Back to Journals » Orthopedic Research and Reviews » Volume 9
Meniscal transplantation: procedures, outcomes, and rehabilitation
Authors Young J, Tudor F, Mahmoud A , Myers P
Received 6 July 2016
Accepted for publication 6 March 2017
Published 15 May 2017 Volume 2017:9 Pages 35—43
DOI https://doi.org/10.2147/ORR.S94378
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Clark Hung
James Young, Francois Tudor, Ahmed Mahmoud, Peter Myers
Brisbane Orthopaedic & Sports Medicine Centre, Brisbane Private Hospital, Spring Hill, Brisbane, Queensland, Australia
Abstract: Meniscal allograft transplantation (MAT) is a possible treatment option for patients with joint pain after meniscectomy. It is necessary that the joint be aligned and stable. Current evidence shows that MAT improves pain and mechanical function in the mid to long term with patients reporting significantly improved outcomes at up to 15 years following surgery. Studies on survivorship showed up to 76% graft survival at 10 years. Recent studies have suggested a chondroprotective effect, but there is, at present, no evidence to support MAT in the prevention of osteoarthritis. This review article reported the current evidence for MAT showing support for fresh frozen, nonirradiated allografts. However, further research is required to determine the ideal indications for MAT, the optimal graft fixation method, and the safest rehabilitation protocol.
Keywords: meniscus, meniscectomy, meniscal allograft transplantation
Introduction
Menisci play a critical role in the mechanical function of the knee. In addition to assisting with the lubrication and proprioception of the knee, they also improve the congruence of an otherwise mismatched joint by increasing the contact surface area, and this results in decreased peak contact pressures during loading and, ultimately, contributes to longevity of the knee joint bearing surfaces.1,2
Damage to the menisci that causes a loss of structural integrity and function, whether by injury, degeneration, or after surgical debridement, leads to altered loading of the chondral bearing surfaces of the knee, resulting in progressive damage and eventual osteoarthritis.3–5 In young, active patients, meniscal loss can be devastating, leading to the loss of knee stability and function, pain, and early-onset arthrosis.5
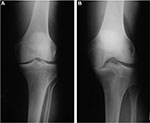
Meniscal allograft transplantation (MAT) is a possible treatment option for the patients with pain after meniscectomy, known as the “post-meniscectomy syndrome,”6 and has been shown to provide predictable symptomatic relief and a return to sporting activity with good long-term survival.7–12 The limitations of this technique are now being explored with MAT having been used in athletes returning to high level sports with good mid-term survival and function,6,13,14 as well as in knees with established degenerative changes where it has been used in combination with other joint restorative or realignment procedures with improvements in both pain and mobility.11,15–18 Normal axial alignment and a stable joint are prerequisites as the presence of both untreated varus lower limb malalignment and anterior cruciate ligament instability increase the risk of MAT graft failure.19–22 As a guide, a minimum of 2 mm of tibiofemoral joint space should be visible on the Rosenberg view (performed with the knee(s) in 45° of flexion, using the X-ray beam directed from posterior to anterior and angled 30° cephalocaudad) before the MAT is considered.19 Figure 1 shows an example.
This review article described the current concepts in the operative procedure, the postoperative rehabilitation protocol, and the expected outcomes of MAT.
MAT planning and allograft considerations
The important considerations that should be taken into account when planning MAT are allograft preparation (including preservation and sizing), graft fixation techniques, and the need for any associated procedures to be performed concomitantly. It is well recognized that MAT (and indeed meniscal repair) should not be performed in a malaligned or unstable knee. If required, realignment osteotomies, chondral repair techniques, and ligament reconstructions must be planned carefully and performed either at the same time or as a separate procedure prior to meniscal transplantation.
Allograft considerations (preservation, disease transmission, and immunogenicity)
The use of allografts is not without specific risks, and this must be well understood by both the surgeon and the patient prior to meniscus implantation. The choice of graft preservation technique has a potentially significant impact on outcome and survival. In addition, there are potential risks of disease transmission and host immune reactions.
