Back to Journals » Pharmacogenomics and Personalized Medicine » Volume 16
Massive Hepatocellular Carcinoma with Situs Inversus Totalis Achieved a Complete Response Following Camrelizumab Plus Apatinib and Combined with Two-Stage Hepatectomy: A Case Report
Authors Wu Y, Ou S , Liao X , Han C, Yang C , Qin W, Tan Y , Lao Q, Peng T , Ye X
Received 14 June 2022
Accepted for publication 18 November 2022
Published 7 February 2023 Volume 2023:16 Pages 111—120
DOI https://doi.org/10.2147/PGPM.S376596
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Martin H Bluth
Yining Wu,1,* Shenjian Ou,1,* Xiwen Liao,1,2 Chuangye Han,1,2 Chengkun Yang,1,2 Wei Qin,1 Yufeng Tan,1 Quan Lao,1 Tao Peng,1,2 Xinping Ye1
1Department of Hepatobiliary Surgery, The First Affiliated Hospital of Guangxi Medical University, Nanning, Guangxi Zhuang Autonomous Region, 530021, People’s Republic of China; 2Guangxi Key Laboratory of Enhanced Recovery After Surgery for Gastrointestinal Cancer, Nanning, Guangxi Zhuang Autonomous Region, 530021, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xinping Ye; Tao Peng, Tel +86-771-5356528, Fax +86-771-5350031, Email [email protected]; [email protected]
Abstract: Situs inversus totalis (SIT) is a rare congenital condition in which abdominal and thoracic organs are transposed from normal positions. Two-stage hepatectomy (TSH) combined with translational therapy for hepatocellular carcinoma (HCC) with SIT has been rarely reported. We report a 41-year-old man with giant hepatocellular carcinoma (71 mm × 55 mm × 51 mm) whose future residual liver (FLR) and standard liver volume (SLV) ratio at first diagnosis was 37.4%. Preoperative volume assessment of portal vein ligation (PVL) revealed inadequate hypertrophy of FLR. After a multidisciplinary group discussion (MDT), the patient decided to follow conversion therapy. Three months later, ratio of the FLR/SLV increased from 37.4% to 71% after operation, which met the surgical requirements. Second hepatectomy, right lobectomy was successful. There was no recurrence after six months of follow-up. In our case, conversion therapy appears to be effective in maintaining residual liver hyperplasia, reducing tumor load, and preventing tumor progression in patients with large HCC during TSH.
Keywords: situs inversus totalis, two-stage hepatectomy, hepatocellular carcinoma, future residual liver, multidisciplinary group discussion
Background
Hepatocellular carcinoma (HCC) is the most common pathological type of primary liver cancer (PLC), accounting for more than 85–90% of PLC, and PLC is the sixth most commonly diagnosed cancer and the third leading cause of cancer death worldwide in 2020, with approximately 906,000 new cases and 830,000 deaths.1,2 Also, HCC diagnosed in China comprise between one-third and one-half of the global incidence burden, the number of new liver cancer cases and cancer deaths in China was 389 thousands and 336 thousands, respectively.3–5 According to the epidemiological survey, Guangxi has a high incidence of HCC in China.6,7 Hepatocarcinogenesis is significantly related to hepatitis B virus (HBV) and hepatitis C virus (HCV) infection,8–14 high exposure to aflatoxin B1 (AFB1) exposure,15–18 drinking water pollution, and other high-risk factors.19 The incidence of HCC with rising numbers in Europe, USA and Japan.20 Current treatment strategies for HCC mainly include surgical resection, liver transplantation, radiofrequency ablation (RFA) or percutaneous ethanol injection (PEI), developing locoregional treatment techniques, transcatheter arterial chemoembolization (TACE), systemic treatments, two or more of them combined therapy.21,22 Moreover, surgical resection is the preferred treatment for HCC. Although, for unresectable liver cancer, such as massive liver cancer, surgical treatment has a great impact on the prognosis and quality of life of patients.23 In the past, extensive liver resection with a marginal future residual liver (FLR) has been addressed by two-stage hepatectomy. Among them, TSH is feasible for patients whose tumor range is not half of the liver but close to the middle hepatic vein, or if the tumor is large and extends to half of the liver, or the ratio of the FLR/SLV is insufficient after hepatectomy. Portal vein occlusion with surgical ligation or radiological embolization is performed in the first stage to induce hypertrophy of FLR. The redistribution of portal blood flow constitutes a stimulating factor for remnant liver hypertrophy.24 The concomitant administration of conversion therapy may convert unresectable tumors into resectable ones, which includes the conversion of surgically unresectable lesions, such as insufficient volume of FLR, to resectable lesions, and it supports the conversion of R1 and R2 resection to R0 resection.25–28 In a study launched in October 2016, the combination of camrelizumab and apatinib showed promising antitumor activity and an acceptable safety profile in advanced HCC. According to the results of this study, combination of camrelizumab and apatinib achieved potent efficacy in terms of objective response rate (ORR), duration of response (DoR) and overall survival (OS) in advanced HCC both in the first-line and second-line settings. The safety was manageable. This study shows that this combined strategy would be suitable as a new choice for first- or second-line treatment in this patient group.29,30
