Back to Journals » Clinical Ophthalmology » Volume 13
Maximum Medical Therapy: Brinzolamide/Brimonidine And Travoprost/Timolol Fixed-Dose Combinations In Glaucoma And Ocular Hypertension
Authors Lerner SF , Oddone F, Lu DW, Sanseau A , Guarro M, Ridolfi A, Hubatsch D
Received 26 August 2019
Accepted for publication 18 October 2019
Published 5 December 2019 Volume 2019:13 Pages 2411—2419
DOI https://doi.org/10.2147/OPTH.S228777
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
S Fabián Lerner,1 Francesco Oddone,2 Da-Wen Lu,3 Ana Sanseau,4 Merce Guarro,5 Antonia Ridolfi,6 Douglas Hubatsch7
1Consultorio Oftalmológico Dr. Fabian Lerner And Facultad de Ciencias Médicas, Universidad Favaloro, Buenos Aires, Argentina; 2Glaucoma Research Unit, IRCCS – Fondazione Bietti, Rome, Italy; 3Department of Ophthalmology, Tri-Service General Hospital, Taipei, Taiwan, Republic of China; 4Instituto de la Visión, Ciudad de Buenos Aires, Argentina; 5Vallès Oftalmologia Recerca-OMIQ and Ophthalmology Department, Hospital de Granollers, Barcelona, Spain; 6Novartis Pharma S.A.S, Paris, France; 7Novartis Pharmaceutical Corporation, Fort Worth, TX, USA
Correspondence: S Fabián Lerner
Consultorio Oftalmológico Dr. Fabián Lerner, Facultad de Ciencias Médicas, Universidad Favaloro, Marcelo T. De Alvear 2010, 2A, Buenos Aires C1122AAF, Argentina
Tel +54 11 4961-9258
Email [email protected]
Introduction: Maximal medical therapy (MMT) is the use of ≥3 classes of topical anti-glaucoma agents to achieve maximal intraocular pressure (IOP) reduction while minimizing adverse effects and compliance challenges.
Purpose: To evaluate the additive IOP-lowering effect of twice-daily brinzolamide 1%/brimonidine 0.2% fixed-dose combination (BBFC) used adjunctively with once daily travoprost 0.004%/timolol 0.5% fixed-dose combination (TTFC) in patients with open-angle glaucoma (OAG)/ocular hypertension (OHT).
Methods: In this phase IV, double-masked study, patients on TTFC for ≥28 days, aged ≥18 years, with mean IOP ≥19 and ≤28 mmHg in at least 1 eye were randomized to receive BBFC+TTFC (n=67) or vehicle+TTFC (n=67) for 6 weeks. The primary endpoint was mean change in diurnal IOP from baseline (BL, averaged over 09:00 and 11:00) at Week 6.
Results: The study was terminated prematurely due to recruitment challenges. BL mean IOP was similar in both groups (BBFC+TTFC: 21.6±1.78 mmHg; vehicle+TTFC: 21.8±1.90 mmHg). Mean change in diurnal IOP from BL at Week 6 was greater with BBFC+TTFC (−4.25 mmHg, 95% confidence interval [CI]: −4.7, −3.8) than with vehicle+TTFC (−2.11 mmHg, 95% CI: −2.6, −1.6, treatment difference, −2.15 mmHg (95% CI: −2.8, −1.5; P<0.001). Ocular adverse events (AEs) were reported in 11.9% of patients given BBFC+TTFC and 7.5% of patients given vehicle+TTFC. The AE with highest frequency was punctate keratitis (3%) in the BBFC+TTFC group; eye irritation (3%) in the vehicle+TTFC group.
Conclusion: BBFC+TTFC as MMT demonstrated clinically relevant and statistically significant reductions in mean diurnal IOP in patients with OAG/OHT. AEs were consistent with known safety profiles of individual medications.