Four methods for graft preservation have been described: fresh allograft, fresh frozen (deep frozen), cryopreserved, and lyophilized (freeze-dried). Fresh allografts work based on the viable cell principle whereby, it has been postulated that an increased number of viable fibrochondrocytes results in improved maintenance of the extracellular matrix and thus the structural integrity of the meniscus.23,24 Although the clinical results are good with excellent survival reported,25 it is an expensive option. In addition, there is an obvious logistical problem of correctly matching a recipient donor before fibrochondrocyte cellularity diminishes and graft viability is lost. Due to this reason, implantation should occur within 14 days of harvest.26–28
Cryopreservation involves deep freezing of the meniscus at −196°C in a cryoprotectant, such as glycerol or dimethyl sulfoxide. This process is to protect cell viability by preventing the formation of intracellular ice crystals. It is difficult to perform and expensive, and recent data have suggested that as little as 4%–10% of cells actually survive although the collagen net architecture does remain largely intact.24–26,29,30 Further evidence suggests that tissue, metabolic, and cell structural changes do occur after cryopreservation.31,32 Although it does allow prolonged storage of the meniscal tissue, it offers no significant benefits over fresh frozen techniques.24,29
The fresh frozen allograft preservation is technically simple, involving the fast freezing of meniscal tissue soaked in sterile saline and an antibiotic solution (usually Rifampicin) and storage at −80°C.29 Once frozen, the grafts are easy to store and remain usable for up to 5 years.33 Although donor fibrochondrocytes may be destroyed by the freezing process, it is postulated that the same process results in denaturation of the histocompatibility antigens and thus decreases immunogenicity within fresh frozen menisci.34 A further advantage is the maintenance of the mechanical properties of the meniscal allograft.35 Most centers use fresh frozen nonirradiated cadaveric allografts, and these have excellent mid- to long-term survival.9,11,36
Lyophilization, or freeze-drying, is no longer a recommended technique due to both a change in the mechanical properties of the meniscus and a reduction in graft size,34 leading to an increased rate of failure with this form of preservation.37 Due to problems with the sterilization of lyophilized grafts, gamma irradiation is also necessary.29 The combined effect of irradiation and lyophilization causes deep changes to the extracellular matrix structure,38 a decrease in tensile strength, graft shrinkage, and poor rehydration.39,40
Disease transmission from the donor tissue has always been of concern.41 The American Association of Tissue Banks has a defined and stringent protocol designed for minimizing the potential for the implantation of diseased donor grafts.42 Donors are assessed for both bacterial infections (aerobic and anaerobic) and viral infections (including human immunodeficiency virus [HIV], hepatitis B and C, human T-lymphocytic virus, and syphilis), and the actual transmission rates are low.43 Historically, the HIV transmission risk was thought to be in the region of 1 in 8 millions;44 however, recent data have suggested that the actual risk is nearer to 1 in 1 million.45 It was proposed that gamma irradiation with at least 3.0 Mrad is necessary to destroy HIV-1 DNA.29 Although this would improve graft sterilization, there is good evidence that this also disrupts the meniscal graft microstructure and thus compromises its mechanical properties.34 Therefore, gamma irradiation is undesirable and should be avoided.
Meniscal allografts express Class I and II histocompatibility antigens and therefore carry the potential for a host immune response.46 Bone grafts are known to be immunogenic, and therefore, when requested, the presence of bone plugs or a bone bridge attached to the meniscal allograft may increase the risk of an immune response.47,48 In practice, however, this has not been reported as a significant problem; in one series, with an average of 4.5 years of follow-up, none of the transplanted fresh meniscal osteochondral allografts provoked an immune response.49
Graft sizing
Matching an appropriately sized meniscus allograft to the knee is vital to the successful outcome of the procedure. There are a number of reported methods for determining the dimensions of the recipient tibial plateau in order to ensure that the graft fits correctly. These methods include X-ray measurements, recipient anthropometric parameters, and calibrated magnetic resonance imaging (MRI) or computed tomography scans. The Pollard’s method is the most widely used method and takes measurements of the tibial plateau from an anterior–posterior X-ray (width) and a lateral X-ray (length) with specific modifiers to estimate the meniscal size, depending on the compartment being measured.12,50 A linear regression analysis has shown that meniscal dimensions can be predicted accurately from radiographic tibial plateau measurements, with only small mean errors.51 The senior author has used these measurements of length and width to calculate an estimated meniscal circumference for both the donor and the recipient; this was done by calculating the circumference of an ellipse by using the width and length dimensions by the way of a mathematical approximation, such as that described by Kepler or Gauss-Kummer.52 This has been helpful in decision making when the Pollard dimensions are not a precise match to the available tissue bank grafts in this study.
Graft shrinkage has been reported in three studies and is associated with the graft preservation technique used.53–55 Lyophilized and gamma-irradiated grafts have shown a significantly increased degree of shrinkage compared with fresh frozen grafts.55 Cryopreserved grafts showed an average of 7% shrinkage on postoperative MRI with a third of grafts showing at least 10% shrinkage.54 In a serial MRI study measuring the meniscal size following the implantation of fresh frozen allografts, only minimal shrinkage in the majority of the grafts was found, and there was no evidence of severe shrinkage.53 These morphological changes did not correlate with clinical outcomes.