Situs inversus totalis (SIT), also known as “mirror person”, manifested as complete inversion of the thoracic and abdominal organs, that is the heart, lung, stomach, liver, spleen and other organs are completely opposite to the normal position, is a rare congenital anatomical variation.31 The incidence ranges between 1:1000 and 1:10,000, depending on the population surveyed.32 At present, the surgical treatment of patients with hepatocellular carcinoma with normal anatomy has been relatively mature. However, due to the rarity of total visceral inversion and hepatocellular carcinoma, the anatomical structure is different from ordinary people, and the operation is difficult. Therefore, we should change the thinking mode of routine operation and overcome the operation habit of general operation, explore the relationship between total visceral transposition and hepatocellular carcinoma, which would improve the understanding of the diagnosis and treatment of the disease. The clinical data of a patient with SIT combined with huge hepatocellular carcinoma in our hospital, combined with literature review, are reported as follows.
Case Presentation
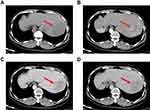
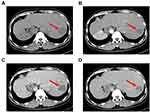
Our study was approved by the ethics committee of The First Affiliated Hospital of Guangxi Medical University, the approval number is speedy trial 2021 (014). Written informed consent was obtained from all patients for the procedures in this study. A 41-year-old man found a “liver space-occupying lesion” two weeks ago by health examination in their local hospital, and admitted to our hospital on May 2021 due to “left upper abdominal pain for one day”, with constantly left-upper-quadrant dull aching pain, each session keep on half a minute, with no aggravating or relieving factors, and the pain could radiate to the left shoulder, with no significant past medical or surgical history, and the patient has had a history of hepatitis B for several years. The abdomen was soft and nontender. The liver was not palpated under the left costal margin. Chest contrast-enhanced computed tomography showed that SIT (Figure 1A and B). The upper-abdominal enhanced computed tomography (CT) scan revealed one high-density areas (71 mm × 55 mm × 56 mm) in segment VII and VIII of the liver (Figure 2A–D), and magnetic resonance imaging presented a distinct liver tumor with about 7 cm in diameter in segment VII and VIII of liver (Figure 3A and B). The contrast-enhanced ultrasound (CEUS) showed a focal liver lesion was identified in segment VII and VIII of liver. The lesion was classified as LI-RADS 5 lesion category according to The Liver Reporting and Data System (LI-RADS), which was likely and almost definitively HCC.33 Laboratory tests suggested that serum aspartate aminotransferase (51U/L), serum alkaline phosphatase (85 U/L), and alanine aminotransferase, serum bilirubin, and total protein were all within normal limits. The results of hepatitis B surface antigen (HBsAg), Hepatitis B e antibody (HBeAb), and hepatitis B core antibody (HBcAb) were 184.08 IU/mL, 4.4 PEIu/mL, 12.6 PEIu/mL, respectively, and hepatitis C core antibody (HCV-Ab) was negative, with a Child Pugh score A (5 points). The indocyanine green retention rate at 15 min (ICG-R15) was 3%. The levels of tumor markers such as des-γ-carboxy prothrombin (DCP) increased to 3399.97 mAU/mL, and alpha-fetoprotein (AFP) was fair. The indication for hepatectomy and the procedure options were based on the tumor location, remnant liver volume, and the hepatic functional reserve assessed by the Model for End-Stage Liver Disease (MELD) score, the indocyanine green retention rate at 15 min, and Child Pugh classification. Consensus on the treatment was reached by a panel of experts from the multidisciplinary team discussion (MDT) including Hepatology, Oncology, Department of Ultrasound, Radiology department, Radiotherapy, Infectious disease. There was no evidence of extrahepatic metastases in a CT scan, so the patient was staged as BCLC-B and CNLC-IIb respectively, according to the staging system of the Barcelona Clinic Liver Cancer (BCLC) and China Liver Cancer (CNLC). The FLR/SLV was 37.4%, radical resection was unattainable due to the bulky tumor, hepatectomy of large tumor load may result in postoperative liver failure and associated complications.34 According to the results of intraoperative ICG, we reassess whether to perform primary radical operation or laparoscopic portal vein branch ligation and conversion therapy.
