Back to Journals » Integrated Blood Pressure Control » Volume 13
Matrix Metalloproteinases and Hypertension-Mediated Organ Damage: Current Insights
Authors Bisogni V, Cerasari A, Pucci G , Vaudo G
Received 22 July 2020
Accepted for publication 12 September 2020
Published 2 November 2020 Volume 2020:13 Pages 157—169
DOI https://doi.org/10.2147/IBPC.S223341
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Turgay Celik
Valeria Bisogni,1,* Alberto Cerasari,1,2,* Giacomo Pucci,1,2 Gaetano Vaudo1,2
1Unit of Internal Medicine, Terni University Hospital, Terni, Italy; 2Department of Medicine, University of Perugia, Perugia, Italy
*These authors contributed equally to this work
Correspondence: Valeria Bisogni
Unit of Internal Medicine, Terni University Hospital, Via Tristano di Joannuccio, 1, Terni, TR 05100, Italy
Tel +39 0744 205328
Email [email protected]
Abstract: Matrix metalloproteinases (MMPs) are important extracellular enzymes involved in many physiological and pathological processes. Changes in the activity and concentration of specific MMPs, as well as the unbalance with their inhibitors (tissue inhibitors of metalloproteinases – TIMPs), have been described as a part of the pathogenic cascade promoted by arterial hypertension. MMPs are able to degrade various protein substrates in the extracellular matrix, to influence endothelial cells function, vascular smooth muscle cells migration, proliferation and contraction, and to stimulate cardiomyocytes changes. All these processes can be activated by chronically elevated blood pressure values. Animal and human studies demonstrated the key function of MMPs in the pathogenesis of hypertension-mediated vascular, cardiac, and renal damage, besides age and blood pressure values. Thus, the role of MMPs as biomarkers of hypertension-mediated organ damage and potential pharmacological treatment targets to prevent further cardiovascular and renal complications in hypertensive population is increasingly supported. In this review, we aimed to describe the main scientific evidence about the behavior of MMPs in the development of vascular, cardiac, and renal damage in hypertensive patients.
Keywords: matrix metalloproteinases, arterial hypertension, hypertension-mediated organ damage
Introduction
Discovered in the early 1960s1 matrix metalloproteinases (MMPs) are a large family of zinc-dependent endopeptidases produced by multiple tissues and cells (ie, fibroblasts, osteoblasts, endothelial cells, vascular smooth muscle cells – VSMCs, macrophages, neutrophils, and lymphocytes), which are initially synthesized as inactive zymogens with a pro-peptide domain that must be removed before the enzymatic activation. According to the structural and substrate specificity, MMPs were originally classified into six main groups,2 as schematically described in Table 1 .
 |
Table 1 Family Members of MMPs, Their Tissue Distribution, and Main Substrates3 |
MMPs and their inhibitors (tissue inhibitors of metalloproteinases – TIMPs, which inhibits the proteolytic action of MMPs) play a key role in several physiological processes, including activation of immune cells, cells proliferation, migration, and differentiation, remodelling of extracellular matrix (ECM), and tissue invasion and vascularization.3 Notwithstanding, changes in MMPs and TIMPs expression and/or activity have also been described in many inflammatory and cardiovascular diseases (CVD).4
Among the multiple risk factors for CVD, high blood pressure (BP) is associated with the strongest evidence for causation, and it has a high prevalence of exposure.5 Systemic arterial hypertension (HT) is indeed the major cause of cardiovascular remodelling, including myocardial ventricular hypertrophy and fibrosis, chronic kidney disease (CKD) and end-stage renal disease (ESRD), intimal-medial thickening, calcification, and fibrosis of vascular wall with increased arterial stiffness.6 The gradual and chronic target organs remodelling in hypertensive subjects is mediated by different pathogenic mechanisms (ie, oxidative stress, endothelial and platelet activation, sympathetic nervous system and renin-angiotensin-aldosterone system overactivity),7 and included changes in the ECM structure.8 In this context, MMPs are activated by many factors induced by chronically elevated BP values, such as proinflammatory signaling molecules (cytokines, interleukins), growth factors, vasoactive agents (angiotensin II, endothelin-1, aldosterone), and reactive oxygen species (ROS), and are likely to be involved in the development of hypertension-mediated organ damage (HMOD) through the interaction with the molecular structure of ECM.9,10 Moreover, increased MMPs activity has also been associated with intracellular effects in cardiomyocytes and VSMCs, altering the smooth muscle tone.11
In the last years, several clinical12–14 and experimental studies15–17 highlighted the role of altered activity and/or concentration of specific MMPs and their inhibitors in the pathogenesis of HMOD. They are proposed as reliable biomarkers and potential pharmacological targets to treat or prevent many cardiovascular and renal complications in patients affected by HT.
Currently, analytical methods for the determination of MMPs in biological samples (plasma, serum, synovial fluid, urine) are multiple: enzyme-linked immunosorbent assays (ELISA) (including multiplexed ELISAs in the form of antibody arrays), zymography, the use of active-site probes followed by enzymatic digestion of the captured MMPs, and liquid chromatography-mass spectroscopy analysis of the digested MMPs,18 each of them showing advantages and disadvantages.19 The standardization of these methods, including sample collection and storage (eg, at −80°C for human samples), is a complex issue and remains crucial to avoid confounding results, but this topic is beyond the scope of this overview. For in-depth information, the readers are referred to more comprehensive researches.18–20
Our aim is to present an overview of the current knowledge about the role of MMPs in the development of vascular and cardiac remodelling, and renal damage in humans affected by HT. We performed a literature search using the MEDLINE database to identify human studies reporting the analysis of MMPs concentrations and/or activity in hypertensive patients with diagnosis of HMOD. The inclusion criteria were: (i) human researches, (ii) English language, (iii) full-length retrievable papers, (iv) since this is a narrative review, we also included the most recent-published systematic reviews to observational, cross-sectional or longitudinal studies, and randomized clinical trials. The following keywords were utilized in the search: “matrix metalloproteinases” AND “hypertension” OR “systemic arterial hypertension” AND “hypertension-mediated organ damage” AND “vascular remodelling” OR “arterial stiffness” OR “cardiac remodelling” OR “left ventricular hypertrophy” OR “diastolic dysfunction” OR “hypertensive-mediated renal impairment” OR “microalbuminuria” OR “chronic kidney disease”.