A number of studies have investigated meniscal extrusion after an MAT. Meniscal extrusion is associated more with the open MAT technique than with the arthroscopic technique56 and is also associated with the suture-only technique when compared to the bone plug technique.56,57 No correlation between graft extrusion and patient-reported outcome measures (PROMs),57–60 radiographs evaluating osteoarthritis,58,60 cartilage changes on MRI,60 or the need for second-look arthroscopy have been identified.60
Operative technique
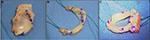
The MAT was initially performed via an open approach, but it has been developed into a predominantly arthroscopic procedure with the use of a mini-arthrotomy for graft insertion.37,61,62 Various methods for MAT fixation have been described. The preferred method in this study was the most commonly used bone fixation technique (Figure 2) whereby the meniscal roots are left attached to allograft bone; two separate bone plugs for the medial meniscus and a single bone bridge for the lateral meniscus (the keyhole or slot technique) were used.12,19 Others have published good results with the use of bone free fixation using only sutures.7,62 Cadaveric studies have shown that bony fixation of the meniscal horns is necessary to restore the near-normal contact forces and recreate normal meniscal hoop stresses, concluding that this is not achieved adequately when using the suture-only fixation.63,64 Animal studies have shown that poor placement of either sutures or bone plugs increases meniscal extrusion and accelerates joint degeneration.65,66 Studies evaluating meniscal extrusion and reporting on clinical outcomes, however, have not shown any significant differences between these two fixation methods.12
Traditional open suture techniques have been replaced with inside-out arthroscopic sutures by the majority of the reporting authors with newer all-inside sutures being used in a number of more recent articles. There are no clinical data currently endorsing one fixation technique over another.
Outcomes after MAT
Clinical outcomes
The clinical outcomes of patients following MAT have been assessed by using a wide range of outcome measures; the Lysholm Knee Scoring Scale, International Knee Documentation Committee Subjective Knee Evaluation Form, Tegner Activity Score (TAS), Visual Analogue Scale, Oxford Knee Score (OKS), and Knee Injury and Osteoarthritis Outcome Score (KOOS) are the most commonly used outcome measures. The published data consistently show a significant improvement in clinical outcomes at both medium- and long-term follow-ups (5–15 years) across all outcome measures with a time-related decay.7,67–69 In some series, although most PROMs improve, the TAS has not shown a significant improvement.6,70,71 It may be that most of the patients undergoing MAT are doing so in order to relieve pain and to improve the quality of life. Increasing the desired activity level is not a primary indication for MAT, and therefore, an improvement in the activity level, as measured by the TAS, would not necessarily be expected. Other measures that have been used to assess the clinical outcomes following MAT include the Short Form (SF-12 and SF-36), Modified Cincinnati Score, Western Ontario and McMaster Universities Osteoarthritis Index, and the Hospital for Special Surgery Knee Score.61,72,73 Studies using these PROMs have also shown a consistent improvement in the clinical outcomes at medium- to long-term follow-ups.62,69,74
Return to sport
The MAT was first used as a salvage procedure; however, some authors suggested its use in aiding an athletes’ return to sport. A study with 4.2 years of follow-up showed that 73% of patients return to some degree of sporting activity following their MAT with 49% of them able to return to their preinjury level.13 When the entire cohort was taken together, however, the mean Tegner score achieved postoperatively was 4; some way short of the cohort’s mean pre-operative Tegner score of 6. Three smaller studies showed that there is a potential for the carefully selected subjects to return to a high-level sporting activity with a success rate between 77% and 92%.6,8,14 However, these studies had only a short follow-up time period (mean follow-up of 36, 39.4, and 39 months). It remains to be seen how patients who return to sport activity will perform in the longer term.
Radiological outcomes
The radiological appearances following an MAT have been studied to assess the progression of graft healing and evaluate any chondroprotective effect. Joint narrowing is the recommended radiological outcome measure when assessing the development of osteoarthritic changes, and an MRI has become the imaging tool of choice when evaluating the transplanted meniscus.75 A recent systematic review has shown evidence to support the theory that the MAT is chondroprotective.75 Meniscal transplants are reported to heal completely or partially to the knee capsule in close to 100% of the cases when assessed arthroscopically and 40%–70% when assessed using an MRI or magnetic resonance arthrography.37,60,72,76,77
Failures and complications
There are a variety of definitions for MAT failure in the literature with the need for joint replacement surgery or excision of the meniscus, the most commonly used end points. Long-term follow-up studies of an average of 12.1, 15, and 13.8 years showed a conversion to knee replacement at a rate of 18%, 28%, and 29%, respectively, with the last study including resection of the graft in their rate.61,78,79 Two studies reporting the survival analysis have shown a mean Kaplan–Meier survival of 9.7 and 12.6 years, respectively, with up to 95% survival at 6 years and 76% survival at 10 years following an MAT.7,67,80 In one recent study, patients receiving an MAT with moderate-to-severe cartilage damage (Outerbridge Grades 3 or 4) showed a failure rate of 22.4% at an average of 8.6 years after transplantation.67 This was echoed in a recent prospective study reporting a 98% and a 78% 2-year survival when MAT is used in a compartment with either good or bare chondral surfaces, respectively.68 A study that considered clinical failure in terms of pain levels and PROMs showed a success rate of 88% at 5 years, 63% at 10 years, and 40% at 15 years.81 When asked if they would have the procedure again, 90% of the patients confirmed that they would.7 Some patients may remain symptomatic after MAT; it is, therefore, suggested that future studies reporting on survivorship should include symptomatic (clinical) failure as well as mechanical failure. Reporting the proportion of patients with ongoing pain and poor PROMs is relevant because one of the main indications for MAT is to relieve knee pain.
