Back to Journals » International Journal of General Medicine » Volume 15
Mean Platelet Volume: A Possible Predictor for Patients with Decompensated Chronic Heart Failure
Authors Andrei CL , Catană A , Sinescu CJ, Mirică A, Ceban O , Chioncel VP , Darabont RO
Received 12 February 2022
Accepted for publication 6 April 2022
Published 16 April 2022 Volume 2022:15 Pages 4131—4140
DOI https://doi.org/10.2147/IJGM.S362257
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Cătălina Liliana Andrei,1 Andreea Catană,1 Crina Julieta Sinescu,1 Andreea Mirică,2 Octavian Ceban,2 Valentin Puiu Chioncel,1 Roxana Oana Darabont1
1University of Medicine and Pharmacy “Carol Davila”, Bucharest, Romania; 2The Bucharest University of Economic Studies, Bucharest, Romania
Correspondence: Andreea Catană, University of Medicine and Pharmacy “Carol Davila”, Dionisie Lupu Street, no. 37, Bucharest, 020021, Romania, Tel +40-745469489, Email [email protected]; [email protected]
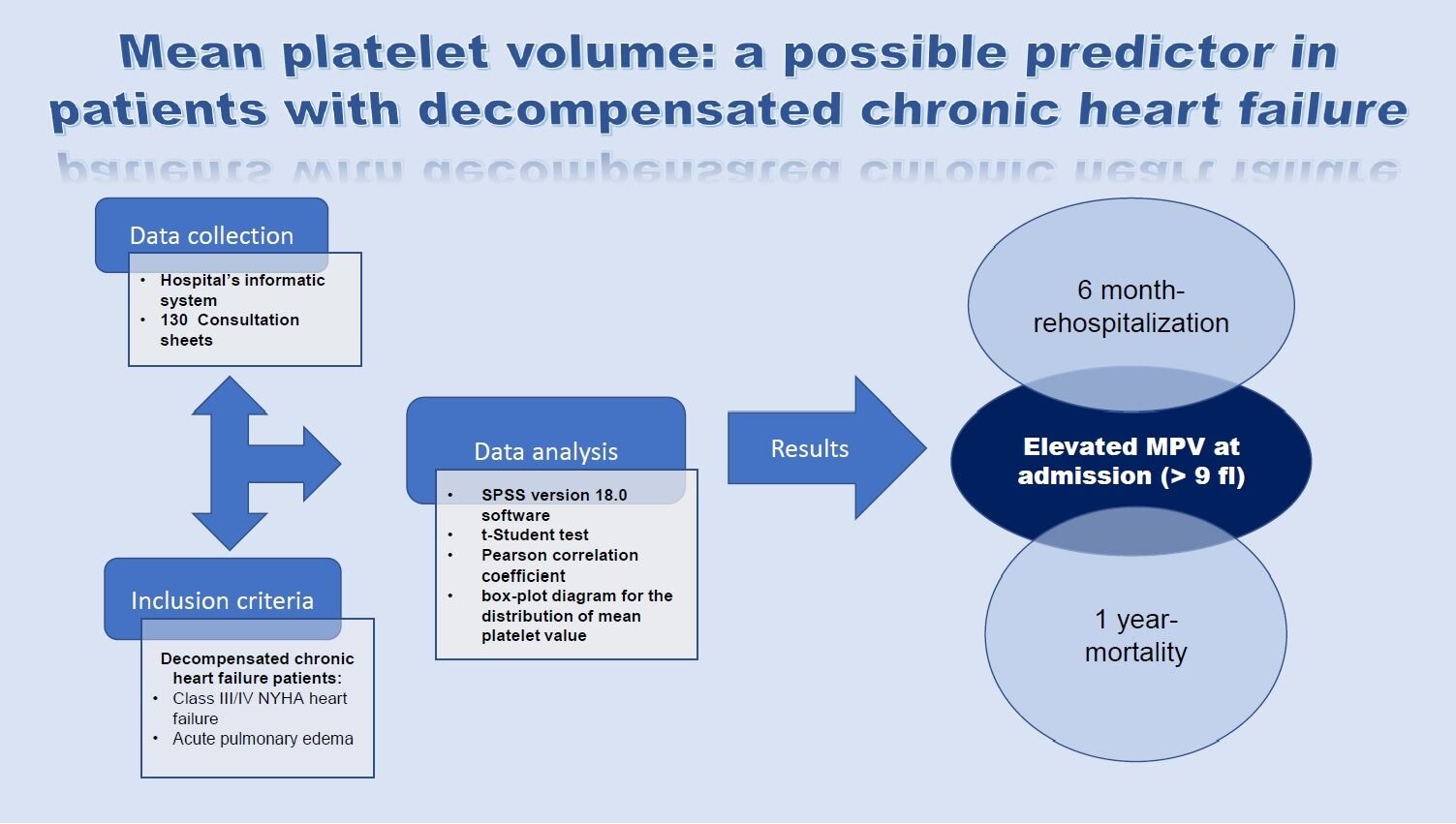
Purpose: Despite all medical efforts and discoveries, heart failure (HF) remains one of the most important and common public health problems, with high mortality and hospitalization rates, due to decompensation of HF. In the present study, we aimed to identify a predictive factor through which we can evaluate the risk of readmission and mortality in the first year, given the initial admission of a patient with decompensated heart failure.