MMPs and Systemic Arterial Hypertension
Systemic HT is a multifactorial disorder resulting from alterations in the renal, neuronal, and vascular control mechanisms of BP.21 The pathogenesis of development and maintenance of high BP values involves multiple systems (eg, increasing levels of vasoactive peptides as plasma catecholamines, angiotensin II and aldosterone, growth factors, inflammation, oxidative stress, endothelial dysfunction), and the role of altered levels of some MMPs and their inhibitors has also been proposed.13,22 Changes in MMPs/TIMPs activity and/or concentrations are promoted by persistently elevated BP levels through the activation of the abovementioned systems. This phenomenon is likely to create a vicious circle, in which changed MMPs are responsible for modification of ECM, VSMCs, and cardiomyocytes, developing organs damage and maintaining HT.
Amongst the large family of MMPs, MMP-2 (or Gelatinase-A), which is constitutively expressed in the heart and vessel wall, and MMP-9 (or Gelatinase-B), an inducible protease whose presence in tissues is associated with the infiltration of inflammatory cells, when changed in their activities or concentrations, contribute to the proteolysis of structural and contractile proteins of ECM, cardiomyocytes, and VSMCs, leading to maintenance of high BP and CVD development and progression.3,11,23–26
Starting from this evidence, many studies and clinical trials25–28 observed a notable higher MMP-9 serum levels in hypertensive patients compared to normotensive controls.27–30 In the Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT) study, Tayebjee and collegues29 demonstrated that in uncontrolled hypertensive patients (eg, stage 3 of HT) circulating plasma concentrations of MMP-9 and TIMP-1, measured by ELISA, were significantly higher compared to normotensive controls at baseline. After a median of three years of antihypertensive treatment, BP values were significantly lower [systolic/diastolic BP±standard deviation (SD): 178±21/94±12 mmHg at baseline vs 139±15/78±10 mmHg, p < 0.0001)], MMP-9 plasma concentrations significantly decreased (p = 0.035), and TIMP-1 increased (p = 0.005). Additionally, concentrations of MMP-9 positively correlated with cardiovascular risk score (calculated by the Framingham equation) (r = 0.317, p = 0.007).29 These findings were subsequently confirmed by Onal et al30 in a population of well-controlled hypertensive patients. Despite no significant differences were found between normotensive and subjects with HT at the beginning of the study in terms of MMP-9 and TIMP-1 serum concentrations, analyzed using a fluorometric assay, after three months of treatment with lisinopril or candesartan, office BP (systolic/diastolic BP±SD: 128±9/82±6 mmHg at baseline vs 118±7/74±6 mmHg, p < 0.001) and MMP-9 concentrations [median and interquartile range (IQR): 71 (0–128) ng/mL at baseline vs 3 (0–128) ng/mL after treatment, p < 0.001)] were significantly lower.
In accordance with these results, it is possible to suggest that in hypertensive patients elevated MMPs levels are strongly involved in the rearrangement of the arterial wall (Figure 1). MMPs are released in exceed concentrations from endothelial and VSMCs after stimulation by HT-related mechanical and hormonal stimuli on the vascular wall. This mechanism could result in increased degradation of elastin relative to collagen, and, associated to altered TIMPs activity, it could lead to the accumulation of poorly cross-linked, immature, and unstable fibril degradation products and misdirected deposition of collagen.30,31 This process leads to a vascular remodelling and a vicious cycle related not only to the maintenance of elevated BP values but also to the development of HMOD, which will be discussed in detail in the following paragraphs.
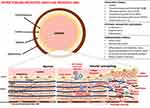 |
Figure 1 Pathological mechanisms of arterial wall remodelling. High blood pressure, by different stimuli, promotes several changes in the vessel wall of both large and small arteries, including recruitment and infiltration of inflammatory cells into the vessel wall, release of free radicals, proinflammatory chemokines, and cytokines, changes in vascular smooth muscle cells (VSMCs) (↑ cells hyperplasia and migration, ↑ VSMCs contraction and ↓ VSMCs relaxation, cells differentiation into osteogenic phenotype), endothelial cells (cells differentiation into osteogenic and VSMCs phenotype), elastic fiber degradation, collagen deposition, and calcification. The activity of several matrix metalloproteinases (MMPs) increases thereby contributing to development of arterial stiffness. Adapted from Van Varik BJ, et al. Mechanisms of arterial remodeling: Lessons from genetic diseases. Front Genet. 2012;3:290. © 2012 van Varik, Rennenberg, Reutelingsperger, Kroon, de Leeuw and Schurgers. This is an open-access article distributed under the terms of the Creative Commons Attribution License http://https://creativecommons.org/licenses/by/3.0/legalcode.33 |
MMPs and Hypertension-Mediated Vascular Remodelling
A key pathological hallmark of HT is the increase in arterial load, a process that leads to structural and functional modifications determining a higher flow impedance at the level of large (conductive) arteries, and growing resistance in small (resistance) vessels. These changes are defined as “vascular remodelling”32 and significantly impact both on the pathogenesis and complications of HT.