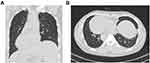 |
Figure 1 The coronal (A) and axial (B) view of chest contrast-enhanced computed tomography showing dextrocardia and SIT. |
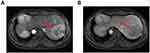 |
Figure 3 Magnetic resonance imaging showing a mass (red arrow) measuring 7.6 × 5.8 × 6.5 cm in segment VII and VIII of liver, (A) T1-weighted image; (B) T2-weighted image. |
Stage I Surgery
These clear images were used to reevaluate the location, size, number of tumor, and the position of adjacent blood vessel, especially the anatomical positional relationship of the right, middle, and left hepatic veins. The gallbladder was removed and separation of right branch of portal vein. The result of intraoperative ICG-R15 test after blocking the right branch of portal vein with portal vein clamp was 23.6%. After full communication with the patient’s family, it was decided to perform laparoscopic ligation of the right branch of the portal vein (Figure 4A and B). Patient was confirmed without bile leakage before closing the abdomen.35 After 18 days of PVL, molecular targeted therapy with apatinib (250 mg orally once daily) and initiated immunotherapy with camrelizumab (200 mg orally once daily) were started, this scheme were administered intravenously over 30 minutes once every 3 weeks. After another two courses of therapy, the follow-up contrast enhanced CT of upper-abdominal scan showed the size of liver lesions was continuous decrease and the lesions have homogeneous or heterogeneous density, most of this was necrotic (Figure 5A–D).
 |
Figure 4 Intraoperative photographs during stage I surgery. (A) Selective isolation and ligation of the right portal vein (white arrow); (B) Ischemic line (white arrow) of the right liver. |
Stage II Surgery
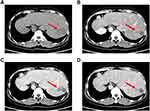
A month later, the patient was readmitted electively on August 2021 for second stage . Viral markers for hepatitis B virus and hepatitis C virus (HCV) were still negative. The levels of tumor markers such as DCP and AFP were fair (Figure 6). The serum alanine transaminase (88U/L) and glutamyltranspeptidase (189 U/L) levels were all abnormal. The upper-abdominal enhanced CT scan revealed one heterogeneous density areas (35 mm × 31 mm × 44 mm) in segment VII and VIII of the liver (Figure 7A–D). Compared with the first upper-abdominal enhanced CT scan, the liver indicates liver cirrhosis, and the mass become more smaller, and the FLR volume was 71%, which meets the requirements of the surgery. Magnetic resonance imaging demonstrated that the tumor load was reduced (Figure 8A and B), and the second contrast-enhanced ultrasound (CEUS) showed the focal liver lesion was still classified as LI-RADS 5 lesion category according to The Liver Reporting and Data System (LI-RADS). However, there was on obvious blood supply in the tumor, and it was smaller than before. The indocyanine green retention rate at 15 min was 8.4%, and the patient was reevaluated and considered as a good candidate for mirror right hemihepatectomy after multi-disciplinary meeting. At initial surgical exploration, ischemic line of the right live could be easily observed (Figure 9A). After separating the adhesion and controlling the hepatic blood flow, along the actual ischemic line of the mirror right liver, the hepatic parenchyma transection from the surface of the liver parenchyma to the superficial layer of the vena cava was performed using an ultrasonic scalpel and bipolar electrocoagulation. The mirror right hepatic pedicle was cut off using a linear cut stapler (LCS). Then, the remaining liver parenchyma close to the first hepatic hilum and in front of the inferior vena cava was continuously separated with an ultrasonic scalpel. The right hepatic vein (RHV) and inferior right posterior vein (IRHV) were both cut off with the LCS. Mirror right hemihepatectomy was carried out until the diseased liver was totally removed (Figure 9B). Lastly, an absorbable gelatin sponge and a surgical drain were placed over the transection plane. The operation time was 285 min with records of the intraoperative blood loss was 600 mL (including ascites)and 300 mL intraoperative autologous blood transfusion. Pathological examination reported that there are necrotizing nodules in the liver with surrounding inflammatory fibrous hyperplasia. There was a large number of inflammatory cells infiltrating the surrounding liver tissue without definite evidence of tumor (Figure 10A–C). The liver function index of the patient decreased to normal at 30 days after operation. The re-examination of enhanced CT in the upper abdomen suggested that there were fluid and gas in the operative area after liver cancer operation. The patient was discharged on postoperative day 10.