Keywords: brinzolamide/brimonidine fixed-dose combination, travoprost/timolol fixed-dose combination, open-angle glaucoma, ocular hypertension, IOP, maximal medical therapy
Introduction
Reduction of intraocular pressure (IOP) can delay the glaucomatous progression and visual field deterioration in patients with open-angle glaucoma (OAG) or ocular hypertension (OHT).1–3 European Glaucoma Society (EGS) treatment guidelines recommend a stepwise approach for IOP reduction with topical drugs, laser or surgical treatment based on disease progression and patient compliance.4 Prostaglandin analogs (PGAs) are the most effective topical ocular hypotensive agents and are recommended as the first-line monotherapy for lowering IOP.5–7
However, when monotherapy is insufficient, combination therapy with multiple IOP medications is warranted for successful management of OAG or OHT.4,7 Although the use of multiple medications is effective in lowering IOP, there is often decreased patient adherence to treatment. Fixed-dose combination (FDC) of ocular hypotensive agents is advantageous as it reduces the number of drops, washout effects, and exposure to preservatives, and increases patient compliance.8–11 Some patients fail to achieve or maintain the target IOP despite treatment with 2 or more medications, and thus require additional anti-glaucoma medications or surgical intervention.4
Maximal medical therapy (MMT) is the use of 3 or more different classes of anti-glaucoma agents to achieve a maximal lowering of IOP.10,12 MMT attempts to achieve the best possible therapeutic outcome with medications while minimizing adverse effects and compliance challenges to the patients. Availability of FDCs helps to achieve MMT with different classes of medications but with a simplified instillation regimen.10–12 Thus, MMT can serve as a convenient medical treatment option for patients requiring additional lowering of IOP.
Travoprost 40 μg/mL (0.004%)/timolol 5 mg/mL (0.5%) FDC (TTFC; DuoTrav ®, Novartis Pharma AG, Basel, Switzerland) is approved as a once daily regimen for the decrease of IOP in patients with OAG or OHT who are insufficiently responsive to topical ß-blockers or PGAs.13 The efficacy and safety of TTFC in patients diagnosed with OAG or OHT has been previously established in well-controlled clinical studies.14–16 Brinzolamide 10 mg (1%)/mL/brimonidine 2 mg/mL (0.2%) FDC (BBFC; Simbrinza®, Novartis Pharma AG, Basel, Switzerland) is approved in the EU as a twice-daily regimen for decreasing IOP in patients with OAG or OHT for whom monotherapy is insufficient.17 Use of BBFC has been effective as a twice- daily regimen in lowering IOP in patients with OAG or OHT inadequately controlled with PGA monotherapy.18,19
A combination therapy with BBFC+TTFC can provide clinicians with the option of managing glaucoma with all 4 classes of medications (PGAs, ß-blockers, carbonic anhydrase inhibitors, and α-agonists) in patients with OAG or OHT who are not contraindicated against the use of ß-blockers and in whom the IOP-lowering is inadequate with TTFC alone. This combination offers clinicians a simplified solution with 2 bottles of eye drops and instillation of 3 drops per day. This is the first study investigating the additive IOP-lowering effect achieved when BBFC, dosed twice daily, is used adjunctively with once-daily TTFC in patients with OAG or OHT inadequately controlled with TTFC.
Materials And Methods
Study Design
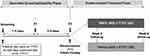
This 6-week, Phase IV, multicenter, parallel-group, double-masked, two-arm randomized study (Clinical trial identifier: NCT02730871) was conducted across 35 sites (Supplementary information S1) in 14 countries between June 2016 and July 2018.
The study consisted of 2 sequential phases (an open-label screening/eligibility phase and a double-masked treatment phase) involving 5 visits. During the screening phase, patients who were on TTFC for ≥28 days continued receiving study TTFC (1 drop, once daily) at 21:00±30 min (if the patient was dosed with TTFC in the evenings prior to screening) or at 09:00±30 min on the following day (if the patient was dosed with TTFC in the mornings prior to screening). It has been demonstrated that there is no significant difference in IOP-lowering efficacy of TTFC between the morning and the evening dosing.20,21 Following the screening phase, 2 eligibility visits were scheduled 3–5 days apart.
Eligible patients were randomized (1:1) using interactive response technology to receive twice-daily BBFC or vehicle (09:00 & 21:00) as an adjunct to TTFC (Figure 1). When both medications were administered, patients waited for 15 min after TTFC instillation before instillation of the masked drug. Both BBFC and vehicle were supplied in identical opaque DROP-TAINER® bottles with masked labels indicating that the product was for investigational use only, and were identified by kit and protocol number. One eye from each patient was chosen as the study eye (Supplementary information S2). Patients had 2 assessment visits at Weeks 2 and 6.