Hypertension-mediated vascular remodelling is a complex and heterogeneous phenomenon (Figure 1), which is subject to dynamic interactions between paracrine stimuli, including MMPs and vasoactive substances, and hemodynamic stimuli such as persistent altered fluid shear stress and circumferential strain, and it differs depending on the vessel type and specific disease state or progression.33,34 All these mechanisms are able to determine recruitment and infiltration of inflammatory cells, changes and apoptosis of VSMCs and endothelial cells,35 and synthesis or degradation of ECM material in the vessel wall of both large and small arteries (Figure 1).10,32,36 The consequences may be represented by increase or decrease of arterial wall thickness and wall-to-lumen ratio, in processes defined as hypertrophic and hypotrophic remodelling, respectively. When the diameter of the vessel changes but the wall-to-lumen ratio remains the same it is called eutrophic remodelling. In conductive vessels, hypertension-mediated remodelling is characterized by increased diameter and thickened intimal and medial layers of the vascular wall, which contributes to increased arterial stiffness and blood pulse wave pressure (outward hypertrophic remodelling).37 By comparison, resistance vessel remodelling more often may present as inwardly either eutrophic or hypertrophic remodelling, depending on the form of HT33,38 and it has been well correlated with clinical disease progression and severity.22,39,40
In hypertension-related remodelling models in both conductive and resistance vessels, several MMPs and their inhibitors are involved, mainly through deregulating the ECM turnover and promoting a shift of endothelial cells and VSMCs to a more secretory, migratory, and proliferative cells (Figure 1).12,13,30,41 MMPs can be detected in all three layers of the vascular wall; basically, endothelium can produce MMP-1 and MMP-2; VSMCs of both intima and media secrete MMP-2 and MMP-9, and they can also produce TIMP-1 and TIMP-2. Adventitia is the layer where MMP-9 can be synthesized. Moreover, other MMPs can be detected in a vascular wall, such as MMP-1, MMP-3, MMP-8, MMP-10, MMP-12, and MMP-13.42
From a clinical perspective, in the literature, several human studies demonstrated a positive correlation between MMPs/TIMPs unbalance and increased arterial stiffness evaluated by pulse wave velocity (PWV) and augmentation index (AIx) in hypertensive patients, confirming the role of these proteinases in the hypertension-mediated vascular remodelling. MMP-9, MMP-2, MMP-1, and MMP-3 are the most frequently described in this context (Table 2).17,43–47 The majority of these studies detected plasma or serum MMPs concentrations using enzyme immunoassays. For example, Tan et al43 in 202 primary hypertensive patients found higher levels of MMP-9 and TIMP-1 compared to 54 age- and gender-matched controls. In the first group, serum levels of MMP-9 (p = 0.014) and TIMP-1 (p = 0.005) were significantly and positively correlated with large arterial stiffness measured by carotid-femoral PWV, but not with peripheral stiffness measured by carotid-radial PWV, suggesting that the contribution of the MMP-9/TIMP-1 system is less crucial in the remodelling of muscular arteries. Furthermore, it has been described that aortic stiffness is related to MMP-9 and MMP-2 levels and serum elastase activity in isolated systolic HT. Indeed, MMP-9 levels correlated significantly with aortic and brachial PWV, even after adjustments for confounding variables.44 Interestingly, in never-treated hypertensive subjects the role of MMP-9 has been studied through the analysis of genetic polymorphism.46 BP values, aortic stiffness measured by carotid-femoral PWV, and serum MMP-9 concentrations were significantly higher in T-allele carriers of the −1562C>T polymorphism. This latter emerged as an independent predictor not only of systolic and diastolic BP but also of PWV (R2 = 0.47, p < 0.0001).46
 |
Table 2 Baseline Characteristics of Studies Providing the Correlation between MMPs Levels and Arterial Stiffness in Hypertensive Patients |
Regarding MMP-1, its plasma levels have been displayed a positive correlation with both carotid-femoral PWV (p < 0.01) 45,47 and augmentation index (AIx) (p < 0.01);47 notably, in the study of McNulty et al this correlation was observed not only in hypertensive subjects but also in healthy controls, suggesting that higher MMP-1 concentrations may reflect an increased collagen type-I degradation that starts in heathy vessels and is probably upregulated by HT.45
Rajzer et al17 confirmed that also MMP-3 (or Stromelysin-1), a matrix metalloproteinase implicated in the age-related vascular remodelling as previously reported in low cardiovascular risk and unmedicated subjects,48 is able to activate MMP-1 and MMP-9 degrading fibrillar collagen and gelatin, respectively.49 MMP-3 might play an initiating role in the hypertension-mediated vascular remodelling and stiffening of the large arterial wall. In particular, in hypertensive patients, carotid-femoral PWV was associated to plasma MMP-3 concentration (B = 0.204, p = 0.0459), and, after 6 months of treatment, regardless of the agent administered, it was described a significant decrease of PWV, systolic BP, MMP-2, and MMP-3, concurrently with an increase of TIMP-1 plasma concentrations.17 Thus, in patients with HT, besides age and BP values, the determinants of arterial stiffness also feature serum MMPs, which levels seem to be lowered by antihypertensive medications.
This evidence confirms that, in studies analyzing samples of patients with relatively low cardiovascular risk, persistent high BP has a crucial role on the abnormal ECM metabolism and the alteration in vascular structure mediated by MMPs/TIMPs unbalance, which leads to increased stiffness and lower arterial compliance. In addition, these findings raise the interesting possibility that antihypertensive treatment may modulate collagen metabolism and VSMCs function through its activity on MMPs. However, to confirm these hypotheses, further prospective studies, well-designed, and with standardized methods of MMPs measurement are necessary.