 |
Figure 6 Dynamic changes in tumor marker levels during treatment process. The decrease of des-γ-carboxy prothrombin level reflects the reduction of tumor load. Alpha-fetoprotein was fair. |
 |
Figure 8 Magnetic resonance imaging showing a mass (red arrow) measuring 4.0×3.4 × 3.0 cm in segment VII and VIII of liver, (A) T1-weighted image; (B) T2-weighted image. |
 |
Figure 9 Intraoperative photographs during stage II surgery. (A) Ischemic line (white arrow) of the right live in stage I surgery; (B) Liver cross-section. |
Discussion
HCC is a malignant tumor with a high fatality rate and poses a serious threat to the health of Chinese people. Radical surgical resection of liver tumors is still the main treatment method for PLC at the present stage. Previous studies have shown that the 5-year survival rate of HCC is about 10%, with poor long-term efficacy. Even for early liver cancer, the 5-year survival rate of patients undergoing surgical treatment is only 50–70%.36,37 Improving postoperative survival is a challenge for surgeons.
Conversion therapy means that after initial evaluation, the lesion does not meet current surgical indications, after giving the patients chemotherapy, radiotherapy, targeted drug therapy, immunotherapy and other treatments, the tumor reach surgical resection indications, prolong patients’ progression-free survival period and improve overall survival rates. In clinical practice, for patients who cannot achieve R0 resection due to causes such as an extremely large tumor volume or vascular invasion, conversion therapy can provide surgical opportunity for R0 resection after achieving the criteria of decreasing tumor volume and tumor stage, complete microvascular tumor thrombus necrosis, evaluation of complete/partial remission, and stable disease lasting for 3–4 months,27 but there is evidence that in some patients who cannot achieve R0 resection, the curative effect of liver tumor resection is not significantly higher than non-surgical treatment.38,39
Nowadays, it is believed that the mirror person with tumor is still a rare coincidence, and the reported cases of mirror person with tumor are sporadic. Although there are few reports of mirror person with tumors, some scholars speculate that some genes are involved in the distribution of left or right organs in the human body, and the mutation or deletion of these genes may be a susceptible factor for tumor formation. In 1600, the first case of human inverted heart disease was reported. In 1983, Kanematsu described the first case of HCC with SIT and only 11 papers of HCC patients with SIT have been reported in PubMed from 2000.32,40–48 At present, there are sporadic reports of gastric cancer, rectal cancer, pancreatic cancer, liver cancer and other tumors. To sum up, we believe that there is no exact relationship between congenital total visceral inversion and the formation of liver cancer. Patients with tumors complicated with SIT generally require surgical treatment. During the operation, the operator’s position, rich clinical experience and solid anatomical basis knowledge are very important. Due to the relatively increased difficulty of surgery in SIT patients, reverse thinking should be adopted during operation. The possibility of vascular variation can not be ruled out. It is important to understand and recognize the anatomy of a mirror-imaged liver.46 Abdominal CT/MRI and other enhanced examination are necessary. To sum up, although liver cancer complicated with SIT is rare in clinic, but it is not difficult to diagnose. The pathophysiology of the disease is normal, it is helpful to the diagnosis and differential diagnosis of chest and abdomen diseases, and avoid misdiagnosis and mistreatment.
The key feature of our case is that an extremely rare patient with visceral inversion complicated with hepatocellular carcinoma underwent secondary surgery after FLR inadequate stage I surgery combined with transformation treatment. In this case, we can know that pre-operation multidisciplinary evaluation and an adequate choice of surgical strategy are essential to ensure the safe operation for HCC patients with SIT. TSH can be obtained after PVL and sequential targeted immunotherapy in patients with advanced hepatocellular carcinoma with anatomical variation and potential resectability. This treatment strategy is a safe and effective method. Similar cases have never been reported before. Therefore, we want to share with readers a new method for the treatment of huge liver cancer.