Patients and Methods: The parameter we have investigated is the mean platelet volume (MPV). Studies have shown that there is a significant correlation between the value of MPV and the risk of cardiovascular disease (CVD) and cardiovascular (CV) death. In this study, we enrolled 130 patients hospitalized for decompensated chronic HF (NYHA class IV HF or acute pulmonary edema) and analyzed whether there is a relationship between the value of the MPV at admission and 6-month rehospitalization, and 1-year mortality, respectively.
Results: The statistical analysis revealed significantly different values (p = 0.041) for MPV at admission between the group of patients without decompensated chronic HF compared to the group of patients with decompensated chronic HF (8.74 fl vs 9.08 fl). Also, the results of our study revealed that patients with decompensated chronic heart failure who were readmitted at 6 months and died at 1 year, respectively, had a higher MPV at admission (> 9 fl), compared to those without the above-mentioned events, with a statistical significance.
Conclusion: A higher MPV at admission can be considered in our study as an independent predictor for rehospitalization and 1-year mortality of patients with decompensated chronic HF.
Keywords: decompensated heart failure, mean platelet volume, 6-month rehospitalization, 1-year mortality
A Letter to the Editor has been published for this article.
Graphical Abstract:
Introduction
Cardiovascular disease (CVD) remains the most common cause of death. The number of patients diagnosed with CVD is increasing in all countries regardless of their socioeconomic status. The prevalence of CVD doubled from 1990 to 2019 (270 million versus 573 million).1,2
Despite successful investigatory methods and elaborate treatment regimens, the number of deaths from CV causes has increased by 66% from 1990 to 1991 (12 million versus 18 million).3
The increasing prevalence (almost double in 2019 compared to 1990) and the high hospitalization rate make HF one of the most expensive diseases that consume financial reserves.
Heart failure (HF) remains one of the most important and common public health problems, with high mortality and hospitalization rate, especially for elderly patients. Among all cardiovascular diseases, it remains the one with the highest degree of disability in terms of quality of life, because most of the patients with HF have a decreased capacity of effort and limiting possibilities of making an individual physical effort.4
In Romania, there are approximately 700,000 patients with HF, and each year 45,000 new cases are diagnosed. Most hospitalizations for HF are due to decompensation episodes. In Romania, 1 in 3 patients die or are hospitalized in the first year after the last admission for HF.5
In the present study, we aimed to identify a possible predictive factor through which we can evaluate the risk of readmission and mortality in the first year, given the initial admission of a patient with decompensated heart failure.
The parameter we have proposed to investigate is the mean platelet volume (MPV). The MPV is a platelet indices that are inexpensive and easy to obtain, by collecting the blood sample, being part of the usual blood count. It is expressed in femtoliters (fl), with normal values between 7.2 and 11.7.6–9 The role of platelets in hemostasis is well known. More recently, the implications of platelets on the pathogenesis of atheromatous plaque and atherothrombosis have been described, and MPV is the simplest method by which platelet reactivity can be assessed. There is a direct relationship between platelet size and the degree of its activity. Therefore, the more voluminous the platelet is, the more metabolically active it is, and it also has a greater amount of prothrombotic material. Studies have shown that there is a direct relationship between platelet size and the degree of its activity and that there is a significant correlation between MPV value and the risk of CVD, and CV death.10–12
In addition, studies conducted by Kaia et al3 and Kandis et al14 pointed out that patients with HF and elevated MPV had negative outcomes expressed by recurrent hospitalizations or death, thus highlighting that there is a possible correlation between MPV value and future negative outcomes in HF patients.