MMPs and Hypertension-Mediated Cardiac Remodelling
Persistent high BP values lead to adaptive processes also in the heart, called “cardiac remodelling”, which are characterized by intrinsic cardiomyocyte modifications and interstitium reorganization with increased collagen deposition, fibrosis, and changes in ECM components.50 MMPs and other proteases overactivation has been proposed as a mediator of cardiac remodelling. This latter is characterized by the interruption of connections between cardiomyocytes and blood vessels. Moreover, the excessive production and accumulation of ECM structural proteins results in enhanced stiffness of the myocardium and impedes ventricular contraction and relaxation, leading to distorted architecture and function of the heart.51 Although these mechanisms are initially compensatory and preserve cardiac contractile performance with development of left ventricular hypertrophy (LVH) and other structural changes, they finally lead to left ventricular dilatation and chronic heart failure (CHF).52
In animal models,15,53 Mmp-2 and Mmp-9, expressed by cardiac myocytes and fibroblasts, have been shown to be the most commonly upregulated Mmps in HT and cardiac hypertrophy. Both of them are highly upregulated in hypertrophic and failing hearts, and they have been implicated in the progression of ventricular dilatation and CHF development.52 More recently, the role of MMP-2, MMP-9, and MMP-1 has been suggested as implicated in hypertensive humans cardiac remodelling, also in cases without other severe comorbidities. The meta-analysis published by Marchesi et al14 reports the results of several studies aimed to evaluate the plasma levels of MMPs and TIMPs in hypertensive patients, and their correlation with LVH, diastolic function, and heart failure. MMP-9 and MMP-2 appear to have the greatest potential as biomarkers of cardiovascular remodelling in HT, even though caution is necessary in the interpretation of results, largely due to the high heterogeneity among the studies, including samples collection and methods of measurement. Not all researches confirmed the increased plasma levels of MMPs in hypertensive patients with cardiac remodelling compared with controls.27,54–56 Conversely, some of the examined papers reported a significant correlation between MMPs levels and the main echocardiographic parameters related to hypertensive cardiac remodelling (ie, left ventricular mass index – LVMI, g/m2 and indices of diastolic dysfunction).57,58 For example, Saglam et al58 showed that not only plasma MMP-9 but also MMP-3 concentrations (determined by enzyme immunoassay method) were significantly higher in patients with LVH than those without LVH, and these two MMPs were correlated with left ventricular posterior wall thickness and Doppler-echocardiographic indices of diastolic dysfunction. Moreover, a study59 conducted comparing healthy controls with 114 subjects with LVH and 61 patients with LVH plus diastolic heart failure (DHF) provided plasma concentrations of four classes of MMPs: gelatinases (MMP-2 and MMP-9), collagenases (MMP-1 and 8), stromelysins (MMP-3), and matrilysins (MMP-7). Interestingly, authors demonstrated that a different plasma MMPs profile emerged for LVH patients compared with those affected by DHF; in these latter there were significantly higher plasma levels of MMP-3 and MMP-7, and lower concentrations of MMP-8. Furthermore, they suggested that multi-biomarker panels for both LVH and DHF including, among others, specific MMPs and TIMPs (MMP-7, MMP-9, TIMP-1, and MMP-2, MMP-8, TIMP-4, respectively) provided prediction algorithms for LVH and DHF with good sensitivity and specificity.59
These findings suggest the promising role of MMPs in the clinical practice as a tool to stratify the risk of cardiac remodelling in hypertensive patients and as a possible target of therapy to prevent changes in myocardial tissue. Nevertheless, data are still contrasting, probably also for the poor standardization of MMPs detection methods, and further prospective studies and clinical trials are needed to confirm these conclusions.
MMPs in Hypertension-Mediated Renal Impairment
The relative risk of renal damage in patients with uncomplicated primary HT is low as compared with other cardiovascular complications. However, given the huge prevalence of HT in the general population, it still remains the second leading cause of renal impairment (eg, increased levels of microalbuminuria), through the development of CKD and ESRD.60 The pathogenetic determinants of hypertensive-mediated renal damage can be primarily summarized into four mechanisms: (i) the systemic BP “load”; (ii) the degree to which such load is transmitted to the renal vascular bed; (iii) the local tissue susceptibility to any given degree of barotrauma; and (iv) the BP-independent tissue damage promoting effects of angiotensin II and aldosterone.60 Collectively, these phenomena lead to the development of glomerulosclerosis, interstitial fibrosis, and arteriosclerosis that represent the main histological patterns of hypertension-mediated renal damage. Nevertheless, data on the histological phenotype of hypertensive-related renal damage, which exhibits differences in models with or without overt renin-angiotensin-aldosterone system activation, suggests that the triggering of downstream molecular mediators of tissue injury may not be exclusive to angiotensin II and/or aldosterone but may represent a response to tissue stress and/or injury per se.60 Increased BP alone can activate many of these downstream pathways such as infiltration of inflammatory cells, increase of monocyte chemoattractant proteins, release of pro-inflammatory (tumor necrosis factor α – TNFα) and profibrotic cytokines (transforming growth factor), as well as the release of ROS. All these conditions are able to increase the MMPs activity.61 Ten members of MMPs (MMP-1, −2, −3, −9, −13, −14, −24, −25, −27, −28) are physiologically expressed in the renal tissue, and, under basal conditions, MMPs are at low levels and are tightly regulated to maintain the homeostatic ECM turnover. Among MMPs expressed in adult kidneys, MMP-1, MMP-2, MMP-3, MMP-9, MMP-13, and MMP-14 have been extensively studied.62 The pathological mechanism by which high free activated MMPs levels alter the renal tissue is complex; briefly, it could be held related to an increase of local markers of inflammation that leads to upregulation of kidney fibrosis by promoting ECM production (Figure 2).