Ethics approval and informed consent
The trial was conducted in accordance with the principles of the Declaration of Helsinki, and in compliance with the International Conference on Harmonization E6, Good Clinical Practice Consolidated Guideline and other regulations as applicable. The trial protocol and all its amendments were approved by an Independent Ethics Committee/Institutional Review Board. All patients provided written informed consent before trial initiation.
Patient Population
Eligible patients aged ≥18 years, diagnosed with OAG or OHT, and on treatment with TTFC for ≥28 days prior to screening were included if they may benefit from further IOP-lowering in the opinion of the investigator. Patients had a mean IOP of ≥19 and ≤28 mmHg at 09:00 on both eligibility visits in at least 1 eye (the same eye[s]) while on TTFC. The mean IOP criteria was ≥21 and ≤28 mmHg at the start of the study. However, this criterion was amended to ≥19 and ≤28 mmHg due to challenges in recruitment. Key exclusion criteria are added as Supplementary information S3.
Study Endpoints
The primary efficacy endpoint was the mean change from baseline (BL) in diurnal IOP at Week 6 (averaged over the 09:00 and 11:00 time points; a 12-h trough and a 2-h peak, respectively). The secondary endpoints were (1) Mean diurnal IOP (averaged over the 09:00 and 11:00 time points) at Week 6; (2) Mean percent change from BL in diurnal IOP (IOP at BL averaged over the 09:00 and 11:00 time points) at Week 6; (3) Mean change and mean percentage change from BL in IOP at 11:00 at Week 6; (4) Mean change and mean percentage change from BL in IOP at 09:00 at Week 6.
Exploratory endpoints included (1) The percentage of patients achieving diurnal IOP targets (i.e., ≤12, ≤13, ≤14 … ≤25 mmHg) at Week 6; (2) Ocular perfusion pressure (OPP) at Week 6 (OPP was calculated at each time point as 2/3* [diastolic blood pressure +1/3 (systolic blood pressure − diastolic blood pressure) − IOP] and was averaged across the time points.
Safety variables included occurrence and characteristics of adverse events (AEs), fundus parameters, best-corrected visual acuity (BCVA), slit-lamp examination, blood pressure, pulse rate, and automated perimetry. Details on assessment of IOP, BCVA, achromatic automated perimetry, dilated fundus examination, and slit-lamp biomicroscopy examination are included as Supplementary information S4.
Statistical Analysis
Sample Size
One hundred and eight evaluable patients per treatment group were required in the primary efficacy analysis, to yield at least 80% power to detect a 1.5 mmHg difference in mean change from BL in IOP at Week 6 between the treatment groups. However, the required sample size to attain the same power was 28 patients per arm if the difference was 3.0 mmHg between treatment groups. Both calculations were based on the assumption of a common standard deviation (SD) for mean IOP of 3.9 mmHg and the use of a two-sample two-sided t-test performed at the α=0.05 level of significance. Assuming a dropout rate of 10%, ~120 patients per treatment group were expected to be enrolled to ensure the required number of evaluable patients in the final efficacy analysis.
Statistical Method
Primary, secondary, and exploratory efficacy analyses were based on the full analysis set (FAS) which included all randomized patients with a BL assessment and who completed at least 1 scheduled on-therapy study visit. The treatment difference (TD) in mean IOP change from BL was examined based upon the least squares (LS) means derived from an analysis of the covariance model. The model had an IOP change from BL as a response variable and included fixed effect terms for BL and treatment. The BL was the average of the 09:00 and the 11:00 IOP measurements at the 2 eligibility visits. Estimates of the difference in mean change from BL in IOP between the BBFC+TTFC and vehicle+TTFC groups, and associated two-sided 95% confidence interval (CI) were also presented.
A closed step-down testing procedure was used for hypothesis testing of primary and secondary endpoints; therefore, no multiplicity adjustment was needed. Following the rejection of the primary efficacy null hypothesis, each secondary hypothesis was tested in a pre-defined order. Significance for a comparison was claimed only if the null hypothesis was rejected (P<0.05) for the previous endpoint in the series. IOP analyses at Week 6 and percentage change from BL in IOP at week 6 used the same statistical methods employed for the primary endpoint. Analyses of IOP change and percent IOP change at each Week 6 time point was based on a mixed model repeated measures with fixed effects of treatment, time point, and the interaction of treatment and time point, with the BL 09:00 IOP as a covariate. Safety results were summarized descriptively for the safety set, which included all patients who received at least 1 dose of study medication.
