Back to Journals » Vascular Health and Risk Management » Volume 19
Markedly Low Cardio-Ankle Vascular Index in Aortic Valve Stenosis: Vital Possible Cause Not to Be Overlooked [Letter]
Authors Takahashi K , Yamamoto T
Received 20 June 2023
Accepted for publication 22 June 2023
Published 27 June 2023 Volume 2023:19 Pages 351—352
DOI https://doi.org/10.2147/VHRM.S426040
Checked for plagiarism Yes
Editor who approved publication: Prof. Dr. Pietro Scicchitano
Koji Takahashi, Tomoyuki Yamamoto
Fukuda Denshi Co., Ltd, Tokyo, Japan
Correspondence: Tomoyuki Yamamoto, Email [email protected]
View the original paper by Dr Plunde and colleagues
Dear editor
We have read with great interest the case report entitled, “Pulse Wave Morphology Changes in Aortic Valve Stenosis Detected with Cardio-Ankle Vascular Index” by Plunde et al.1 They reported the pulse wave morphology changes after aortic valve replacement (AVR) surgery due to aortic valve stenosis (AVS), showing steeper upstroke slope as well as increased cardio-ankle vascular index (CAVI) measurements, and emphasized the usefulness of CAVI in AVS screening. Their findings provide a meaningful new perspective on the relationship between pulse waveforms and CAVI. However, this case report needs and deserves further consideration. The purpose of this letter is to present an important possible cause for the markedly low CAVI measurements in AVS.
We reported that the reasons for the decrease in CAVI in AVS can be mainly explained by the prolongation of ejection time associated with the decrease in average blood flow velocity, and the decrease in dynamic elastic modulus of the arteries due to decreased frequency of the pulse wave.2 Those are sufficient to explain the changes in CAVI from around 7 to 9, as previously reported.3 However, in this case report, CAVI increased sharply from 4.7 to 9.35 before and after surgery, which is difficult to explain only based on the reasons we have clarified to date. As a result of further investigation, here we present a vital possible cause not to be overlooked.
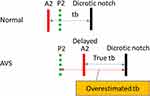
That is the abnormal second heart sound (HS2) known as “Paradoxical Splitting”.4 CAVI detects the HS2 as the point of aortic valve closure. The HS2 normally consists of the sounds for aortic valve closure (A2), followed by pulmonary valve closure (P2). However, in AVS, the order of A2 and P2 may be reversed due to the delay of aortic valve closure from the prolonged period in which left ventricular pressure is higher than aortic pressure. In this case, tb, which is the time from HS2 to the dicrotic notch of the brachial pulse, is overestimated, resulting in a decrease in CAVI (Figure 1).
Systolic heart murmur associated with AVS is seen in the phonocardiogram of the case report, but it does not appear to affect the CAVI measurements as the authors reported. Nevertheless, tb estimated from the figure is significantly long, and the Paradoxical Splitting of HS2 is suspected, though it is not clear enough to see. This HS2 abnormality can occur not only in AVS but also in left ventricular dysfunction, such as left bundle branch block and hypertrophic cardiomyopathy.5
The measurement range of CAVI is from heart to the ankle, and if there is an abnormality in the heart which affects heart sounds, the result will also be abnormal. This is a special feature of CAVI which is not found in other arterial indices, because CAVI utilizes the direct information from the heart. Therefore, careful observation is necessary when markedly low CAVI is measured; it may lead to the discovery of hidden heart diseases, as Plunde et al emphasized.
Further research and development are expected in the future.
Disclosure
Koji Takahashi and Tomoyuki Yamamoto are employees of Fukuda Denshi Co., Ltd. and involved in the development of CAVI. The authors report no other conflicts of interest in this communication.
References
1. Plunde O, Franco-Cereceda A, Bäck M. Pulse wave morphology changes in aortic valve stenosis detected with cardio-ankle vascular index. Vasc Health Risk Manag. 2023;19:325–328. doi:10.2147/VHRM.S401221
2. Takahashi K, Yamamoto T, Shirai K. Reasons for the decline of cardio-ankle vascular index (CAVI) in aortic valve stenosis (AVS) [Letter]. Vasc Health Risk Manag. 2022;18:18 887–888. doi:10.2147/VHRM.S399737
3. Plunde O, Franco-Cereceda A, Bäck M. Cardiovascular risk factors and hemodynamic measures as determinants of increased arterial stiffness following surgical aortic valve replacement. Front Cardiovasc Med. 2021;8:754371. doi:10.3389/fcvm.2021.754371
4. Kumar S, Luisada AA. Mechanism of changes in the second heart sound in aortic stenosis. Am J Cardiol. 1971;28:162–167. doi:10.1016/0002-9149(71)90364-x
5. Felner JM. The Second Heart Sound. In: Walker HK, Hall WD, Hurst JW, editors. Clinical Methods: The History, Physical, and Laboratory Examinations.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.