In the last years, MMPs gelatinase-subgroup (eg, MMP-2 and MMP-9) and TIMP-1 have been demonstrated to be involved in unfavorable tissue remodelling associated with HT in renal disease.62–66 MMP-9 seems to act in self-propagating inflammation by creating collagen fragments that are both chemotactic for neutrophils and stimulate them to release more MMP-9,67 and, together with MMP-2, induces epithelial–mesenchymal transition (EMT), a potentially significant step in fibrosis.68 In the early stage of renal damage associated with chronic high BP, MMPs activation in the ECM acts as a compensatory mechanism to degrade the excessive collagen synthesis and to prevent the development of renal fibrosis. However, as the CKD progresses, protective MMPs activity begins to wane.63 Hence, in the subsequent stages of hypertension-mediated renal disease, MMPs and TIMPs play a relevant role because of directly involved in the pathogenic remodelling of ECM, leading to the progression of nephrosclerosis and CKD. Specifically, changes in the activity of MMP-9 isoform are involved in structural alterations of the renal tubule and glomerulus, especially in advanced CKD where kidney fibrosis is preponderant.61
Notably, recent studies demonstrated a significant association between hypertension-mediated renal dysfunction and the increased activity of specific MMPs, such as MMP-2 and MMP-9, even in stages where the decline in renal function is still moderate.63,64,66 Rodríguez-Sánchez et al63 showed significant active concentrations of MMP-9, detected by ELISA, in the plasma samples of a subgroup of hypertensive patients with estimated glomerular filtration rate (eGFR) ranged between 60 and 30 mL/min/1.73m2 despite the systemic elevation of TIMP-1, with a significantly negative correlation between the concentration of the active isoform of MMP-9 and eGFR (R = −0.531, p > 0.001). Compared with hypertensive patients with eGFR > 90 mL/min/1.73m2, they found a significant reduction in the protein-protein interaction between MMP-9 and TIMP-1 in those subjects with worse eGFR, suggesting that these latter have more circulating TIMP-1 but it is not exerting its inhibitory capacity on MMP-9. Similar results have been found in a greater number of patients by Xu et al66 where an ELISA method was performed to measure serum levels of MMPs and TIMPs. The MMP-9/TIMP-1 ratio was lower in the group with eGFR < 60 mL/min/1.73 m2 and it was associated with microalbuminuria, even after adjustment of confounding factors such as other inflammatory biomarkers. Interestingly, in a population of 39 well-controlled hypertensive patients64 during chronic renin-angiotensin-aldosterone system blockade, resistant albuminuria was associated with (i) a strong increase in the plasma circulating active MMP-9 form, despite unchanged plasma circulating MMP-9 protein levels, and (ii) a significant reduction in the MMP-9/TIMP-1 interaction as a consequence of an increase in oxidized-TIMP-1 levels. In the same study, the animal experiment confirmed that increased Mmp-9 activity at a circulatory level is parallel to the increased Mmp-9 activity in the renal tissue, suggesting that Mmp-9 is a circulating biomarker specifically involved in albuminuria development.63
In advanced stage of renal damage, in addition to MMP-9, higher plasma concentrations of MMP-2 and MMP-10 were observed in hypertensive-mediated ESRD patients compared to both hypertensives without complications and normotensive subjects.65 This evidence suggests that MMP-2 and MMP-10 do not play a major role in the initial stage of HT but instead may be involved in the development of renal injury once primary HT has been established and the renal damage has been already started. Interestingly, MMP-2 and MMP-10 plasma concentrations, based on electrochemiluminescence detection technology, have a strong positive correlation (R = 0.7422, p < 0.001), highlighting a co-regulation of such MMPs in effecting end-stage renal damage in HT.65
Conclusion
Systemic arterial hypertension, MMPs, and their inhibitors play a crucial role in the complex interaction between vascular cells, cardiomyocytes, and ECM components. They are involved in the development of the hypertension-mediated damage of the main “target” organs, such as vascular system, heart, and renal tissue, leading to end-stage diseases and many other cardiovascular complications. Among the large family of MMPs, plasma MMP-9, MMP-2, MMP-3, and MMP-1 appear the most frequently studied in relation to the HMOD and seem to have the greatest potential as plasma biomarkers of “target” organs remodelling. These findings suggest the way to potential new tools to better stratify the cardiovascular risk in hypertensive patients and provides the basis for a specific and targeted therapy that might reduce the organ damage mediated by chronically elevated blood pressure values. Further prospective and method-standardized studies are required aiming at use plasma or serum MMPs concentrations or activity in clinical practice.
Abbreviations
AIx, augmentation index; BP, blood pressure; CHF, chronic heart failure; CKD, chronic kidney disease; CVD, cardiovascular diseases; DBP, diastolic blood pressure; DHF, diastolic heart failure; DM, diabetes mellitus; ECM, extracellular matrix; eGFR, estimated glomerular filtration rate; ELISA, enzyme-linked immunosorbent assay; EMT, epithelial–mesenchymal transition; ESRD, end-stage renal disease; HT, arterial hypertension; LVH, left ventricular hypertrophy; LVMI, left ventricular mass index; M, males; Mmps, matrix metalloproteinases referred to animal models; MMPs, matrix metalloproteinases referred to human models; PWV, pulse wave velocity; ROS, reactive oxygen species; SBP, systolic blood pressure; SD, standard deviation; TIMPs, tissue inhibitors of metalloproteinases; TNFα, tumor necrosis factor α; VSMCs, vascular smooth muscle cells; yrs, years.