Complications following an MAT include meniscal tears (including root detachment), synovitis, restriction in a range of movements, and infection. Studies have shown that the reoperation rate following complications can be as high as 46%, but it is usually ~20%–30% in most studies, and around half of these reoperations are to treat a symptomatic meniscal allograft tears or to repair a detachment. 13,67,68,79,82,83 Furthermore, a reoperation within 2 years postoperatively has been found to be a poor prognostic factor with an odds ratio of 8.4 for future conversion to a joint replacement or revision of the graft.82 Concomitant procedures, such an anterior cruciate ligament reconstruction or a corrective osteotomy for malalignment, are regularly performed and may increase the risk of complications.
Graft extrusion is a commonly discussed radiological complication following an MAT. Major graft extrusion is defined as >3 mm displacement of the meniscus out of the tibiofemoral compartment when viewed on an MRI. Although minor extrusions occur in almost all the meniscal transplants, major extrusion is said to occur in between 26% and 75% of cases with a higher rate associated with suture-only fixation, known chondral damage, and open surgery.56,57,59,60,62,84 However, graft extrusion does not appear to correlate with worse clinical or radiological results when compared to nonextruded grafts outcomes.62,75,85
Rehabilitation
A comparison of different rehabilitation protocols following an MAT has not been performed in the literature. Although the fine details vary in almost every study, there is a general agreement throughout the literature that, after a period of restricted weight-bearing, full weight-bearing can be achieved by the end of the 6th postoperative week. Similarly, the majority of the authors aimed at achieving a range of motion progressing to >0° to 90° within the same time period.7,13,67,68,83 One author devised a more aggressive approach to rehabilitation with a return to full weight-bearing by 2 weeks postoperatively and a return to sport at 4 months postoperatively. Others extended the period of restricted weight-bearing for >6 weeks.
Recommendations regarding return to sport after MAT remain a point of contention. Although some studies suggest lifetime avoidance of full sporting activity,60,86 others have allowed participants to return to sport after as little as 3 months.61 Most of the literature, however, recommends a return to sport at a period of 6–12 months postoperatively.6,8,14
General orthopedic rehabilitation principles should be incorporated to improve patients’ outcomes after MAT. Aerobic exercise, tailored to any necessary weight-bearing and range restrictions, should be incorporated early on during the rehabilitation program as this accelerates wound healing recovery.87 Hydrotherapy after orthopedic surgery improves function without increasing the risk of wound complications; however, research has not specifically evaluated its use following MAT.88
Table S1 summarizes the rehabilitation protocol preferred by the senior authors. For the first 2 weeks, the knee is braced and locked in 10° of flexion and touch weight-bearing is permitted. The brace is then removed, and weight-bearing increased by 25% of body weight per week to achieve full weight-bearing by the end of the 6th postoperative week. Active range of motion is also encouraged after the removal of the brace, aiming for a range from 0° to 90° by 6 weeks. Flexion to 120° is expected by 12 weeks. Because of the rollback of the meniscus with flexion and the increased loads applied to the posterior horn with deep flexion, exercises that involve flexion to beyond 90° under load are discouraged. The senior author recommends against dynamic sport for 12 months and only social or noncompetitive sport thereafter.
Future developments
Future high-quality research projects should focus on answering key questions regarding MAT: What are the long-term outcomes of MAT? Does MAT prevent the development of end-stage arthritis? Is there a role for MAT in the management of asymptomatic patients following a significant partial or subtotal meniscectomy?
A randomized control trial, the MeTEOR study, is currently being conducted in the UK whereby meniscus-deficient patients either receive an MAT or undergo nonoperative management with a personalized knee therapy protocol. The primary end point is to investigate the mean change in cartilage volume and thickness over the course of 1 year, as shown by serial MRI investigations. Secondary outcome measures include PROMs and complications following an MAT.89 It is hoped that high-quality randomized trials such as this will better define the chondroprotective role of MAT. If proven, there may be a role for prophylactic MAT in selected patients.75,89 The indications for MAT are being continuously expanded. Further long-term research is required to confirm the benefits of MAT to other patient populations, including those with moderate-to-severe chondral damage, those wishing to return to a high level of sport, and children.
Conclusion
The clinical outcomes of patients following subtotal or total meniscectomy are worrying, and there remains a high risk for the development of future osteoarthritis. MAT is of benefit to patients who have undergone meniscectomy with improvements in knee function, pain, and radiographic outcomes. Patients with a stable, well-aligned, but painful knee following subtotal or total meniscectomy are suitable for the procedure. When the knee is either malaligned or unstable, the results of concurrent or staged surgery to treat these associated conditions in addition to a meniscal transplant are good. There is the potential for patients to return to their preinjury sporting level following an MAT, but the long-term outcome in this group remains unclear.