Abbreviations
SIT, situs inversus totalis; TSH, two-stage hepatectomy; FLR, future residual liver; SLV, standard liver volumes; HBV, hepatitis B virus; AFB1, aflatoxin B1; RFA, radiofrequency ablation; PEI, percutaneous ethanol injection; TACE, transcatheter arterial chemoembolization; ORR, objective response rate; DoR, duration of response; OS, overall survival; CEUS, contrast-enhanced ultrasound; LI-RADS, Liver Reporting and Data System; HBsAg, hepatitis B surface antigen; HBeAb, hepatitis B e antibody; HBcAb, hepatitis C core antibody; ICG-R15, indocyanine green retention rate at 15 min; AFP, alpha-fetoprotein; DCP, des-γ-carboxy prothrombin; MELD, Model for End-Stage Liver Disease; MDT, multidisciplinary team discussion; BCLC, Barcelona Clinic Liver Cancer; CNLC, China Liver Cancer; PVL, portal branch ligation; HCV, hepatitis C virus; LCS, linear cut stapler; RHV, right hepatic vein; IRHV; inferior right posterior vein.
Ethics Approval and Consent to Participate
This study involving patient was reviewed and approved by the Ethics Committee of the First Affiliated Hospital of Guangxi Medical University, and the approval number is speedy trial 2021 (014). The patient provided his written informed consent to participate in this study.
Consent for Publication
Written informed consent was obtained from the patient to publish this case report.
Acknowledgments
We would like to acknowledge the helpful comments on this article received from our reviewers.
Author Contributions
All authors made a significant contribution to the work reported, whether that taking part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported in part by the Natural Science Foundation of Guangxi Province of China (No. 2020GXNSFAA159127).
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. doi:10.3322/caac.21590
3. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi:10.3322/caac.21338
4. Petrick JL, Florio AA, Znaor A, et al. International trends in hepatocellular carcinoma incidence, 1978–2012. Int J Cancer. 2020;147(2):317–330.
5. Zheng R, Zhang S, Zeng H, et al. Cancer incidence and mortality in China, 2016. J Natl Cancer Center. 2022;2(1):1–9.
6. Yuan JM, Govindarajan S, Henderson BE, Yu MC. Low prevalence of hepatitis C infection in hepatocellular carcinoma (HCC) cases and population controls in Guangxi, a hyperendemic region for HCC in the People’s Republic of China. Br J Cancer. 1996;74(3):491–493.
7. Wang XY, Huang JM, Lu XM, et al. Changing risk factors for hepatocellular carcinoma in hyperendemic regions in the era of universal hepatitis B vaccination. Cancer Epidemiol. 2020;67:101775.
8. Lin MV, King LY, Chung RT. Hepatitis C virus-associated cancer. Annu Rev Pathol. 2015;10:345–370.
9. Yamashita T, Honda M, Kaneko S. Molecular mechanisms of hepatocarcinogenesis in chronic hepatitis C virus infection. J Gastroenterol Hepatol. 2011;26(6):960–964.