Despite all medical efforts, HF remains one of the most important and common public health problems, with high mortality and hospitalization rate, especially for elderly patients, and one of the most expensive diseases. Finding novel ways to prevent future hospitalization or death due to decompensated heart failure remains a challenge. Therefore, finding a parameter that can help the physician to predict future outcomes for decompensated HF patients, could be helpful in creating certain therapeutic possibilities to prevent negative outcomes for these categories of patients.
Materials and Methods
Materials
The study took place in the Cardiology Department of the Emergency Hospital “Bagdasar-Arseni”, in Bucharest, Romania. This department is one of the most advanced Cardiology Departments in Romania, where most complex cases from the entire country are redirected. The study type was retrospective, and data was collected between January 2017 and January 2018. Data regarding rehospitalization and mortality were followed for one year until January 2019. The inclusion criteria were as follows: patients over 18 years, with decompensated chronic HF – New York Heart Association (NYHA) class IV or acute pulmonary edema (APE). The exclusion criteria were as follows: patients with NYHA I–II classes, neoplasia, chronic kidney disease, acute coronary syndrome, acute stroke, acute pulmonary thromboembolism (PE), thrombocytopenia, and autoimmune disease. Regarding the terminology used in heart failure for the studied population, according to the ESC Guidelines for HF 2021, decompensated chronic HF is defined as a sudden or progressive deterioration of chronic heart failure, and patients with chronic heart failure are described as those who had an established diagnosis of HF (typical symptomatology with or without signs of congestion, systolic dysfunction, echocardiographic evidence of structural and functional abnormalities consistent with diastolic dysfunction, objective evidence of increased LV filling pressure consistent with raised natriuretic peptides).13 These were documented based on the cardiologic consultation sheets from the hospital archive and the hospital’s informatics system (Hipocrate), using the diagnostic criteria ICD 10 (International Classification of Disease).
Two hundred and ten consultation sheets were analyzed from the hospital archive, with the approval of the Medical and Health Services Manager of “Bagdasar-Arseni” Emergency Hospital. Finally, using the inclusion and exclusion criteria, 130 consultation sheets remained eligible for our study.
Our study brings significant value added to the literature as the sample size is similar to that used by Kandis et al in their research.14
Demographic data (age, sex, environment), anthropometric measurements (body mass index-BMI), pathological medical history (history of myocardial infarction – MI, history of PE, history of stroke), associated comorbidities (hypertension – HTA, type 2 diabetes, dyslipidemia, anemia, infections, COPD – chronic obstructive pulmonary disease), laboratory data (NT-proBNP, presepsin, erythrocyte sedimentation rate (ESR), fibrinogen, serum creatinine at admission and on discharge, serum ionogram – sodium (Na) and potassium (K), complete blood count (hemoglobin (Hb), erythrocyte count, mean corpuscular volume (MCV), mean corpuscular hemoglobin concentration (MCHC), mean corpuscular hemoglobin (MCH), leukocytes count, platelet count, MPV), echocardiographic data (systolic/diastolic HF), and electrocardiographic (ECG) data (cardiac rhythm, left bundle/right bundle branch block), were analyzed.
In our study, we defined renal dysfunction, anemia, electrolyte disorders, and infections, based on several parameters and their values.
Renal dysfunction is defined as an elevated serum creatinine >1.3 mg/dl for men and >1.1 mg/dl for women.
Regarding anemia and type of anemia, anemia is defined as a level of Hb <13 g/dl for men and <12 g/dl for women, macrocytic anemia as a MCV > 100 fl, microcytic anemia as a MCV < 80 fl, hypochromic anemia as MCH < 27 pg/cell and MCHC < 33 g/dl.