Acknowledgment
No author had received any incentives, financial or otherwise, in the collating of this review article.
Disclosure
The authors report no conflicts of interest in this work.
References
Henning C, Lynch M. Current concepts of meniscal function and pathology. Clin Sports Med. 1985;4(2):259–265. | ||
Levy IM, Torzilli P, Warren R. The effect of medial meniscectomy on anterior-posterior motion of the knee. J Bone Joint Surg Am. 1982;64(6):883–888. | ||
Roos H, Laurén M, Adalberth T, Roos EM, Jonsson K, Lohmander LS. Knee osteoarthritis after meniscectomy: prevalence of radiographic changes after twenty-one years, compared with matched controls. Arthritis Rheum. 1998;41(4):687–693. | ||
Andersson-Molina H, Karlsson H, Rockborn P. Arthroscopic partial and total meniscectomy: a long-term follow-up study with matched controls. Arthroscopy. 2002;18(2):183–189. | ||
Jorgensen U, Sonne-Holm S, Lauridsen F, Rosenklint A. Long-term follow-up of meniscectomy in athletes. A prospective longitudinal study. J Bone Joint Surg Br. 1987;69(1):80–83. | ||
Chalmers PN, Karas V, Sherman SL, Cole BJ. Return to high-level sport after meniscal allograft transplantation. Arthroscopy. 2013;29(3):539–544. | ||
Zaffagnini S, Grassi A, Muccioli GMM, et al. Survivorship and clinical outcomes of 147 consecutive isolated or combined arthroscopic bone plug free meniscal allograft transplantation. Knee Surg Sports Traumatol Arthrosc. 2016;24(5):1432–1439. | ||
Alentorn-Geli E, Vazquez RS, Diaz PA, Cusco X, Cugat R. Arthroscopic meniscal transplants in soccer players: outcomes at 2- to 5-year follow-up. Clin J Sport Med. 2010;20(5):340–343. | ||
ElAttar M, Dhollander A, Verdonk R, Almqvist KF, Verdonk P. Twenty-six years of meniscal allograft transplantation: is it still experimental? A meta-analysis of 44 trials. Knee Surg Sports Traumatol Arthrosc. 2011;19(2):147–157. | ||
Noyes FR, Barber-Westin SD, Rankin M. Meniscal transplantation in symptomatic patients less than fifty years old. J Bone Joint Surg Am. 2004;86-A(7):1392–1404. | ||
Stone K, Adelson W, Pelsis J, Walgenbach A, Turek T. Long-term survival of concurrent meniscus allograft transplantation and repair of the articular cartilage. J Bone Joint Surg Br. 2010;92(7):941–948. | ||
Myers P, Tudor F. Meniscal allograft transplantation: how should we be doing it? A systematic review. Arthroscopy. 2015;31(5):911–925. | ||
Zaffagnini SP, Grassi AMD, Muccioli GMMMD, et al. Is sport activity possible after arthroscopic meniscal allograft transplantation? Midterm results in active patients. Am J Sports Med. 2016;44(3):625–632. | ||
Marcacci M, Marcheggiani Muccioli GM, Grassi A, et al. Arthroscopic meniscus allograft transplantation in male professional soccer players: a 36-month follow-up study. Am J Sports Med. 2014;42(2):382–388. | ||
Saltzman BM, Bajaj S, Salata M, et al. Prospective long-term evaluation of meniscal allograft transplantation procedure: a minimum of 7-year follow-up. J Knee Surg. 2012;25(2):165–175. | ||
Stone KR, Walgenbach AW, Turek TJ, Freyer A, Hill MD. Meniscus allograft survival in patients with moderate to severe unicompartmental arthritis: a 2-to 7-year follow-up. Arthroscopy. 2006;22(5):469–478. | ||
Rue LJ-PH, Yanke AB, Busam ML, McNickle AG, Cole BJ. Prospective evaluation of concurrent meniscus transplantation and articular cartilage repair minimum 2-year follow-up. Am J Sports Med. 2008;36(9):1770–1778. | ||
Hommen JP, Applegate GR, Del Pizzo W. Meniscus allograft transplantation: ten-year results of cryopreserved allografts. Arthroscopy. 2007;23(4):388–393. | ||
Noyes FR, Heckmann TP, Barber-Westin SD. Meniscus repair and transplantation: a comprehensive update. J Orthop Sports Phys Ther. 2012;42(3):274–290. | ||
Van Arkel E, De Boer H. Human meniscal transplantation. Preliminary results at 2 to 5-year follow-up. Bone Joint J. 1995;77(4):589–595. | ||
van Arkel ERA, De Boer H. Survival analysis of human meniscal transplantations. Bone Joint J. 2002;84(2):227–231. | ||
