Back to Journals » International Journal of Nephrology and Renovascular Disease » Volume 12
Medullary Sponge Kidney: Current Perspectives
Authors Imam TH , Patail H , Patail H
Received 14 February 2019
Accepted for publication 17 September 2019
Published 26 September 2019 Volume 2019:12 Pages 213—218
DOI https://doi.org/10.2147/IJNRD.S169336
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Pravin Singhal
Talha H Imam,1 Haris Patail,2 Hassan Patail3
1Division of Nephrology, Kaiser Permanente, Fontana, CA, USA; 2St. George’s University School of Medicine, West Indies, Grenada; 3Department of Medicine, Division of Pulmonary Critical Care and Sleep Medicine, Stony Brook University Hospital, Stony Brook, NY, USA
Correspondence: Talha H Imam
Division of Nephrology, Kaiser Permanente, 9985 Sierra Ave, Fontana, CA 92335, USA
Tel +1 909-427-7521
Email [email protected]
Abstract: Medullary Sponge Kidney (MSK) disease is a rare congenital malformation of the distal nephron where cystic dilatation is appreciable in the collecting ducts and renal papillae. Most cases of the malformation are thought to arise from a malfunction within neurotrophic factor and tyrosine kinase interactions. Presentation and prognosis are usually indolent; however, they include urinary tract infections (UTI), nephrolithiasis and nephrocalcinosis, distal renal tubular acidosis (dRTA) and hypocitraturia. With an insidious and asymptomatic onset, MSK is a difficult renal manifestation to both diagnose and treat. Difficulty diagnosing MSK today arises from clinical settings deviating from the usage of contrast methods when assessing the urogenital tract. Many healthcare standards for kidney disorders center diagnosis around imaging techniques rather than contrast methods. This ultimately leads to a decrease in the total number of confirmed cases of MSK. Though intra-venous urogram (IVU) remains as the current gold standard to diagnose MSK, other methods such as endoscopy and Multi-detector computed tomography (MDCT) are being put into place. Endoscopic examination and renal biopsy may allow definitive diagnosis; however, such invasive methods may be considered excessive. Moving forward, differential diagnoses for MSK can be made more precisely when patients present with other renal manifestations, especially in groups at risk. These groups include patients between the age of 20 and 30, patients with other renal malformations, high sodium diet patients, hyperparathyroid patients, and patients with family history of MSK. Basic treatment is aimed at controlling stone formation by stabilizing urinary pH. Treatment for patients, especially those prone to forming stones, includes the application of potassium citrate compounds, prophylactic water and diet control, surgical intervention or lithotripsy for removal of symptomatic kidney stones.
Keywords: medullary sponge kidney, nephron, nephrocalcinosis, renal stones
Introduction
Medullary sponge kidney (MSK) disease is a rare condition affecting the distal nephron within the renal medulla and typically presents itself with nephrocalcinosis, distal renal tubular acidosis, and hypocitraturia.1–3 MSK is defined as dilatation of medullary and papillary portions of the collecting ducts due to cystic damage to the distal nephron.4 MSK acquires its name from the classical cysts found within the nephron which can grow from 1 to 8 millimeters and appear as “sponges” upon cross-sectional examination.4,5 This disorder normally affects the medulla where the cortical structures are almost always spared.
Though it was first recognized in the 1930’s by Lendarduzzi, many aspects of MSK as a disease are still not well understood. The disease is often sporadic, rarely familial, and is shown to present bilaterally with a prevalence between 5/10,000 and 5/100,000.2,4,5 Despite its sporadic nature, MSK rarely presents with familial inheritance in an autosomal dominant fashion.2 With an autosomal dominant presentation, MSK has also shown to display characteristics of familial clustering, reduced penetrance and variable expressivity.6 It typically presents between age 20 and 30. Women are slightly more affected than men.2,4 Rare cases of MSK have been reported as a disease occurrence in neonates.1
Most patients are asymptomatic. Episodic renal stones and recurrent urinary tract infections (UTI) are its common clinical implications.7,8 Imaging and diagnostic modalities allow appreciation of pre-calyceal dilation and enlarged inner medullary collecting ducts.9 The gold standard for diagnoses is using intra-venous urogram (IVU), however other procedures such as CT, X-ray, and endoscopy can also be used.