Regarding electrolyte disorders, hyponatremia is defined as seric Na <135 mmol/l and hyperpotassemia as seric K > 5.5 mmol/l.
Regarding the presence or the absence of infections, the increased value of presepsin (P-Sep >300 pg/mL) and the presence of biological inflammatory syndrome (elevated ESR, fibrinogen, leukocytes) were biomarkers used for the diagnosis of infections.
Blood samples collected from peripheral venous punction were processed in the hospital lab on the same day. For the determination of the complete blood count, 2 mL of whole blood was collected in the vacutainer tube that contained 3.6 mg EDTA (Ethylenediaminetetraacetic acid), and then blood analysis was performed on a Beckman Coulter ACT 5 diff AL autoanalyzer. Seric creatinine was determined using the Jaffe photometric method on the Dimension RXL autoanalyzer, the seric ionogram was determined on the Ilyte autoanalyzer, and NT-proBNP and p-sepsin were determined using the ELISA method on the Pathfast-compact immunolyzer autoanalyzer.
Echocardiography was performed in our Cardiology Department on Aloka Prosound Alpha 7 diagnostic ultrasound system.
ECG recordings at admission and on discharge were made with a portable ECG device (Fukuda Denshi) with 12 derivations, by which heart rate and QRS complex changes were analyzed.
Rehospitalizations and 1-year death were followed by consulting the hospital’s informatics system (Hipocrate) or by phone call.
The primary objective of this study was to analyze whether there exists a correlation between MPV value and 6-month-rehospitalization and 1-year mortality, respectively, in patients with decompensated HF.
We used statistical analysis to measure the correlation between MPV and 6-month rehospitalization and between MPV and 1-year mortality.
Statistical Analysis
All data were performed using SPSS version 18.0 software. For the parameter MPV, the following were calculated: the mean, standard deviation, and confidence interval for the p-value of 1% depending on the laboratory parameters of the study population. Using the t-Student test, we compared the mean MPV in the group of patients who had this variable and the group of patients who did not have this variable. The Pearson correlation coefficient was used to analyze the correlation between NT-proBNP and MPV. For the graphical representation of the results for the distribution of MPV values, the box-plot diagram was used.
Results
Demographic data show that the mean age for the enrolled patients was 72.5 years. Anthropometric data show that of the total enrolled patients 30.0% were normal weight, 6.2% underweight, 31.5% overweight, and 32.3% obese. Normal weight was defined as BMI between 18.5 and 24.9 kg/m2, underweight <18.5 kg/m2, overweight >24.9 kg/m2, obese >29.9 kg/m2. The basic data of our patients are statistically recorded in Table 1.
 |
Table 1 Patient Characteristics |
Starting from the fact that renal dysfunction, anemia and hyponatremia are already known as negative prognostic factors in patients with HF, we included in our analysis, variables related to these factors.
For patients with increased serum creatinine, both at admission (55.4%) and on discharge (49.21%), there were no significant correlations with MPV values compared to patients with a normal value of creatinine.
For the patients with anemia (57 patients at admission and 47 patients on discharge), the results showed that there were no significant changes in MPV compared to those without anemia. Analyzing the type of anemia, statistical analysis showed that in patients with microcytic anemia, MPV value was increased significantly (p = 0.041) compared to those without microcytic anemia (9.16 fl vs 8.79 fl).
Regarding electrolyte disorders, statistical analysis showed no significant changes in MPV for patients with hyponatremia and hyperpotassemia.
Regarding the presence or the absence of infections, the increased value of presepsin (P-Sep >300 pg/mL) and the presence of biological inflammatory syndrome (elevated ESR, fibrinogen, leukocytes) were biomarkers used for the diagnosis of infections. According to the analysis of data from our study, which showed comparable values for MPV in patients with infection and those without infection (8.51 fl vs 8.86 fl), we could not find a significant relationship between the value of presepsin and the value of MPV at admission (p = 0.13), because the group of patients for whom the value of presepsin at admission was available, was far too small to have a statistical significance and because during the patient enrollment period, presepsin was not constantly available at the hospital laboratory.
The laboratory data is statistically resumed in Table 2.
 |
Table 2 Laboratory Parameters Corroborated with High MPV |
Regarding NT-proBNP, which is a marker of cardiac decompensation, we obtained a statistically significant correlation between the value from discharge and MPV at admission (Pearson coefficient 0.249) – see Supplementary Material.