Cameron JC, Saha S. Meniscal allograft transplantation for unicompartmental arthritis of the knee. Clin Orthop Relat Res. 1997;337:164–171. | ||
Rodeo SA, Seneviratne A, Suzuki K, Felker K, Wickiewicz TL, Warren RF. Histological analysis of human meniscal allografts. J Bone Joint Surg Am. 2000;82(8):1071–1082. | ||
Fabbriciani C, Lucania L, Milano G, Panni AS, Evangelisti M. Meniscal allografts: cryopreservation vs deep-frozen technique. An experimental study in goats. Knee Surg Sports Traumatol Arthrosc. 1997;5(2):124–134. | ||
Verdonk PC, Demurie A, Almqvist KF, Veys EM, Verbruggen G, Verdonk R. Transplantation of viable meniscal allograft. J Bone Joint Surg. 2005;87(4):715–724. | ||
Crook T, Ardolino A, Williams L, Barlow I. Meniscal allograft transplantation: a review of the current literature. Ann R Coll Surg Engl. 2009;91(5):361–365. | ||
Hulet C, Pereira H, Peretti G, Denti M, eds. Surgery of the Meniscus. 1st ed. London: Springer; 2016. | ||
Verdonk R, Kohn D. Harvest and conservation of meniscal allografts. Scand J Med Sci Sports. 1999;9(3):158–159. | ||
Mickiewicz P, Binkowski M, Bursig H, Wrobel Z. X-ray microtomography-based measurements of meniscal allografts. Orthop Traumatol Surg Res. 2015;101(3):319–324. | ||
Gelber PE, Gonzalez G, Torres R, Giralt NG, Caceres E, Monllau JC. Cryopreservation does not alter the ultrastructure of the meniscus. Knee Surg Sports Traumatol Arthrosc. 2009;17(6):639–644. | ||
Pegg DE, Wusteman MC, Wang L. Cryopreservation of articular cartilage. Part 1: conventional cryopreservation methods. Cryobiology. 2006;52(3):335–346. | ||
Villalba R, Peña J, Navarro P, et al. Cryopreservation increases apoptosis in human menisci. Knee Surg Sports Traumatol Arthrosc. 2012;20(2):298–303. | ||
Schubert T, Cornu O, Delloye C. Organization: type of grafts, conservation, regulation. In: Beaufils P, Verdonk R, editors. The Meniscus. 1st ed. London: Springer; 2010:315–321 | ||
Binnet MS, Akan B, Kaya A. Lyophilised medial meniscus transplantations in ACL-deficient knees: a 19-year follow-up. Knee Surg Sports Traumatol Arthrosc. 2012;20(1):109–113. | ||
Sekiya JK, Ellingson CI. Meniscal allograft transplantation. J Am Acad Orthop Surg. 2006;14(3):164–174. | ||
LaPrade RF, Wills NJ, Spiridonov SI, Perkinson S. A prospective outcomes study of meniscal allograft transplantation. Am J Sports Med. 2010;38(9):1804–1812. | ||
Wirth CJ, Peters G, Milachowski KA, Weismeier KG, Kohn D. Long-term results of meniscal allograft transplantation. Am J Sports Med. 2002;30(2):174–181. | ||
Delloye C, Naets B, Cnockaert N, Cornu O. Harvest, storage and microbiological security of bone allografts. In: Delloye C, Bannister G, editors. Impaction Bone Grafting in Revision Arthroplasty. New York: Marcel Dekker; 2004:11–22. | ||
Lubowitz JH, Verdonk PC, Reid JB, Verdonk R. Meniscus allograft transplantation: a current concepts review. Knee Surg Sports Traumatol Arthrosc. 2007;15(5):476–492. | ||
Gelber PE, Gonzalez G, Lloreta JL, Reina F, Caceres E, Monllau JC. Freezing causes changes in the meniscus collagen net: a new ultrastructural meniscus disarray scale. Knee Surg Sports Traumatol Arthrosc. 2008;16(4):353–359. | ||
Rijk PC. Meniscal allograft transplantation—part I: background, results, graft selection and preservation, and surgical considerations. Arthroscopy. 2004;20(7):728–743. | ||
Hornicek FJ, Woll JE, Kasprisin D, eds. Standards for Tissue Banking. 10th ed. McLean, VA: American Association of Tissue Banks; 2002. | ||
Frank RM, Cole BJ. Meniscus transplantation. Curr Rev Musculoskelet Med. 2015;8(4):443–450. | ||
Buck B, Resnick L, Shah S, Malinin T. Human immunodeficiency virus cultured from bone: implications for transplantation. Clin Orthop Relat Res. 1990;251:249–253. | ||
McAllister DR, Joyce MJ, Mann BJ, Vangsness CT. Allograft update the current status of tissue regulation, procurement, processing, and sterilization. Am J Sports Med. 2007;35(12):2148–2158. | ||
Khoury MA, Goldberg VM, Stevenson S. Demonstration of HLA and ABH antigens in fresh and frozen human menisci by immunohistochemistry. J Orthop Res. 1994;12(6):751–757. | ||