This rare renal malformation has been seen with other renal manifestations including Beckwith-Wiedemann syndrome, polycystic renal disease, Wilms Tumor, Horseshoe Kidney, Rabson-Mendenhall syndrome, Cakut syndrome, and Caroli syndrome.2,3,10,11 The genetic association between MSK and Caroli’s disease (CD) is known. Renal anomalies which may be associated with CD include MSK, cortical cysts, adult recessive polycystic kidney disease, and rarely autosomal dominant polycystic kidney disease. However, exact incidence of MSK in patients of CD is not known but it has been reported in around 3.3% patients. CD is usually inherited in autosomal recessive or less commonly in autosomal dominant pattern while MSK is mostly sporadic.12 As a disease itself, MSK can coexist with other renal developmental manifestations since it is considered a renal developmental malformation itself. Abnormalities in developmental genes including GDNF and RET as well as the association with Multiple Endocrine Neoplasia (MEN 2A) can lead to several different renal pathologies, extending further than MSK.
Epidemiology
The incidence of MSK is similar worldwide compared to the United States.4 Since MSK is associated with renal and urinary tract stone formation, it is hypothesized that up to 20% of patients with calcified renal stones will have the disease.4,13 Though MSK may have a sporadic prevalence of 5/10,000 to 5/100,000 according to Geavlete et al, other sources state that the epidemiology of MSK is difficult to predict. This may be due to the cause of a decreased amount of diagnoses since MSK is known to have an indolent and asymptomatic behavior. The mean age for MSK is 27 years old.4
Pathogenesis
There are several unknowns in regards to the pathogenesis of MSK.14 However, Fabris et al expand on the involvement and interaction of two genes including the survival factor, glial cell derived neurotrophic factor (GDNF) and receptor tyrosine kinase (RET).2 The number of MSK cases reported to be associated with GDNF and RET mutations is low, however, it still remains as the only current well-understood pathogenesis of the disease.1 Despite its prominent neuronal role, GDNF is also involved in renal development in inducing the ureteric bud and its growth, with branching from Wolff’s duct.2 During nephrogenesis, the metanephric blastema synthesizes GDNF which allows GDNF and RET to functionally interact with one another.2 Without proper GDNF induction of the metanephric blastema, the lower portions of the nephron fail to grow and differentiate.2 When GDNF is experimentally knocked out, “GDNF-null” mice show lack of proper renal development or complete renal agenesis.15 In other studies, involving animals who were heterozygous for the GDNF gene, different variations of renal abnormalities were found including small kidneys, cortical cysts, and unilateral dysgenesis.2 Without the presence of a function GDNF from the metanephric blastema, proper renal development is absent. These few cases of GDNF absence or malfunction can help determine and understand the main pathogenesis behind MSK.
Additionally, RET malformations can lead to improper renal formation.16 RET mutations are seen nearly 20% of the time in patients who have any type of renal agenesis from birth.2 Since RET is required for GDNF function, abnormalities or absence of RET can also lead to failure of ureteric bud outgrowth, renal agenesis or aplasia, and failure of the distal nephron and urinary system to develop.16 The RET malformation in MSK has been seen in rare occurrences in association MEN 2A.1
Mutations and abnormalities in genes imperative for proper renal formation lead to diminished distal nephron development, where pre-calyceal and collecting ducts are mostly affected.2 This leads to cyst formation, causing nephrocalcinosis and distal renal tubular acidosis as subsequent consequences of urine concentration defects.1 The occurrence of distal acidification in the nephron is thought to be the initial cause of the series of events including hypercalciuria, hypocitraturia, stone formation, and defective bone mineralization.2