Results
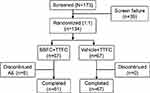
The study was terminated prematurely when only ~56% of the planned patients were enrolled due to recruitment challenges. In total, 173 patients were screened of whom 134 (77.5%) were randomized to BBFC+TTFC (n=67) or vehicle+TTFC (n=67). In all, 128 (95.5%) patients completed the study. Six patients discontinued the study in the BBFC+TTFC group due to an AE. There were no discontinuations in the vehicle+TTFC group (Figure 2). The FAS and safety set included 134 patients. Demographics and BL characteristics were balanced between both treatment groups although there were more female patients in the vehicle+TTFC than the BBFC+TTFC group (Table 1). The mean±SD age of patients was 65.7±12.60 years; 54.5% of patients were female and 79.9% patients were White. Primary OAG was diagnosed in 75.4% patients and 17.9% patients had OHT. The mean IOP at BL was similar in BBFC+TTFC (21.6±1.78 mmHg) and vehicle+TTFC (21.8±1.90 mmHg) groups. The mean OPP (SD) at BL was similar between the 2 groups (BBFC+TTFC 49.9±6.0 mmHg; vehicle+TTFC 50.0±7.3 mmHg).
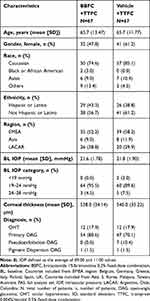 |
Table 1 Demographics And Baseline Characteristics (Full Analysis Set) |
Efficacy Outcomes
The study met its primary objective. The mean change in diurnal IOP from BL at Week 6 was significantly greater with BBFC+TTFC (−4.25 mmHg, 95% CI: −4.7, −3.8) than with vehicle+TTFC (−2.11 mmHg, 95% CI: −2.6, −1.6) with a TD of −2.15 mmHg (95% CI: −2.8, −1.5; P<0.001, Figure 3).
The mean diurnal IOP (averaged over the 09:00 and 11:00 time points) at Week 6 was 17.42 mmHg (95% CI: 16.9, 17.9) with BBFC+TTFC and 19.57 mmHg (95% CI: 19.1, 20.0) with vehicle+TTFC; the TD was statistically significant (−2.15 mmHg; 95% CI: −2.8, −1.5; P<0.001, Figure 4). The mean percent change in diurnal IOP from BL at Week 6 (averaged over the 09:00 and 11:00 time points) was greater in the BBFC+TTFC than vehicle+TTFC group with a TD of −9.98% (95% CI: −13.1, −6.9; P< 0.001) (Supplementary figure SF1).
Similarly, at Week 6, BBFC+TTFC showed a greater mean change and mean percent change in IOP than vehicle+TTFC, at the 11:00 (peak) and 09:00 (trough) efficacy time points (both P<0.001; Supplementary figure SF2A and B).
The target IOP of ≤18 mmHg at Week 6 was achieved by 62.7% of patients with BBFC+TTFC versus 35.8% of patients with vehicle+TTFC. The proportion of patients achieving an IOP target of ≤13, ≤14, ≤15, ≤16, ≤17, ≤19, ≤20, and ≤21 mmHg were also higher with BBFC+TTFC than with vehicle+TTFC (Supplementary figure SF3).
The mean change from BL at Week 6 in OPP was 2.6±4.68 mmHg with BBFC+TTFC and 2.1±5.29 mmHg with vehicle+TTFC (Supplementary table ST1). The mean change from BL (SD) at Week 6 in OPP at 11:00 was similar between the 2 groups (BBFC+TTFC 1.9±5.68 mmHg; vehicle+TTFC 1.3±5.87 mmHg). The corresponding mean percentage changes were: 4.4%±12.28 and 3.2%±11.70, respectively. At the 09:00 time point, the mean change from BL in OPP was 2.6±5.17 mmHg in the BBFC+TTFC group and 1.9±6.17 mmHg in the vehicle+TTFC group; the corresponding mean percentage change from BL±SD was 5.5±10.20% and 4.2±11.37%, respectively (Supplementary table ST1).