Disclosure
There are no relationships with industry. The authors report no conflicts of interest in this work.
References
1. Gross J, Lapiere CM. Collagenolytic activity in amphibian tissues: a tissue culture assay. Proc Natl Acad Sci U S A. 1962;48(6):1014–1022. doi:10.1073/pnas.48.6.1014
2. Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92(8):827–839. doi:10.1161/01.RES.0000070112.80711.3D
3. Cui N, Hu M, Khalil RA. Biochemical and biological attributes of matrix metalloproteinases. Prog Mol Biol Transl Sci. 2017;147:1–73.
4. Fontana V, Silva PS, Gerlach RF, Tanus-Santos JE. Circulating matrix metalloproteinases and their inhibitors in hypertension. Clin Chim Acta. 2012;413(7–8):656–662. doi:10.1016/j.cca.2011.12.021
5. Fuchs FD, Whelton PK. High blood pressure and cardiovascular disease. Hypertension. 2020;75(2):285–292. doi:10.1161/HYPERTENSIONAHA.119.14240
6. Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021–3104.
7. Rubattu S, Pagliaro B, Pierelli G, et al. Pathogenesis of target organ damage in hypertension: role of mitochondrial oxidative stress. Int J Mol Sci. 2015;16(1):823–839. doi:10.3390/ijms16010823
8. Morillas P, Quiles J, De Andrade H, et al. Circulating biomarkers of collagen metabolism in arterial hypertension: relevance of target organ damage. J Hypertens. 2013;31(8):1611–1617. doi:10.1097/HJH.0b013e3283614c1c
9. Nyhan D, Steppan J, Barodka V, Berkowitz DE. Vascular stiffness and increased pulse pressure in the aging cardiovascular system. Cardiol Res Pract. 2011;263585.
10. Harvey A, Montezano AC, Lopes RA, Rios F, Touyz RM. Vascular fibrosis in aging and hypertension: molecular mechanisms and clinical implications. Can J Cardiol. 2016;32(5):659–668. doi:10.1016/j.cjca.2016.02.070
11. Parente J, de Castro M. Matrix metalloproteinase in the cardiovascular remodeling of hypertension: current insights and therapeutic potential. Met Med. 2018;5(3):13–30.
12. Lin J, Davis HB, Dai Q, et al. Effects of early and late chronic pressure overload on extracellular matrix remodeling. Hypertens Res. 2008;31(6):1225–1231. doi:10.1291/hypres.31.1225
13. Wang X, Khalil RA. Matrix metalloproteinases, vascular remodeling, and vascular disease. Adv Pharmacol. 2018;81:241–330.
14. Marchesi C, Dentali F, Nicolini E, et al. Plasma levels of matrix metalloproteinases and their inhibitors in hypertension: a systematic review and meta-analysis. J Hypertens. 2012;30(1):3–16. doi:10.1097/HJH.0b013e32834d249a
15. Heymans S, Lupu F, Terclavers S, et al. Loss or inhibition of uPA or MMP-9 attenuates LV remodeling and dysfunction after acute pressure overload in mice. Am J Pathol. 2005;166(1):15–25. doi:10.1016/S0002-9440(10)62228-6
16. Matsusaka H, Ide T, Matsushima S, et al. Targeted deletion of matrix metalloproteinase 2 ameliorates myocardial remodeling in mice with chronic pressure overload. Hypertension. 2006;47(4):711–717. doi:10.1161/01.HYP.0000208840.30778.00
17. Rajzer M, Wojciechowska W, Kameczura T, et al. The effect of antihypertensive treatment on arterial stiffness & serum concentration of selected matrix metalloproteinases. Arch Med Sci. 2017;13(4):760–770. doi:10.5114/aoms.2016.58825
18. Lopez-Avila V, Spencer JV. Methods for detection of matrix metalloproteinases as biomarkers in cardiovascular disease. Clin Med Cardiol. 2008;2:75–87. doi:10.4137/CMC.S484
19. Cheng XC, Fang H, Xu WF. Advances in assays of matrix metalloproteinases (MMPs) and their inhibitors. J Enzyme Inhib Med Chem. 2008;23(2):154–167.
20. Catterall JB, Cawston TE. Assays of matrix metalloproteinases (MMPs) and MMP inhibitors: bioassays and immunoassays applicable to cell culture medium, serum, and synovial fluid. Methods Mol Biol. 2003;225:353–364.
21. Pintérová M, Kuneš J, Zicha J. Altered neural and vascular mechanisms in hypertension. Physiol Res. 2011;60:381–402. doi:10.33549/physiolres.932189
22. Intengan HD, Schiffrin EL. Vascular remodeling in hypertension: roles of apoptosis, inflammation, and fibrosis. Hypertension. 2001;38(3 Pt 2):581–587. doi:10.1161/hy09t1.096249
23. Beaudeux JL, Giral P, Bruckert E, Foglietti MJ, Chapman MJ. Matrix metalloproteinases, inflammation and atherosclerosis: therapeutic perspectives. Clin Chem Lab Med. 2004;42(2):121–131. doi:10.1515/CCLM.2004.024
24. Kadoglou NP, Daskalopoulou SS, Perrea D, Liapis CD. Matrix metalloproteinases and diabetic vascular complications. Angiology. 2005;56(2):173–189. doi:10.1177/000331970505600208
25. Dai X, Kaul P, Smith SC, Stouffer GA. Predictors, treatment, and outcomes of STEMI occurring in hospitalized patients. Nat Rev Cardiol. 2016;13(3):148–154.
