016;61:545–549. doi:10.4103/0019-5154.190115
70. Jih MH, Friedman PM, Goldberg LH, Robles M, Glaich AS, Kimyai-Asadi A. The 1450-nm diode laser for facial inflammatory acne vulgaris: dose-response and 12-month follow-up study. J Am Acad Dermatol. 2006;55:80–87.
71. Barbaric J, Abbott R, Posadzki P, et al. Light therapies for acne: abridged Cochrane systematic review including GRADE assessments. Br J Dermatol. 2018;178:61–75. doi:10.1111/bjd.15495
72. Chularojanamontri L, Tuchinda P, Kulthanan K, Pongparit K. Moisturizers for acne: what are their constituents? J Clin Aesthet Dermatol. 2014;7(5):36–44.
73. Goh CL, Noppakun N, Micali G, et al. Meeting the challenges of acne treatment in asian patients: a review of the role of dermocosmetics as adjunctive therapy. J Cutan Aesthet Surg. 2016;9(2):85–92. doi:10.4103/0974-2077.184043
74. Shahmoradi Z, Iraji F, Siadat AH, Ghorbaini A. Comparison of topical 5% nicotinamide gel versus 2% clindamycin gel in the treatment of the mild-moderate acne vulgaris: a double-blinded randomized clinical trial. J Res Med Sci. 2013;18(2):115–117.
75. Sinha P, Srivastava S, Mishra N, Yadav NP. New perspectives on antiacne plant drugs: contribution to modern therapeutics. Biomed Res Int. 2014;2014:301304. doi:10.1155/2014/301304
76. Barros BS, Zaenglein AL. The use of cosmeceuticals in acne: help or hoax? Am J Clin Dermatol. 2017;18:159–163. doi:10.1007/s40257-016-0249-6
77. Melnik BC, Schmitz G. Role of insulin, insulin-like growth factor-1, hyperglycaemic food and milk consumption in the pathogenesis of acne vulgaris. Exp Dermatol. 2009;18(10):833–841. doi:10.1111/j.1600-0625.2009.00924.x
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