There were 38 patients in the NYHA Class IV HF group (29.2%). Statistical analysis showed that in NYHA Class IV HF the MPV value was increased at admission (9.08 fl for NYHA Class IV) and the same results for the number of patients with APE and the value of MPV at admission were registered. The statistical analysis revealed significantly different values (p = 0.041) for MPV at admission between the group of patients without decompensated chronic heart failure compared to the group of patients with NYHA class IV heart failure (8.74 fl vs 9.08 fl). The same conclusion was obtained for patients with APE as a form of decompensated chronic heart failure. Those data are resumed in Table 3. Similar data were published by Kandis et al which showed that the value of MPV is increased in patients with decompensated HF versus patients with stable HF.14
 |
Table 3 Patients’ Characteristics Corroborated with High MPV |
Regarding the comorbidity analysis, we recorded the following data: 96 patients with hypertension (73.8%), 49 patients with type 2 diabetes (37.7%), 44 patients with dyslipidemia (33.8%), 6 patients with a history of stroke (4.6%), 24 patients with a history of MI (18.5%), 10 patients with a history of PE (7.7%), and 20 patients with COPD (15.4%). Aside from patients with a history of MI (Table 3), where we observed a statistically significant higher MPV value than those without this history (9.13 fl vs 8.78 fl; p = 0.047), for none of the other comorbidities studied we did not obtain a meaningful relationship with MPV.
Analyzing the ECG changes of enrolled patients, we observed that patients who had atrial fibrillation (AFib) rhythm at admission (63 patients) had a statistically significant higher MPV value than patients with sinus rhythm (SR) (67 patients) – 9.04 fl vs 8.65 fl (p-value 0.01). We obtained the same correlations for patients who had at discharge AFib rhythm (68 patients) compared to patients discharged with SR (62 patients) – 9.05 fl vs 8.61 fl (p-value 0.003). During the hospitalization, we registered patients who had SR at admission, but who installed persistent AFib until the moment of discharge. It is already known in the literature that patients with AFib and acute HF have an increased value of MPV and, implicitly, of platelet reactivity. A limited number of studies have shown that paroxysmal episodes of AFib, which are decompensating factors of HF, are associated with increased MPV in those patients, in which case MPV is an independent predictor for hospitalizations.15–17
Regarding rehospitalizations, our study showed that 64 patients were rehospitalized after 6 months (49.2%). The MPV value was 9.14 fl, much higher than in the case of those who were not rehospitalized (8.55 fl), with a statistical significance (p = 0.000). Regarding death at 1 year, our study showed that 20 patients (15.3%) died in the first year of the last admission. The MPV value for this group was significantly higher (9.40 fl, p = 0.006). Furthermore, our study shows that MPV of at least 9.1 fl is encountered in patients with decompensated chronic HF – NYHA class IV HF and acute pulmonary edema (APE) who were rehospitalized at 6 months or died at 1 year, as revealed in Figures 1 and 2.
 |
Figure 1 MPV value in patients with APE (1) and NYHA class IV HF (0), and 6-month rehospitalization. |
 |
Figure 2 MPV value in patients with APE (1) and NYHA class IV HF (0), and 1-year mortality. |
Based on the above-mentioned data, we can state that a high MPV value (>9 fl) is an independent predictor of age, sex, weight, and other laboratory data compared to lower MPV values. However, we cannot exclude the fact that patients with MPV < 9 fl might have a higher survival rate at 1 year or a lower rate of rehospitalizations.