Bos G, Goldberg VM, Zika J, Heiple K, Powell A. Immune responses of rats to frozen bone allografts. J Bone Joint Surg Am. 1983;65(2):239–246. | ||
Stevenson S. The immune response to osteochondral allografts in dogs. J Bone Joint Surg Am. 1987;69(4):573–582. | ||
Zukor D, Cameron J, Brooks P, et al. The fate of human meniscal allografts. In: Ewing JW, ed. Articular Cartilage and Knee Joint Function: basic science & arthroscopy. New York: Raven Press; 1990:147–152. | ||
Pollard ME, Kang Q, Berg EE. Radiographic sizing for meniscal transplantation. Arthroscopy. 1995;11(6):684–687. | ||
McDermott ID, Sharifi F, Bull AMJ, Gupte CM, Thomas RW, Amis AA. An anatomical study of meniscal allograft sizing. Knee Surg Sports Traumatol Arthrosc. 2004;12(2):130–135. | ||
Abbott A. On the perimeter of an ellipse. Mathematica J. 2009;11(2):172–185. | ||
Carter T, Economopoulos KJ. Meniscal allograft shrinkage—MRI evaluation. J Knee Surg. 2013;26(03):167–172. | ||
Lee BS, Chung JW, Kim JM, Cho WJ, Kim KA, Bin SI. Morphologic changes in fresh-frozen meniscus allografts over 1 year a prospective magnetic resonance imaging study on the width and thickness of transplants. Am J Sports Med. 2012;40(6):1384–1391. | ||
Milachowski KA, Weismeier K, Wirth CJ. Homologous meniscus transplantation. Experimental and clinical results. Int Orthop. 1989;13(1):1–11. | ||
De Coninck T, Huysse W, Verdonk R, Verstraete K, Verdonk P. Open versus arthroscopic meniscus allograft transplantation: magnetic resonance imaging study of meniscal radial displacement. Arthroscopy. 2013;29(3):514–521. | ||
Abat F, Gelber PE, Erquicia JI, Pelfort X, Gonzalez-Lucena G, Monllau JC. Suture-only fixation technique leads to a higher degree of extrusion than bony fixation in meniscal allograft transplantation. Am J Sports Med. 2012;40(7):1591–1596. | ||
Jang SH, Kim JG, Ha JG, Shim JC. Reducing the size of the meniscal allograft decreases the percentage of extrusion after meniscal allograft transplantation. Arthroscopy. 2011;27(7):914–922. | ||
Koh Y, Moon H, Kim Y, Park Y, Jo S, Kwon S. Comparison of medial and lateral meniscal transplantation with regard to extrusion of the allograft, and its correlation with clinical outcome. J Bone Joint Surg Br. 2012;94(2):190–193. | ||
Ha JK, Shim JC, Kim DW, Lee YS, Ra HJ, Kim JG. Relationship between meniscal extrusion and various clinical findings after meniscus allograft transplantation. Am J Sports Med. 2010;38(12):2448–2455. | ||
van der Wal RJP, Thomassen BJW, van Arkel ERA. Long-term clinical outcome of open meniscal allograft transplantation. Am J Sports Med. 2009;37(11):2134–2139. | ||
Verdonk PCM, Verstraete KL, Almqvist KF, et al. Meniscal allograft transplantation: long-term clinical results with radiological and magnetic resonance imaging correlations. Knee Surg Sports Traumatol Arthrosc. 2006;14(8):694–706. | ||
Alhalki MM, Howell SM, Hull ML. How three methods for fixing a medial meniscal autograft affect tibial contact mechanics. Am J Sports Med. 1999;27(3):320–328. | ||
Chen MI, Branch TP, Hutton WC. Is it important to secure the horns during lateral meniscal transplantation? A cadaveric study. Arthroscopy. 1996;12(2):174–181. | ||
Aagaard H, Jörgensen U, Bojsen-Möller F. Reduced degenerative articular cartilage changes after meniscal allograft transplantation in sheep. Knee Surg Sports Traumatol Arthrosc. 1999;7(3):184–191. | ||
Aagaard H, Jørgensen U, Bojsen-Møller F. Immediate versus delayed meniscal allograft transplantation in sheep. Clin Orthop Relat Res. 2003;406(1):218–227. | ||
Stone KR, Pelsis JR, Surrette ST, Walgenbach AW, Turek TJ. Meniscus transplantation in an active population with moderate to severe cartilage damage. Knee Surg Sports Traumatol Arthrosc. 2015;23(1):251–257. | ||
Kempshall PJ, Parkinson B, Thomas M, et al. Outcome of meniscal allograft transplantation related to articular cartilage status: advanced chondral damage should not be a contraindication. Knee Surg Sports Traumatol Arthrosc. 2015;23(1):280–289. | ||