Comorbidities
Hyperparathyroidism is a rare secondary manifestation of MSK, though the exact mechanisms are still unclear.17 It is hypothesized that the interaction of the RET gene may also contribute to the occurrence of hyperparathyroidism in patients with MSK.13 With long term complications of undetected MSK, patients may simply experience negative calcium balances due to hypercalciuria. This negative calcium balance can potentially lead to or exacerbate hyperparathyroidism.17 Studies conducted by Janjua et al included MSK patients whose nephrocalcinosis and hypercalciuria symptoms preceded the occurrence of hyperparathyroidism.17 Excessive loss of calcium through the crystallization in the nephron can cause an up-regulation of parathyroid hormone (PTH) attempting to readjust the calcium imbalance. By confirming these findings with their observed patients, Janjua et al concluded that hyperparathyroidism is secondary to MSK. A case report of a fifty-two-year-old patient showed that she had a history of MSK for six years which was followed by high levels of PTH.17 It was found that after resection of parathyroid glands, the patients serum values of both calcium and phosphate returned to normal.17 Janjua et al then concluded that though there may be an association of MSK with hyperparathyroidism with MSK, however, more studies are required to definitively conclude that the two occur together.17 Researchers also concluded that since MSK has been rarely associated with MEN2A, where the co-occurrence of hyperparathyroidism and MSK is plausible.17
Clinical Presentation
Major complications associated with MSK include nephrocalcinosis leading to nephrolithiasis, distal renal tubular acidosis, and UTI secondary to renal stones.8,14,18 Nephrolithiasis can be found in 70% of MSK patients.5 Though MSK is generally considered a benign disease, moderate complications following nephrolithiasis can include hematuria and renal failure if left untreated.1 Frequent episodes of nephrolithiasis may also lead to pyelonephritis and hematuria due to frequent UTIs.1,5,9 If present, the disorder’s typical clinical manifestations include hematuria, vague loin pain and burning sensations.8 In some cases, nephrocalcinosis associated with MSK has caused patients extreme chronic pain, where everyday pain drastically effects quality of life.19 Despite the regular occurrence of renal stones located in the distal nephron, collecting ducts, and papillae, MSK has not been found to be associated with papillary interstitial fibrosis.9
MSK is usually associated with increased levels of urinary calcium along with normal urinary potassium and bicarbonate levels in most patients.9 Urine pH, ammonia, and titratable acidity is increased in MSK patients; however, this finding does not differ from other patients experiencing nephrolithiasis by a separate cause.9 This conclusion shows that several abnormal serum findings may attributed to recurrent renal stones rather than a direct consequence of the MSK distal tubular malformation.9
Renal stones associated with MSK carry equal amounts of both calcium phosphate and calcium oxalate.9 Both calcium phosphate and calcium oxalate stones can be appreciated as radiopaque on imagining.
It is important to distinguish renal stones versus renal plugs in MSK patients, where stones do not adhere to renal tubule linings.9 MSK patients can often present with inner medullary collecting duct plugs which do not pass due to their elongated structure that adheres to renal epithelia and basement membranes.9 Evan et al theorized that due to the possible renal tubule epithelial malformation, MSK patients can be more prone to experiencing mineral plugs compared to the general population.9 Plugs within the renal tubules can present with similar complications as stones which can include but are not limited to urinary stasis, UTIs, and pyelonephritis.