Discussions
The mechanisms by which patients with increased MPV have an evolution towards worsening HF are not clear, but the role of platelets in the pathology of atherothrombosis is well known.18–20 There is a correlation between the size and functionality of platelets, which makes them have a higher prothrombotic potential, therefore platelets with higher volume are more reactive.21–24 By contributing high-volume platelets to the progression of atherosclerotic lesions, and to the appearance of complications in CVD, we show that MPV can be considered a marker of detection in patients with increased risk of developing both complications and a more severe evolution of the CVD.25–27
On the other hand, antiplatelet medication (acetylsalicylic acid, clopidogrel) whose role in maintaining a good control of mortality and morbidity in cardiovascular diseases is proven by studies, has no different effects depending on the value of MPV. Their effects are the same regardless of MPV value. This could be a possible explanation of why patients with increased MPV, even if they are being treated with antiplatelet drugs, have a more severe progression, with more frequent rehospitalizations and a higher death rate.28,29
Regarding the impact of comorbidities on the prognosis of patients with HF, as it is revealed in the medical literature, anemia and renal dysfunction are associated with negative outcomes. While anemia doubles the risk of death in patients with HF, the presence of renal dysfunction further increases the risk of death.30 Data from our research did not indicate a significant correlation between MPV and renal dysfunction or anemia. However, regarding the type of anemia, our study showed that patients with microcytic anemia had statistically increased MPV values compared to those without microcytic anemia. Since microcytic anemia is commonly caused by iron deficiency, which itself is a strong independent predictor of HF outcomes,31 this may indirectly suggest that our studied groups of patients with increased MPV and microcytic anemia are more susceptible to recurrent hospitalization and 1-year death.
Concerning the presence or absence of infections, in our study, we used the value of presepsin alongside biological inflammatory syndrome as the cornerstone for the diagnosis of infections. Infections predispose to the formation of immune complexes and the release of cytokines, which lead to thrombocytopenia, through a compensatory mechanism, thus leading to an increase in MPV. Based on this hypothesis, it can be stated that in patients with infections, increased MPV can be considered a negative prognostic factor. Data from our study showed no significant relationship between the value of presepsin and the value of MPV at admission because, as we commented in the “Results” section, the group of patients for whom the value of presepsin at admission was available, was too small to have a statistical relevance.
NT-proBNP is a marker of decompensation and prognosis of HF. It is known that patients who maintain at discharge an elevated NT-proBNP value above 300 pg/mL (despite a complete and complex treatment), have an unfavorable medium- and long-term prognosis of HF. Our study found a significant correlation between the NT-proBNP value at discharge and the MPV value at admission (Pearson coefficient 0.249). This makes us consider that an increased MPV value at admission may be considered an unfavorable prognostic indicator for patients with decompensated chronic HF.
Conclusion
In conclusion, MPV value >9 fl may be considered in our study as a possible predictor marker for rehospitalization and 1-year mortality for patients with decompensated chronic HF. Certain comorbidities (hypertension, history of myocardial infarction, atrial fibrillation, and microcytic anemia) are elements associated with an increase in MPV in patients with decompensated chronic HF and can be considered elements of a possible “portrait” of these patients at increased risk of rehospitalization and death.
Limitations of the Study
We believe that our study has a few limitations: it was a retrospective study, which has not made it possible for us to establish a nonbiased design for the selection of the patients; the patient groups were not numerous enough, so we could not provide the statistical strength for some of the variables discussed (eg serum creatinine, serum sodium, serum potassium, presepsin); there was not always a uniformity in the collection, transport, and processing of blood samples, which may have influenced the correct determination of MPV.
Ethical Approval and Informed Consent
This study was conducted according to the guidelines of the Declaration of Helsinki, and also our research in the hospital and data extraction from the hospital archive was approved by the Medical and Health Services Manager of “Bagdasar-Arseni” Emergency Hospital. Throughout our study, we did not provoke emotional or physical harm to the patients since our research only implicated data collection and data analysis from the medical sheets or the hospitals’ medical informatics system. Because this was a retrospective study and the waiver of informed consent would not adversely affect the rights and welfare of the subjects, written informed consent from the patients was waived. Patient data confidentiality was protected. Our study was conducted under the specific guidelines of the Ethical Committee of the University of Medicine and Pharmacy “Carol Davila”, Bucharest which states that since this is a non-interventional study that implies data collection from a public database (in this case, the informatics system of the Emergency Hospital “Bagdasar-Arseni”), ethical approval is not required. The Ethical Committee of the University of Medicine and Pharmacy “Carol Davila”, Bucharest requires ethical approval only for interventional studies (experimental procedures/medications that imply humans or animals). Please see the guidelines in the following link: https://umfcd.ro/cercetare-si-dezvoltare/informatii-utile/norme-etice/.