Vundelinckx B, Vanlauwe J, Bellemans J. Long-term subjective, clinical, and radiographic outcome evaluation of meniscal allograft transplantation in the knee. Am J Sports Med. 2014;42(7):1592–1599. | ||
Vundelinckx B, Bellemans J, Vanlauwe J. Arthroscopically assisted meniscal allograft transplantation in the knee: a medium-term subjective, clinical, and radiographical outcome evaluation. Am J Sports Med. 2010;38(11):2240–2247. | ||
Lee BS, Bin SI, Kim JM. Articular cartilage degenerates after subtotal/total lateral meniscectomy but radiographic arthrosis progression is reduced after meniscal transplantation. Am J Sports Med. 2016;44(1):159–165. | ||
Rath E, Richmond JC, Yassir W, Albright JD, Gundogan F. Meniscal allograft transplantation two-to eight-year results. Am J Sports Med. 2001;29(4):410–414. | ||
Stone KR, Pelsis J, Surrette S, Stavely A, Walgenbach AW. Meniscus allograft transplantation allows return to sporting activities. Arthroscopy. 2013;10(29):e52–e53. | ||
Yanke AB, Chalmers PN, Frank RM, Friel NA, Karas V, Cole BJ. Clinical outcome of revision meniscal allograft transplantation: minimum 2-year follow-up. Arthroscopy. 2014;30(12):1602–1608. | ||
Smith NA, Parkinson B, Hutchinson CE, Costa ML, Spalding T. Is meniscal allograft transplantation chondroprotective? A systematic review of radiological outcomes. Knee Surg Sports Traumatol Arthrosc. 2015;24(9):2923–2935. | ||
Hardy PP, Roumazeille T, Klouche S, et al. Arthroscopic meniscal allograft transplantation without bone blocks: evaluation with MR-arthrography. Arthroscopy. 2013;29(10):e106. | ||
Noyes FR, Barber-Westin SD. Irradiated meniscus allografts in the human knee. A two- to five- year follow-up study. Ortho Trans. 1995;19:417. | ||
Verdonk PC, Verstraete KL, Almqvist KF, et al. Meniscal allograft transplantation: long-term clinical results with radiological and magnetic resonance imaging correlations. KSSTA. 2006;14(8):694–706. | ||
Kazi HA, Abdel-Rahman W, Brady PA, Cameron JC. Meniscal allograft with or without osteotomy: a 15-year follow-up study. Knee Surg Sports Traumatol Arthrosc. 2015;23(1):303–309. | ||
Matava MJ. Meniscal allograft transplantation: a systematic review. Clin Orthop Relat Res. 2007;455:142–157. | ||
Noyes FR, Barber-Westin SD. Meniscal transplantation in symptomatic patients under fifty years of age. J Bone Joint Surg Am. 2015;97(15):1209–1219. | ||
McCormick F, Harris JD, Abrams GD, et al. Survival and reoperation rates after meniscal allograft transplantation: analysis of failures for 172 consecutive transplants at a minimum 2-year follow-up. Am J Sports Med. 2014;42(4):892–897. | ||
Riboh JC, Tilton AK, Cvetanovich GL, Campbell KA, Cole BJ. Meniscal allograft transplantation in the adolescent population. Arthroscopy. 2016;32(6):1133–1140. | ||
Lee BS, Kim JM, Kim KA, Bin SI. Patient-related risk factors for the extrusion of lateral meniscal allograft transplants. Arthroscopy. 2015;31(4):699–706. | ||
Lee DH, Kim SB, Kim TH, Cha EJ, Bin SI. Midterm outcomes after meniscal allograft transplantation comparison of cases with extrusion versus without extrusion. Am J Sports Med. 2010;38(2):247–254. | ||
Sekiya JK, West RV, Groff YJ, Irrgang JJ, Fu FH, Harner CD. Clinical outcomes following isolated lateral meniscal allograft transplantation. Arthroscopy. 2006;22(7):771–780. | ||
Emery CF, Kiecolt-Glaser JK, Glaser R, Malarkey WB, Frid DJ. Exercise accelerates wound healing among healthy older adults: a preliminary investigation. J Gerontol A Biol Sci Med Sci. 2005;60(11):1432–1436. | ||
Villalta EM, Peiris CL. Early aquatic physical therapy improves function and does not increase risk of wound-related adverse events for adults after orthopedic surgery: a systematic review and meta-analysis. Arch Phys Med Rehabil. 2013;94(1):138–148. | ||
Smith NA, Achten J, Parsons N, et al. Meniscal transplantation and its effect on osteoarthritis risk: an abridged protocol for the MeTEOR study: a comprehensive cohort study incorporating a pilot randomised controlled trial. Bone Joint Res. 2015;4(6):93–98. |
Supplementary material
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.