10. Bartosch B, Thimme R, Blum HE, Zoulim F. Hepatitis C virus-induced hepatocarcinogenesis. J Hepatol. 2009;51(4):810–820. doi:10.1016/j.jhep.2009.05.008
11. Jeong SW, Jang JY, Chung RT. Hepatitis C virus and hepatocarcinogenesis. Clin Mol Hepatol. 2012;18(4):347–356. doi:10.3350/cmh.2012.18.4.347
12. Liu W, Guo TF, Jing ZT, et al. Hepatitis B virus core protein promotes hepatocarcinogenesis by enhancing Src expression and activating the Src/PI3K/Akt pathway. FASEB J. 2018;32(6):3033–3046. doi:10.1096/fj.201701144R
13. Tsuge M. The association between hepatocarcinogenesis and intracellular alterations due to hepatitis B virus infection. Liver Int. 2021;41(12):2836–2848. doi:10.1111/liv.15065
14. Lefeuvre C, Le Guillou-Guillemette H, Ducancelle A, Pleiotropic A. Role of the hepatitis B virus core protein in hepatocarcinogenesis. Int J Mol Sci. 2021;22:24. doi:10.3390/ijms222413651
15. Han C, Yu T, Qin W, et al. Genome-wide association study of the TP53 R249S mutation in hepatocellular carcinoma with aflatoxin B1 exposure and infection with hepatitis B virus. J Gastrointest Oncol. 2020;11(6):1333–1349. doi:10.21037/jgo-20-510
16. Zhu Q, Ma Y, Liang J, et al. AHR mediates the aflatoxin B1 toxicity associated with hepatocellular carcinoma. Signal Transduct Target Ther. 2021;6(1):299. doi:10.1038/s41392-021-00713-1
17. Zhou R, Liu M, Liang X, Su M, Li R. Clinical features of aflatoxin B1-exposed patients with liver cancer and the molecular mechanism of aflatoxin B1 on liver cancer cells. Environ Toxicol Pharmacol. 2019;71:103225. doi:10.1016/j.etap.2019.103225
18. Long XD, Ma Y, Zhou YF, Ma AM, Fu GH. Polymorphism in xeroderma pigmentosum complementation group C codon 939 and aflatoxin B1-related hepatocellular carcinoma in the Guangxi population. Hepatology. 2010;52(4):1301–1309. doi:10.1002/hep.23807
19. Su H, Han C, He Y, et al. Molecular mechanism of CK19 involved in the regulation of postoperative recurrence of HBV-associated primary hepatocellular carcinoma in Guangxi. Ann Transl Med. 2021;9(24):1780. doi:10.21037/atm-21-6020
20. Thomas MB, Zhu AX. Hepatocellular carcinoma: the need for progress. J Clin Oncol. 2005;23(13):2892–2899. doi:10.1200/JCO.2005.03.196
21. Graf D, Vallbohmer D, Knoefel WT, et al. Multimodal treatment of hepatocellular carcinoma. Eur J Intern Med. 2014;25(5):430–437. doi:10.1016/j.ejim.2014.03.001
22. Zhou J, Sun H, Wang Z, et al. Guidelines for the diagnosis and treatment of hepatocellular carcinoma (2019 edition). Liver Cancer. 2020;9(6):682–720. doi:10.1159/000509424
23. Zhuo W, Li A, Yang W, Duan J, Min J, Wei J. Case report: hepatic artery infusion chemotherapy after stage I ALPPS in a patient with huge HCC. Front Surg. 2021;8:746618. doi:10.3389/fsurg.2021.746618
24. Au KP, Chan ACY. Current status of associating liver partition with portal vein ligation for staged hepatectomy: comparison with two-stage hepatectomy and strategies for better outcomes. World J Gastroenterol. 2019;25(43):6373–6385. doi:10.3748/wjg.v25.i43.6373
25. Clavien P-A, Petrowsky H, Michelle L, Graf R. Strategies for safer liver surgery and partial liver transplantation. N England J Med. 2007;356(15):1545–1559. doi:10.1056/NEJMra065156
26. Adam R, Laurent A, Azoulay D, Castaing D, Bismuth H. Two-stage hepatectomy: a planned strategy to treat irresectable liver tumors. Ann Surg. 2000;232(6):777–785. doi:10.1097/00000658-200012000-00006
27. Zhao HT, Cai JQ. Chinese expert consensus on neoadjuvant and conversion therapies for hepatocellular carcinoma. World J Gastroenterol. 2021;27(47):8069–8080. doi:10.3748/wjg.v27.i47.8069
28. Sun HC, Zhou J, Wang Z, et al. Chinese expert consensus on conversion therapy for hepatocellular carcinoma (2021 edition). Hepatobiliary Surg Nutr. 2022;11(2):227–252. doi:10.21037/hbsn-21-328
29. Xu J, Shen J, Gu S, et al. Camrelizumab in combination with apatinib in patients with advanced hepatocellular carcinoma (RESCUE): a nonrandomized, open-label, phase II trial. Clin Cancer Res. 2021;27(4):1003–1011. doi:10.1158/1078-0432.CCR-20-2571