Though it is relatively rare, MSK can present in neonates and children with more severe complications compared to adults.2 Distal rental tubular acidosis in young children with MSK can lead to severe bone disorders, where children will present with short stature, failure to thrive, and rickets-like symptoms.2
Diagnosis
Though MSK is a rare renal defect, differentiation from other causes of nephrolithiasis is possible due to MSK’s patent rounded, enlarged, and puffy papillae on imaging.9 Confirming diagnosis is important due to differential diagnoses such as hyperparathyroidism, renal tubular acidosis type I, hypervitaminosis D, milk-alkali syndrome, and sarcoidosis also causing nephrolithiasis due to nephrocalcinosis.4 Aside from differential diagnoses, making a confirmed diagnosis for MSK is not influential on the management of the disease itself.13 Diagnostic evidence via imaging can have benefits, however the definitive diagnosis is not necessary to treat individual episodes of renal stones caused by MSK.13
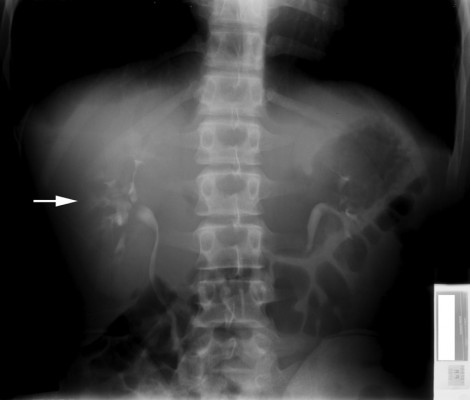
Diagnosis of MSK has slightly evolved, however, the cornerstone is centered around radiographic intra-venous urography (IVU).1,5 Contrast medium collection in papillary ducts lead to a classic image of papillary blush or bouquets (Figure 1).1 When observable, MSK imaging either produces patterns of bouquets, brush-like striation, or linear striations.13 Observing renal papillae via endoscopy, diffuse damage to renal papillae with contour rounding causing a billowy appearance leading to distal papillae blunting is appreciable.9 Endoscopy has shown to properly establish a diagnosis of MSK.9 Other imaging modalities such as roentgenogram and computed tomography (CT) (Figure 2) have been explored in the past leading to limited diagnostic accuracy.13 Common UTI and kidney disorders are now being diagnosed and monitored more by imaging techniques rather than usage of contrast mediums.2 The replacement of IVU by CT imaging is a drawback in MSK management due to CT being unable to properly demonstrate the classic signs of MSK.3,13,20 CT imaging has been proven to better establish diagnoses for most urological conditions, however this unfortunately does not apply to MSK.13 This transition can negatively affect accurate diagnoses of MSK due to a deviation from contrast mediums.20 Renal ultrasound also provides a newer option where the renal medulla may appear hyperechogenic (increased ultrasound signal) due to renal stones.5 Gambaro et al concluded that the number of diagnoses of MSK may be reduced over time due to imaging techniques moving away from contrast methods, which is considered the gold standard.1 If MSK is not diagnosed early leading to long term irreversible complications, nephrectomy may be required.20
 |
Figure 1 Intravenous pyelogram showing brush-like striations (arrow) within dilated contrast-filled medullary tubules. Note: Reprinted from Perm J. 18(2), Imam TH, Taur AS, Patail H, image diagnosis: medullary sponge kidney. e130-e131, doi:10.7812/TPP/13-145, copyright(2014), with permission from The Permanente Press. Available from: www.thepermanentejournal.org.7 |
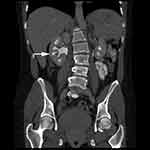 |
Figure 2 Computerized tomography urogram (post-contrast) coronal view demonstrating dilated medullary contrast-filled tubules and medullary calcifications (arrow). Note: Reprinted from Perm J. 18(2), Imam TH, Taur AS, Patail H, image diagnosis: medullary sponge kidney. e130-e131, doi:10.7812/TPP/13-145, copyright(2014), with permission from The Permanente Press. Available from: www.thepermanentejournal.org.7 |
Multi-detector computed tomography (MDCT) can accurately diagnose MSK comparable to IVU.13 MDCT has a slightly lower sensitivity compared to IVU in regard to MSK, however studies show that the method is still a suitable replacement for diagnoses if needed.13 A study conducted by Guany et al showed how MDCT was diagnostic for MSK in nine out of ten patients who were previously diagnosed by IVU, where no false positives occurred. On MDCT, MSK presents with brush-like striations within the renal papillae as well as cystic dilations of the distal tubules.13 MDCT is superior to traditional CT imaging in MSK patients due to MDCT utilizing higher resolution due to thin section axial imaging.13 Further trials must be explored to determine whether MDCT is a high diagnostic indicator of MSK.