Funding
No funding was received for this work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Chu SG, Becker RC, Berger PB, et al. Mean platelet volume as a predictor of cardiovascular risk: a systematic review and meta-analysis. J Thromb Haemost. 2010;8(1):148–156. doi:10.1111/j.1538-7836.2009.03584.x
2. Greisenegger S, Endler G, Hsieh K, Tentschert S, Mannhalter C, Lalouschek W. Is elevated mean platelet volume associated with a worse outcome in patients with acute ischemic cerebrovascular events? Stroke. 2004;35(7):1688–1691. doi:10.1161/01.STR.0000130512.81212.a2
3. Kaya H, Yıldırımlı MK, Kurt R, Beton O, Yilmaz MB. Mean platelet volume as a predictor of heart failure-related hospitalizations in stable heart failure outpatients with sinus rhythm. Acta Cardiol Sin. 2017;33(3):292–300. doi:10.6515/acs20160930a
4. Slavka G, Perkmann T, Haslacher H, et al. Mean platelet volume may represent a predictive parameter for overall vascular mortality and ischemic heart disease. Arterioscler Thromb Vasc Biol. 2011;31(5):1215–1218. doi:10.1161/ATVBAHA.110.221788
5. Vinereanu D, Ovidiu C, Mircea C, Dan G. Romanian heart failure awareness weeks. Prevalence and incidence of heart failure in Romania. București. 2018. Available from: https://www.escardio.org/static-file/Escardio/Subspecialty/HFA/Advocacy%20and%20Awareness/Awareness%20day/Documents/HFAD%202018/ROMANIA%20HF%202018.pdf.
6. Henning BF, Zidek W, Linder B, Tepel M. Mean platelet volume and coronary heart disease in hemodialysis patients. Kidney Blood Press Res. 2002;25(2):103–108. doi:10.1159/000063516
7. Kim S, Molnar MZ, Fonarow GC, et al. Mean platelet volume and mortality risk in a national incident hemodialysis cohort. Int J Cardiol. 2016;220:862–870. doi:10.1016/j.ijcard.2016.06.074
8. Lancé MD, Sloep M, Henskens YM, Marcus MA. Mean platelet volume as a diagnostic marker for cardiovascular disease: drawbacks of preanalytical conditions and measuring techniques. Clin Appl Thromb Hemost. 2012;18(6):561–568. doi:10.1177/1076029612458147
9. Lano G, Sallée M, Pelletier M, et al. Mean platelet volume predicts vascular access events in hemodialysis patients. J Clin Med. 2019;8(5):608. doi:10.3390/jcm8050608
10. Martin JF, Trowbridge EA, Salmon G, Plumb J. The biological significance of platelet volume: its relationship to bleeding time, platelet thromboxane B2 production and megakaryocyte nuclear DNA concentration. Thromb Res. 1983;32(5):443–460. doi:10.1016/0049-3848(83)90255-4
11. Noris P, Melazzini F, Balduini CL. New roles for mean platelet volume measurement in the clinical practice? Platelets. 2016;27(7):607–612. doi:10.1080/09537104.2016.1224828
12. Victor LS, Selva RM, Anitha P, et al. Increased soluble platelet/endothelial cellular adhesion molecule‐1 and osteonectin levels in patients with severe congestive heart failure. Independence of disease etiology, and antecedent aspirin therapy. Eur J Heart Fail. 1999;1:243–249. doi:10.1016/S1388-9842(99)00029-X
13. Theresa AM, Marco M, Marianna A, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599–3726. doi:10.1093/eurheartj/ehab368
14. Kandis H, Ozhan H, Ordu S, et al. The prognostic value of mean platelet volume in decompensated heart failure. Emerg Med J. 2011;28(7):575–578. doi:10.1136/emj.2009.088401
15. Bayar N, Arslan S, Cagirci G, et al. Usefulness of mean platelet volume for predicting stroke risk in paroxysmal atrial fibrillation patients. Blood Coagul Fibrinolysis. 2015;26(6):669–672. doi:10.1097/MBC.0000000000000334
16. Tekin G, Tekin YK, Sivri N, Yetkin E. Mean platelet volume in patients with nonvalvular atrial fibrillation. Blood Coagul Fibrinolysis. 2013;24(5):537–539. doi:10.1097/MBC.0b013e32835facb3