In a subset of patients, the descriptive mean IOP change from BL at the 15:00 time point was reported as a sensitivity analysis. Consistent with the findings of the predefined endpoints, the mean change in IOP from BL at 15:00 was higher with BBFC+TTFC (−5.2±3.41 mmHg, [n=20],) versus vehicle+TTFC (−1.9±2.88 mmHg [n=22]) at Week 6 (Supplementary table ST2).
Safety Outcomes
Overall, 23.9% of patients in the BBFC+TTFC group and 14.9% of patients in the vehicle+TTFC group experienced at least 1 AE (Table 2). Treatment-related AEs were more frequent in the BBFC+TTFC (14.9%) than the vehicle+TTFC group (1.5%, Supplementary table ST3). The most common ocular AE was punctate keratitis (3.0%) in the BBFC+TTFC group and eye irritation (3.0%) in the vehicle+TTFC group. The most common non-ocular AE was asthenia (3%) in the BBFC+TTFC group (Table 2). One serious AE (dysuria) was reported in the vehicle+TTFC group; this event was reported by the investigator as not related to the study drug. Six (9%) patients in the BBFC+TTFC group experienced 1 of the following AEs each that led to their discontinuation from the study: allergic conjunctivitis, eye pruritus, punctate keratitis, drug hypersensitivity, dizziness, or dyspnea. No deaths were reported during the study. There were no differences observed between treatments for changes in vital signs, BCVA, perimetry, slit-lamp biomicroscopy, or fundus examination (Supplementary information S5).
 |
Table 2 Adverse Events (Safety Set) |
Discussion
The study results demonstrated that use of BBFC, dosed twice daily for 6 weeks, as an adjunct to TTFC, dosed once daily, provided clinically meaningful IOP reduction in patients with OAG or OHT inadequately controlled on TTFC.
Previous studies have established the individual efficacy of BBFC and TTFC in lowering IOP in patients with OAG or OHT.22–27 It has been demonstrated that adding a third drug (brinzolamide) to TTFC resulted in a 6–9% additional reduction in IOP without major safety concerns.28 Also, addition of BBFC to a PGA has resulted in a combined greater IOP reduction.18,19 This is the first study evaluating the IOP-lowering effect of the BBFC added to TTFC as MMT.
In this study, the mean reduction in IOP from BL at Week 6 was significantly greater (P<0.001) with BBFC+TTFC (4.25 mmHg) than with vehicle+TTFC (2.11 mmHg). Patients treated with BBFC+TTFC adjunctive therapy had an additional >2 mmHg IOP-lowering than those treated with TTFC alone. The reduction with BBFC+TTFC was also greater compared with a triple combination therapy (brinzolamide twice daily+TTFC once daily) reported in an earlier study where patients with OAG showed a mean diurnal IOP reduction of 2.8 mmHg.28 Thus, BBFC+TTFC provides a suitable option for optimal management of patients on TTFC who require further IOP-lowering.
The Early Manifest Glaucoma Trial has demonstrated that the risk of disease progression decreases by 10% with each 1 mmHg decrease in mean IOP.29 Further, an IOP of <18mmHg prevents the progression of disease and visual field deterioration according to the Advanced Glaucoma Intervention Study.2 In this study, the mean IOP reduction with BBFC+TTFC was 4.25 mmHg and >60% of patients in this group achieved the target IOP of ≤18mmHg, indicating that BBFC+TTFC has the potential to reduce disease progression.
In addition, BBFC+TTFC showed a significant reduction in IOP at both the 11:00 (peak) and 09:00 (trough) time points. This is similar to the findings of previous studies in which BBFC dosed thrice daily was combined with PGA or travoprost once daily.18,19
Fluctuations in OPP is an important risk factor for the progression of OAG.30–32 In this study, BBFC+TTFC showed no significant change in OPP from BL after 6 weeks of treatment, similar to a previous study in which BBFC did not adversely affect OPP after 4 weeks of treatment.33
Safety is a concern when multiple medications are used. Occurrence of AEs negatively affects patient compliance. In this study, AEs were consistent with the known safety profiles of the individual drugs; no new AEs were reported. The relatively higher incidence of AEs in the BBFC+TTFC group compared with the vehicle+TTFC group can be attributed to the increased number of medications used.