Prognosis
Aside from recurrent episodes of nephrolithiasis, MSK can be considered a benign condition. Typical complications of nephrolithiasis are observed in patients including UTIs, pyelonephritis, and end stage renal disease (ESRD), especially if urinary tract obstructions are not addressed clinically.18 In a retrospective cohort study, MSK patients were divided into nephrectomy and non-nephrectomy groups, where the investigators found that kidney stone-related events were decreased following nephrectomy in MSK patients, however the incidence of ESRD was not decreased.18 The investigational findings by Cheungpasitporn et al are plausible due to MSK being a bilateral condition. By affecting both kidneys, unilateral nephrectomy reduces the total number of stones present in a patient, however the patients remain at risk of ESRD and mortality is not significantly decreased.18 With renal stone formation being the most important prognostic factor, Fabris et al concluded that MSK patients with at least one stone risk factor (hypercalciuria, hypocitraturia, hyperuricosuria, hyperoxaluria) were at a higher risk for renal stone complications and should prophylactically be treated with potassium citrate.8 Patients without stone risk factors had better prognoses in regard to stone formation.8
Treatment
Due to its rare occurrence and dearth of literature, MSK treatment remains unclear. Most treatment regimens are centered around prophylactic measures compared to symptomatic care. Prophylaxis for controlling symptoms associated with MSK is very similar to nephrolithiasis. Prevention of recurrent renal stones includes a well-balanced diet consisting of fruits, vegetables, and adequate fluid intake.2,8 Symptomatic care is focused on pH stabilization of urine, resulting in citrate and alkaline compound usage.8,14 Potassium citrate has shown to decrease distal renal tubular acidosis and reduce the renal stone precipitation in patients with MSK.8 When given to patients, citrate compounds have also shown to improve calciuria and decrease the negative effects of calcium mobilization from bone.8 The acid-base shift in MSK patients is a leading factor for hypercalciuria and stone formation.8 Citrate treatment is shown to improve the acid-base deviation and ultimately improve calcium oxalate and phosphate stones.8 Despite determining that dRTA is a major cause of nephrolithiasis in MSK patients, potassium citrate compounds have shown a reduction in recurrent stones in idiopathic patients as well.8
Thiazide diuretics can be added as an adjunctive measure to decrease rates of calcium stone formation in the distal nephron.2 Thiazide diuretics allow the reabsorption of calcium in the distal convoluted tubules, ultimately helping in the prevention in the recurrence of new stone formation.8
As a last line choice, surgical intervention including the removal of stones may be a choice for patients who experience both recurrent stone formation and harsh clinical symptoms. Though stone removal is advantageous due to the removal of symptoms, the actual prognosis of the disease does not change.2 Next to surgical management, vaporization of the urothelium where cystic dilation is present has been explored, followed by lithotripsy which allows the breakdown and passage of stones in the urine.5 Both open surgical remedies and shock wave lithotripsy can be advantageous in patients with medullary sponge disease if reoccurring stones or clinical symptoms representing nephrolithiasis become excessive.
Conclusion
Due to the slow progression, lack of understanding, and vague symptomatology, MSK has become a rare disease that is difficult to diagnose and manage clinically. Its symptoms including nephrolithiasis allow the disease progression to become vague and misunderstood. Alongside nephrolithiasis, patients with MSK can present with recurrent UTIs, dRTA, and hypocitraturia. With its vague presentation, a decreased number of diagnoses has been shown due to the decreased usage of the gold standard method - IVU. MSK can occur with other renal anomalies, however the treatment for MSK alone has been shown to be conservative, managing mostly symptoms for patients. Patients who have already been diagnosed with MSK should be treated accordingly and further monitored to prevent further complications with recurrent stone formation. Further research should move towards better diagnosing patients with MSK, possibly by implementing the usage of IVU more with patients with nephrolithiasis as well as research centered around better treatment for MSK as a disease itself.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Gambaro G, Feltrin GP, Lupo A, Bonfante L, D’Angelo A, Antonello A. Medullary sponge kidney (Lenarduzzi–cacchi–ricci disease): a Padua Medical School discovery in the 1930s. Kidney Int. 2006;69(4):663–670. doi:10.1038/sj.ki.5000035
2. Fabris A, Anglani F, Lupo A, Gambaro G. Medullary sponge kidney: state of the art. Nephrol Dial Transplant. 2013;28(5):1111–1119. doi:10.1093/ndt/gfs505
3. Xiang H, Han J, Ridley WE, Ridley LJ. Medullary sponge kidney. J Med Imaging Radiat Oncol. 2018;62:93–94. doi:10.1111/1754-9485.40_12784
4. Garfield K, Leslie SW. Medullary Sponge Kidney. StatPearls Publishing; January 2018.
5. Geavlete P, Nita G, Alexandrescu E, Geavlete B. The impact of modern endourological techniques in the treatment of a century old disease - medullary sponge kidney with associated nephrolithiasis. J Med Life. 2013;6(4):482–485.