26. Blankenberg S, Rupprecht HJ, Poirier O, et al. Plasma concentrations and genetic variation of matrix metalloproteinase 9 and prognosis of patients with cardiovascular disease. Circulation. 2003;107(12):1579–1585. doi:10.1161/01.CIR.0000058700.41738.12
27. Li-Saw-Hee FL, Edmunds E, Blann AD, Beevers DG, Lip GYH. Matrix metalloproteinase-9 and tissue inhibitor metalloproteinase-1 levels in essential hypertension. Relationship to left ventricular mass and anti-hypertensive therapy. Int J Cardiol. 2000;75(1):43–47. doi:10.1016/S0167-5273(00)00274-6
28. Schieffer B, Bünte C, Witte J, et al. Comparative effects of AT1-antagonism and angiotensin-converting enzyme inhibition on markers of inflammation and platelet aggregation in patients with coronary artery disease. J Am Coll Cardiol. 2004;44(2):362–368. doi:10.1016/j.jacc.2004.03.065
29. Tayebjee MH, Nadar S, Blann AD, Gareth Beevers D, MacFadyen RJ, Lip GYH. Matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 in hypertension and their relationship to cardiovascular risk and treatment: a substudy of the Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT). Am J Hypertens. 2004;17(9):764–769. doi:10.1016/S0895-7061(04)00855-6
30. Onal IK, Altun B, Onal ED, Kirkpantur A, Gul OS, Turgan C. Serum levels of MMP-9 and TIMP-1 in primary hypertension and effect of antihypertensive treatment. Eur J Intern Med. 2009;20(4):369–372. doi:10.1016/j.ejim.2008.10.003
31. Donnelly R, Collinson DJ, Manning G. Hypertension, matrix metalloproteinases and target organ damage. J Hypertens. 2003;21(9):1627–1630. doi:10.1097/00004872-200309000-00009
32. Brown IAM, Diederich L, Good ME, et al. Vascular smooth muscle remodeling in conductive and resistance arteries in hypertension. Arterioscler Thromb Vasc Biol. 2018;38(9):1969–1985. doi:10.1161/ATVBAHA.118.311229
33. Van Varik BJ, Rennenberg RJMW, Reutelingsperger CP, Kroon AA, De Leeuw PW, Schurgers LJ. Mechanisms of arterial remodeling: lessons from genetic diseases. Front Genet. 2012;3:290. doi:10.3389/fgene.2012.00290
34. Renna NF, De Las Heras N, Miatello RM. Pathophysiology of vascular remodeling in hypertension. Int J Hypertens. 2013;2013:808353.
35. Savoia C, Sada L, Zezza L, et al. Vascular inflammation and endothelial dysfunction in experimental hypertension. Int J Hypertens. 2011;2011:281240. doi:10.4061/2011/281240
36. Wagenseil JE, Mecham RP. Elastin in large artery stiffness and hypertension. J Cardiovasc Transl Res. 2012;5(3):264–273. doi:10.1007/s12265-012-9349-8
37. Roman MJ, Saba PS, Pini R, et al. Parallel cardiac and vascular adaptation in hypertension. Circulation. 1992;86(6):1909–1918. doi:10.1161/01.CIR.86.6.1909
38. Munroe PB, Olgunturk RO, Fryns JP, et al. Mutations in the gene encoding the human matrix Gla protein cause keutel syndrome. Nat Genet. 1999;21(1):142–144. doi:10.1038/5102
39. Rizzoni D, Porteri E, Boari GEM, et al. Prognostic significance of small-artery structure in hypertension. Circulation. 2003;108(18):2230–2235. doi:10.1161/01.CIR.0000095031.51492.C5