17. Turgut O, Zorlu A, Kilicli F, et al. Atrial fibrillation is associated with increased mean platelet volume in patients with type 2 diabetes mellitus. Platelets. 2013;24(6):493–497. doi:10.3109/09537104.2012.725876
18. Huczek Z, Kochman J, Filipiak KJ, et al. Mean platelet volume on admission predicts impaired reperfusion and long-term mortality in acute myocardial infarction treated with primary percutaneous coronary intervention. J Am Coll Cardiol. 2005;46(2):284–290. doi:10.1016/j.jacc.2005.03.065
19. Jagroop IA, Mikhailidis DP. The effect of tirofiban on fibrinogen/agonist-induced platelet shape change and aggregation. Clin Appl Thromb Hemost. 2008;14(3):295–302. doi:10.1177/1076029608316014
20. Roy-Chaudhury P, Sukhatme VP, Cheung AK. Hemodialysis vascular access dysfunction: a cellular and molecular viewpoint. JASN. 2006;17(4):1112–1127. doi:10.1681/ASN.2005050615
21. Abeles RD, Mullish BH, Forlano R, et al. Derivation and validation of a cardiovascular risk score for prediction of major acute cardiovascular events in non-alcoholic fatty liver disease; the importance of an elevated mean platelet volume. Aliment Pharmacol Ther. 2019;49(8):1077–1085. doi:10.1111/apt.15192
22. Badrnya S, Schrottmaier WC, Kral JB, et al. Platelets mediate oxidized low-density lipoprotein-induced monocyte extravasation and foam cell formation. Arterioscler Thromb Vasc Biol. 2014;34(3):571–580. doi:10.1161/ATVBAHA.113.302919
23. Braekkan SK, Mathiesen EB, Njølstad I, Wilsgaard T, Størmer J, Hansen JB. Mean platelet volume is a risk factor for venous thromboembolism: the Tromsø Study, Tromsø, Norway. J Thromb Haemost. 2010;8(1):157–162. doi:10.1111/j.1538-7836.2009.03498.x
24. Pikija S, Cvetko D, Hajduk M, Trkulja V. Higher mean platelet volume determined shortly after the symptom onset in acute ischemic stroke patients is associated with a larger infarct volume on CT brain scans and with worse clinical outcome. Clin Neurol Neurosurg. 2009;111(7):568–573. doi:10.1016/j.clineuro.2009.04.002
25. Bal Z, Bal U, Okyay K, et al. Hematological parameters can predict the extent of coronary artery disease in patients with end-stage renal disease. Int Urol Nephrol. 2015;47(10):1719–1725. doi:10.1007/s11255-015-1073-2
26. Klovaite J, Benn M, Yazdanyar S, Nordestgaard BG. High platelet volume and increased risk of myocardial infarction: 39,531 participants from the general population. J Thromb Haemost. 2011;9(1):49–56. doi:10.1111/j.1538-7836.2010.04110.x
27. Thompson CB, Jakubowski JA, Quinn PG, Deykin D, Valeri CR. Platelet size as a determinant of platelet function. J Lab Clin Med. 1983;101(2):205–213.
28. Matsagas M, Jagroop IA, Geroulakos G, Mikhailidis DP. The effect of a loading dose (300 mg) of clopidogrel on platelet function in patients with peripheral arterial disease. Clin Appl Thromb Hemost. 2003;9(2):115–120. doi:10.1177/107602960300900204
29. Yi YH, Yin WJ, Gu ZC, et al. A simple clinical pre-procedure risk model for predicting thrombocytopenia associated with periprocedural use of tirofiban in patients undergoing percutaneous coronary intervention. Front Pharmacol. 2018;9:1456. doi:10.3389/fphar.2018.01456
30. Herzog CA, Muster HA, Li S, Collins AJ. Impact of congestive heart failure, chronic kidney disease, and anemia on survival of medicare population. J Card Fail. 2004;10:467–472. doi:10.1016/j.cardfail.2004.03.003
31. Caterina R, Rosa C, Robersta R, Andrea P, Domenico S. Iron deficiency: a new target for patients with heart failure. Front Cardiovasc Med. 2021;2021:908.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