30. Mei K, Qin S, Chen Z, Liu Y, Wang L, Zou J. Camrelizumab in combination with apatinib in second-line or above therapy for advanced primary liver cancer: cohort A report in a multicenter phase Ib/II trial. J Immunother Cancer. 2021;9:3. doi:10.1136/jitc-2020-002191
31. Xu Q, Liu W, Lin C, Dang Y, Lin C. Transverse colon cancer with obstruction in a patient with situs inversus totalis: a case report and review of literature. Asian J Surg. 2020;43(12):1186–1188. doi:10.1016/j.asjsur.2020.09.003
32. Patel RB, Gupta NR, Vasava NC, Khambholja JR, Chauhan S, Desai A. Situs Inversus Totalis (SIT) with Hepatocellular Carcinoma (HCC): a rare case report and review of 12 other cases. Indian J Surg. 2013;75(6):424–429. doi:10.1007/s12262-012-0744-9
33. Schima W, Heiken J. LI-RADS v2017 for liver nodules: how we read and report. Cancer Imaging. 2018;18(1):14. doi:10.1186/s40644-018-0149-5
34. Maupoey Ibanez J, Montalva Oron EM, Bosca Robledo A, et al. From conventional two-stage hepatectomy to ALPPS: fifteen years of experience in a hepatobiliary surgery unit. Hepatobiliary Pancreat Dis Int. 2021;20(6):542–550. doi:10.1016/j.hbpd.2021.08.001
35. Deng Z, Jin Z, Qin Y, et al. Efficacy of the association liver partition and portal vein ligation for staged hepatectomy for the treatment of solitary huge hepatocellular carcinoma: a retrospective single-center study. World J Surg Oncol. 2021;19(1):95. doi:10.1186/s12957-021-02199-1
36. Zeng H, Zheng R, Guo Y, et al. Cancer survival in China, 2003–2005: a population-based study. Int J Cancer. 2015;136(8):1921–1930. doi:10.1002/ijc.29227
37. European Association for the Study of the Liver. Electronic address: eee, European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236.
38. Xie DY, Ren ZG, Zhou J, Fan J, Gao Q. 2019 Chinese clinical guidelines for the management of hepatocellular carcinoma: updates and insights. Hepatobiliary Surg Nutr. 2020;9(4):452–463.
39. Wang K, Guo WX, Chen MS, et al. Multimodality treatment for hepatocellular carcinoma with portal vein tumor thrombus: a large-scale, multicenter, propensity mathching score analysis. Medicine. 2016;95(11):e3015.
40. Niki Y, Shiraki K, Enokimura N, et al. Hepatocellular carcinoma associated with situs inversus totalis. J Clin Gastroenterol. 2004;38(4):382–383.
41. Kakinuma D, Tajiri T, Yoshida H, et al. A case of hepatocellular carcinoma with situs inversus totalis. J Nippon Med Sch. 2004;71(3):209–212.
42. Li T, Wang L, Chen RX, et al. Hepatocellular carcinoma with situs inversus totalis and polysplenia syndrome. Liver Int. 2007;27(10):1430–1431.
43. Fu RD, Li JY, Zhang XH, Chen HW. Right hemihepatectomy via an anterior approach for hepatocellular carcinoma in a situs inversus totalis patient. Case Rep Gastroenterol. 2020;14(1):91–97.
44. Liao KX, Li JW, Author Reflections ASO, New Minimally A. Invasive procedure for hepatocellular carcinoma with situs inversus totalis. Ann Surg Oncol. 2021;28(11):6832–6833.
45. Liao KX, Cao L, Zheng SG, Li JW. Pure laparoscopic anatomic right hemihepatectomy for hepatocellular carcinoma with situs inversus totalis using indocyanine green fluorescence staining (with video). Ann Surg Oncol. 2021;28(11):6830–6831.
46. Harada K, Masuda T, Beppu T, et al. Hepatic resection using a liver-hanging maneuver and Glissonean pedicle transection for hepatocellular carcinoma in a patient with situs inversus totalis: report of a case. Surg Today. 2012;42(8):801–804.
47. Xu JB, Xu G, Chen GF, Gu DH, Zhang JH, Qi FZ. Hepatocellular carcinoma with hypersplenic thrombocytopenia and situs inversus totalis: a case report. Chin Med Sci J. 2016;31(2):134–136.
48. Kamiike W, Itakura T, Tanaka H, et al. Hepatic segmentectomy on primary liver cancer with situs inversus totalis. HPB Surg. 1996;9(3):169–172.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.