40. Mulvany MJ, Baumbach GL, Aalkjaer C, et al. Vascular remodeling. Hypertension. 1996;28(3):505–506.
41. You T, Nicklas BJ. Inflammation and the metabolic syndrome. Med Sci Sports Exerc. 2004;36(Supplement):S1.
42. Li Y, Wang W, Li L, Khalil RA. MMPs and ADAMs/ADAMTS inhibition therapy of abdominal aortic aneurysm. Life Sci. 2020;253:117659. doi:10.1016/j.lfs.2020.117659
43. Tan J, Hua Q, Xing X, Wen J, Liu R, Yang Z. Impact of the metalloproteinase-9/tissue inhibitor of metalloproteinase-1 system on large arterial stiffness in patients with essential hypertension. Hypertens Res. 2007;30(10):959–963. doi:10.1291/hypres.30.959
44. Wallace SY, McEniery CM, Dakham Z, et al. Matrix metalloproteinase-9 (MMP-9), MMP-2, and serum elastase activity are associated with systolic hypertension and arterial stiffness. Arterioscler Thromb Vasc Biol. 2005;25(2):372–378. doi:10.1161/01.ATV.0000151373.33830.41
45. McNulty M, Mahmud A, Spiers P, Feely J. Collagen type-I degradation is related to arterial stiffness in hypertensive and normotensive subjects. J Hum Hypertens. 2006;20(11):867–873. doi:10.1038/sj.jhh.1002015
46. Zhou S, Feely J, Spiers JP, Mahmud A. Matrix metalloproteinase-9 polymorphism contributes to blood pressure and arterial stiffness in essential hypertension. J Hum Hypertens. 2007;21(11):861–867. doi:10.1038/sj.jhh.1002244
47. Stakos DA, Tziakas DN, Chalikias GK, Mitrousi K, Tsigalou C, Boudoulas H. Associations between collagen synthesis and degradation and aortic function in arterial hypertension. Am J Hypertens. 2010;23(5):488–494. doi:10.1038/ajh.2010.2
48. Medley TL, Kingwell BA, Gatzka CD, Pillay P, Cole TJ. Matrix metalloproteinase-3 genotype contributes to age-related aortic stiffening through modulation of gene and protein expression. Circ Res. 2003;92(11):1254–1261. doi:10.1161/01.RES.0000076891.24317.CA
49. Kingwell BA, Medley TL, Waddell TK, Cole TJ, Dart AM, Jennings GL. Large artery stiffness: structural and genetic aspects. Clin Exp Pharmacol Physiol. 2001;28(12):1040–1043. doi:10.1046/j.1440-1681.2001.03580.x
50. Riaz S, Zeidan A, Mraiche F. Myocardial proteases and cardiac remodeling. J Cell Physiol. 2017;232(12):3244–3250. doi:10.1002/jcp.25884
51. Lu P, Takai K, Weaver VM, Werb Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol. 2011;3(12):a005058. doi:10.1101/cshperspect.a005058
52. Panek AN, Bader M. Matrix reloaded: the matrix metalloproteinase paradox. Hypertension. 2006;47(4):640–641. doi:10.1161/01.HYP.0000208603.97395.44
53. Hayashidani S, Tsutsui H, Ikeuchi M, et al. Targeted deletion of MMP-2 attenuates early LV rupture and late remodeling after experimental myocardial infarction. Am J Physiol Heart Circ Physiol. 2003;285(3):H1229–H1235. doi:10.1152/ajpheart.00207.2003
54. Laviades C, Varo N, Fernández J, et al. Abnormalities of the extracellular degradation of collagen type I in essential hypertension. Circulation. 1998;98(6):535–540. doi:10.1161/01.CIR.98.6.535
55. Zervoudaki A, Economou E, Stefanadis C, et al. Plasma levels of active extracellular matrix metalloproteinases 2 and 9 in patients with essential hypertension before and after antihypertensive treatment. J Hum Hypertens. 2003;17(2):119–124. doi:10.1038/sj.jhh.1001518
56. Zervoudaki A, Economou E, Pitsavos C, et al. The effect of Ca2+ channel antagonists on plasma concentrations of matrix metalloproteinase-2 and −9 in essential hypertension. Am J Hypertens. 2004;17(3):273–276. doi:10.1016/j.amjhyper.2003.11.007
57. López B, González A, Querejeta R, Larman M, Díez J. Alterations in the pattern of collagen deposition may contribute to the deterioration of systolic function in hypertensive patients with heart failure. J Am Coll Cardiol. 2006;48(1):89–96. doi:10.1016/j.jacc.2006.01.077
58. Saglam M, Karakaya O, Esen AM, et al. Contribution of plasma matrix metalloproteinases to development of left ventricular hypertrophy and diastolic dysfunction in hypertensive subjects. Tohoku J Exp Med. 2006;208(2):117–122. doi:10.1620/tjem.208.117
59. Zile MR, DeSantis SM, Baicu CF, et al. Plasma biomarkers that reflect determinants of matrix composition identify the presence of left ventricular hypertrophy and diastolic heart failure. Circ Hear Fail. 2011;4(3):246–256. doi:10.1161/CIRCHEARTFAILURE.110.958199
60. Bidani AK, Griffin KA. Pathophysiology of hypertensive renal damage: implications for therapy. Hypertension. 2004;44(5):595–601. doi:10.1161/01.HYP.0000145180.38707.84
61. Cheng Z, Limbu MH, Wang Z, et al. MMP-2 and 9 in chronic kidney disease. Int J Mol Sci. 2017;18(4):1–11. doi:10.3390/ijms18040776
62. Toba H, Lindsey ML. Extracellular matrix roles in cardiorenal fibrosis: potential therapeutic targets for CVD and CKD in the elderly. Pharmacol Ther. 2019;193:99–120. doi:10.1016/j.pharmthera.2018.08.014
63. Rodríguez-Sánchez E, Navarro-García JA, Aceves-Ripoll J, et al. Association between renal dysfunction and metalloproteinase (MMP)-9 activity in hypertensive patients. Nefrología. 2019;39(2):184–191. doi:10.1016/j.nefro.2018.08.009
64. Pulido-Olmo H, García-Prieto CF, Álvarez-Llamas G, et al. Role of matrix metalloproteinase-9 in chronic kidney disease: a new biomarker of resistant albuminuria. Clin Sci. 2016;130(7):525–538. doi:10.1042/CS20150517
65. Friese RS, Rao F, Khandrika S, et al. Matrix metalloproteinases: discrete elevations in essential hypertension and hypertensive end-stage renal disease. Clin Exp Hypertens. 2009;31(7):521–533. doi:10.3109/10641960802668730
66. Xu TY, Zhang Y, Li Y, Zhu DL, Gao PJ. The association of serum inflammatory biomarkers with chronic kidney disease in hypertensive patients. Ren Fail. 2014;36(5):666–672. doi:10.3109/0886022X.2014.890002
67. Sormani MP, Calabrese M, Signori A, Giorgio A, Gallo P, De Stefano N. Modeling the distribution of new MRI cortical lesions in multiple sclerosis longitudinal studies. PLoS One. 2011;6(10):e26712. doi:10.1371/journal.pone.0026712
68. Tan RJ, Liu Y. Matrix metalloproteinases in kidney homeostasis and diseases. Am J Physiol Renal Physiol. 2012;302(11):F1351–F1361. doi:10.1152/ajprenal.00037.2012
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.