Patient adherence is important to achieve optimal treatment response in glaucoma.34 However, use of multiple IOP-lowering agents administered individually to patients can reduce adherence to treatment.35 In this study, BBFC+TTFC showed clinically relevant IOP reduction with only 2 bottles and administration of 3 drops of medication per day. Thus, the results of this study demonstrate that MMT with 2 FDCs containing 4 drug classes not only helps in further IOP-lowering in glaucoma patients but also offers a simplified dosing regimen to increase patient comfort and compliance.
This study was conducted in multiple countries and is the first to evaluate the efficacy of BBFC+TTFC as MMT in lowering IOP. This study was terminated prematurely and recruited fewer (56%) patients than planned. However, the results of the study are statistically significant. The recruitment of patients in this study was a challenge due to varying practice patterns. There is no single treatment pathway to TTFC and even in those patients treated with TTFC, not all patients were suitable for an add-on treatment with BBFC.
One limitation is that it was a short-term study, which did not allow for long-term assessment of efficacy and safety parameters of the study medications. However, pivotal studies have reported the efficacy and safety of BBFC and its individual components (dosed twice daily) for up to 6 months.26,27 Another limitation is that as BBFC was used as an adjunct to TTFC, the efficacy of the individual components (BBFC or TTFC) in patients with OAG or OHT could not be evaluated. Twenty-four hour monitoring of IOP would have helped in a better understanding of treatment response.
Conclusions
MMT with BBFC+TTFC demonstrated a clinically relevant and statistically significant reduction in mean diurnal IOP in patients with OAG or OHT. The AEs with BBFC+TTFC were consistent with the known safety profile of the individual medications. Addition of BBFC to TTFC may provide an effective treatment option for patients requiring additional IOP reduction beyond that achieved with TTFC alone.
Data Sharing Statement
The datasets generated during the current study are not publicly available due to ethical considerations but are available from the corresponding author on reasonable request.
Acknowledgments
The medical writing support and editorial assistance during the development of the manuscript was provided by Indumathy Pinnamaneni and Swati Bhandari (Novartis Healthcare Pvt. Ltd., Hyderabad, India). Dr Oddone’s work for this study was partly supported by the Italian Ministry of Health and by Fondazione Roma. The study was supported by Novartis Pharma AG, Basel and is registered with ClinicalTrials.gov as NCT02730871. The results of this study have been presented at: Lu et al Poster presented at the 34th Asia-Pacific Academy of Ophthalmology Congress, Bangkok, Thailand (6–9 March, 2019) and Lerner et al Poster presented at the 8th World Glaucoma Congress, Melbourne, Australia (27–30 March, 2019).
Disclosure
Fabian Lerner reports grants/research support/speaker fees from Allergan, Glaukos, Iridex, Novartis/Alcon. Francesco Oddone reports personal fees from Allergan, Centervue, Glaucoom and Omikron Italia, honoraria from Centervue, Glaucoom Omikron Italia and Santen, and unrestricted grants from Allergan and Omikron Italia. Ana Sanseau reports Honoraria and Consultation fees from Novartis, Alcon, Poen, Baush & Lomb, Speaker’s bureau: Novartis. Merce Guarro reports grants/research support from Novartis, Alcon, and Santen. Antonia Ridolfi and Doug Hubatsch are both employees of Novartis. The authors report no other conflicts of interest in this work.
References
1. Maier PC, Funk J, Schwarzer G, Antes G, Falck-Ytter YT. Treatment of ocular hypertension and open angle glaucoma: meta-analysis of randomised controlled trials. BMJ. 2005;331(7509):134. doi:10.1136/bmj.38506.594977.E0
2. AGISInvestigators T. The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration.The AGIS investigators. Am J Ophthalmol. 2000;130(4):429–440.
3. Ernest PJ, Schouten JS, Beckers HJ, Hendrikse F, Prins MH, Webers CA. An evidence-based review of prognostic factors for glaucomatous visual field progression. Ophthalmology. 2013;120(3):512–519.