6. Fabris A, Lupo A, Ferraro PM, et al. Familial clustering of medullary sponge kidney is autosomal dominant with reduced penetrance and variable expressivity. Kidney Int. 2013;83(2):272–277. doi:10.1038/ki.2012.378
7. Imam TH, Taur AS, Patail H. Image diagnosis: medullary sponge kidney. Perm J. 2014;18(2):2. doi:10.7812/TPP/13-145
8. Fabris A, Lupo A, Bernich P, et al. Long-term treatment with potassium citrate and renal stones in medullary sponge kidney. Clin J Am Soc Nephrol. 2010;5(9):1663–1668. doi:10.2215/CJN.00220110
9. Evan AP, Worcester EM, Williams JC, et al. Biopsy proven medullary sponge kidney: clinical findings, histopathology, and role of osteogenesis in stone and plaque formation: pathogenesis of MSK stones. Anat Rec. 2015;298(5):865–877. doi:10.1002/ar.23105
10. Cheungpasitporn W, Pawar A, Erickson S. Recurrent renal calculi in coexistence of horseshoe kidney and medullary sponge kidney. Urol Ann. 2017;9(2):214. doi:10.4103/UA.UA_173_16
11. Shan D, Rezonzew G, Mullen S, et al. Heterozygous Pkhd1C642 mice develop cystic liver disease and proximal tubule ectasia that mimics radiographic signs of medullary sponge kidney. Am J Physiol Renal Physiol. 2019;316:F463–F472. doi:10.1152/ajprenal.00181.2018
12. Sinha RJ, Sharma A, Singh V, Pandey S. Medullary sponge kidney and Caroli’s disease in a patient with stricture urethra: look for the hidden in presence of the apparent. BMJ Case Rep. 2018;11(1):
13. Gaunay GS, Berkenblit RG, Tabib CH, Blitstein JR, Patel M, Hoenig DM. Efficacy of multi-detector computed tomography for the diagnosis of medullary sponge kidney. Curr Uroly. 2017;11(3):139–143. doi:10.1159/000447208
14. El-Sawi M, Shahein A-R. Medullary sponge kidney presenting in a neonate with distal renal tubular acidosis and failure to thrive: a case report. J Med Case Rep. 2009;3(1). doi:10.1186/1752-1947-3-6656
15. Sánchez MP, Silos-Santiago I, Frisén J, He B, Lira SA, Barbacid M. Renal agenesis and the absence of enteric neurons in mice lacking GDNF. Nature. 1996;382:70. doi:10.1038/382070a0
16. Chatterjee R, Ramos E, Hoffman M, et al. Traditional and targeted exome sequencing reveals common, rare and novel functional deleterious variants in RET-signaling complex in a cohort of living US patients with urinary tract malformations. Hum Genet. 2012;131(11):1725–1738. doi:10.1007/s00439-012-1181-3
17. Janjua MU, Long X, Mo Z, Dong C, Jin P. Association of medullary sponge kidney and hyperparathyroidism with RET G691S/S904S polymorphism: a case report. J Med Case Rep. 2018;12(1). doi:10.1186/s13256-018-1736-6
18. Cheungpasitporn W, Thongprayoon C, Brabec BA, Kittanamongkolchai W, Erickson SB. Outcomes of living kidney donors with medullary sponge kidney. Clin Kidney J. 2016;9(6):866–870. doi:10.1093/ckj/sfv107
19. Gambaro G, Goldfarb DS, Baccaro R, et al. Chronic pain in medullary sponge kidney: a rare and never described clinical presentation. J Nephrol. 2018;31:537. doi:10.1007/s40620-018-0480-8
20. Cheungpasitporn W, Erickson S. Medullary sponge kidneys and the use of dual-energy computed tomography. Urol Ann. 2015;7(1):129. doi:10.4103/0974-7796.148666
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