4. EGS. European glaucoma society terminology and guidelines for glaucoma, 4th Edition - Chapter 3: treatment principles and options supported by the egs foundation: part 1: foreword; introduction; glossary; chapter 3 treatment principles and options. Br J Ophthalmol. 2017;101(6):130–195.
5. Law SK. First-line treatment for elevated intraocular pressure (IOP) associated with open-angle glaucoma or ocular hypertension: focus on bimatoprost. Clin Ophthalmol. 2007;1(3):225–232.
6. Eyawo O, Nachega J, Lefebvre P, et al. Efficacy and safety of prostaglandin analogues in patients with predominantly primary open-angle glaucoma or ocular hypertension: a meta-analysis. Clin Ophthalmol. 2009;3:447–456.
7. Li F, Huang W, Zhang X. Efficacy and safety of different regimens for primary open-angle glaucoma or ocular hypertension: a systematic review and network meta-analysis. Acta Ophthalmol. 2018;96(3):e277–e284.
8. Khouri AS, Realini T, Fechtner RD. Use of fixed-dose combination drugs for the treatment of glaucoma. Drugs Aging. 2007;24(12):1007–1016.
9. Crawley L, Zamir SM, Cordeiro MF, Guo L. Clinical options for the reduction of elevated intraocular pressure. Ophthalmol Eye Dis. 2012;4:43–64.
10. Fechtner RD, Singh K. Maximal glaucoma therapy. J Glaucoma. 2001;10(5 Suppl 1):S73–75. doi:10.1097/00061198-200110001-00026
11. Fechtner RD, Realini T. Fixed combinations of topical glaucoma medications. Curr Opin Ophthalmol. 2004;15(2):132–135. doi:10.1097/00055735-200404000-00013
12. Zimmerman TJ, Fechtner RD. Maximal medical therapy for glaucoma. Arch Ophthalmol. 1997;115(12):1579–1580. doi:10.1001/archopht.1997.01100160749014
13. EMA. <duotrav-epar-product-information_en.pd.pdf>. 2019. Available from: https://wwwemaeuropaeu/en/documents/product-information/duotrav-epar-product-information_enpdf.
14. Jordao ML, Hatanaka M, Ogundele A, de Moraes Silva MR, Vessani RM. Safety and efficacy of fixed-combination travoprost/timolol in patients with open-angle glaucoma or ocular hypertension not controlled with timolol monotherapy. Clin Ophthalmol. 2014;8:1527–1534. doi:10.2147/OPTH
15. Schnober D, Hubatsch DA, Scherzer ML. Efficacy and safety of fixed-combination travoprost 0.004%/timolol 0.5% in patients transitioning from bimatoprost 0.03%/timolol 0.5% combination therapy. Clin Ophthalmol. 2015;9:825–832. doi:10.2147/OPTH.S80880
16. Nakano T, Mizoue S, Fuse N, Iwase A, Matsumoto S, Yoshikawa K. Fixed combination of travoprost and timolol maleate reduces intraocular pressure in Japanese patients with primary open-angle glaucoma or ocular hypertension: a prospective multicenter open-label study. Adv Ther. 2015;32(9):823–837. doi:10.1007/s12325-015-0246-9
17. Simbrinza EMA Summary of Product Characteristics. 2019. Available from: https://wwwemaeuropaeu/documents/product-information/simbrinza-epar-product-information_enpdf.
18. Feldman RM, Katz G, McMenemy M, Hubatsch DA, Realini T. A randomized trial of fixed-dose combination brinzolamide 1%/brimonidine 0.2% as adjunctive therapy to travoprost 0.004. Am J Ophthalmol. 2016;165:188–197. doi:10.1016/j.ajo.2016.02.026
19. Fechtner RD, Myers JS, Hubatsch DA, Budenz DL, DuBiner HB. Ocular hypotensive effect of fixed-combination brinzolamide/brimonidine adjunctive to a prostaglandin analog: a randomized clinical trial. Eye (Lond). 2016;30(10):1343–1350. doi:10.1038/eye.2016.126
20. Denis F, Andrew R, Wells D, Friren B. A comparison of morning and evening instillation of a combination travoprost 0.004%/timolol 0.5% ophthalmic solution. Eur J Ophthalmol. 2006;16(3):407–415. doi:10.1177/112067210601600308
21. Suic SP, Laus KN, Dosen VM, Ekert M, Mandic Z, Bojic L. Comparison of evening and morning dosing of travoprost 0.004%/timolol 0.5% fixed combination in 6 month period. Coll Antropol. 2010;34(3):847–852.
22. Pfeiffer N, Scherzer ML, Maier H, et al. Safety and efficacy of changing to the travoprost/timolol maleate fixed combination (DuoTrav) from prior mono- or adjunctive therapy. Clin Ophthalmol. 2010;4:459–466. doi:10.2147/opth.s10694
23. Costa VP, Moreira H, Paolera MD. de Moraes Silva MR. Efficacy and safety of travoprost 0.004%/timolol 0.5% fixed combination as transition therapy in patients previously on prostaglandin analog monotherapy. Clin Ophthalmol. 2012;6:699–706. doi:10.2147/OPTH.S30717
24. Lerner SF, Park KH, Hubatsch DA, Erichev V, Paczka JA, Roberts TV. Efficacy and tolerability of travoprost 0.004%/timolol 0.5% fixed-dose combination for the treatment of primary open-angle glaucoma or ocular hypertension inadequately controlled with beta-blocker monotherapy. J Ophthalmol. 2017;2017:1917570.
25. Whitson JT, Realini T, Nguyen QH, McMenemy MG, Goode SM. Six-month results from a Phase III randomized trial of fixed-combination brinzolamide 1% + brimonidine 0.2% versus brinzolamide or brimonidine monotherapy in glaucoma or ocular hypertension. Clin Ophthalmol. 2013;7:1053–1060. doi:10.2147/OPTH.S46881
26. Aung T, Laganovska G, Hernandez Paredes TJ, Branch JD, Tsorbatzoglou A, Goldberg I. Twice-daily brinzolamide/brimonidine fixed combination versus brinzolamide or brimonidine in open-angle glaucoma or ocular hypertension. Ophthalmology. 2014;121(12):2348–2355. doi:10.1016/j.ophtha.2014.06.022
27. Gandolfi SA, Lim J, Sanseau AC, Parra Restrepo JC, Hamacher T. Randomized trial of brinzolamide/brimonidine versus brinzolamide plus brimonidine for open-angle glaucoma or ocular hypertension. Adv Ther. 2014;31(12):1213–1227.
28. Goldberg I, JG C, Jasek MC, JA S, WC S; Group ASI. Intraocular pressure-lowering efficacy of brinzolamide when added to travoprost/timolol fixed combination as adjunctive therapy. J Glaucoma. 2012;21(1):55–59.
29. Heijl A, Leske MC, Bengtsson B, et al. Reduction of intraocular pressure and glaucoma progression: results from the early manifest glaucoma trial. Arch Ophthalmol. 2002;120(10):1268–1279.
30. Klein BE, Klein R, Knudtson MD. Intraocular pressure and systemic blood pressure: longitudinal perspective: the Beaver Dam Eye Study. Br J Ophthalmol. 2005;89(3):284–287.
31. Hulsman CA, Vingerling JR, Hofman A, Witteman JC, de Jong PT. Blood pressure, arterial stiffness, and open-angle glaucoma: the Rotterdam study. Arch Ophthalmol. 2007;125(6):805–812.
32. Leske MC, Wu SY, Hennis A, Honkanen R, Nemesure B; Group BES. Risk factors for incident open-angle glaucoma: the Barbados Eye Studies. Ophthalmology. 2008;115(1):85–93.
33. Seibold LK, DeWitt PE, Kroehl ME, Kahook MY. The 24 hr effects of brinzolamide/brimonidine fixed combination and timolol on intraocular pressure and ocular perfusion pressure. J Ocul Pharmacol Ther. 2017;33(3):161–169.
34. Atey TM, Shibeshi W, SW A. The impact of adherence and instillation proficiency of topical glaucoma medications on intraocular pressure. J Ophthalmol. 2017;2017:1683430.
35. Barnebey HS, Robin AL. Adherence to fixed-combination versus unfixed travoprost 0.004%/timolol 0.5% for glaucoma or ocular hypertension: a randomized trial. Am J Ophthalmol. 2017;176:61–69.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.