Back to Journals » Drug Design, Development and Therapy » Volume 18
Mapping the Research of Ferroptosis in Parkinson’s Disease from 2013 to 2023: A Scientometric Review
Authors Chen Y , Wu Z, Li S, Chen Q, Wang L , Qi X, Tian C, Yang M
Received 4 January 2024
Accepted for publication 5 March 2024
Published 3 April 2024 Volume 2024:18 Pages 1053—1081
DOI https://doi.org/10.2147/DDDT.S458026
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Anastasios Lymperopoulos
Yingfan Chen,1,2,* Zhenhui Wu,3,* Shaodan Li,2 Qi Chen,2 Liang Wang,2 Xiaorong Qi,1 Chujiao Tian,1 Minghui Yang2
1Medical School of Chinese People’s Liberation Army, Beijing, People’s Republic of China; 2Department of Traditional Chinese Medicine, the Six Medical Center of Chinese People’s Liberation Army General Hospital, Beijing, People’s Republic of China; 3College of Pharmacy, Jiangxi University of Chinese Medicine, Nanchang, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Minghui Yang, Department of Traditional Chinese Medicine, the Six Medical Center, Chinese People’s Liberation Army General Hospital, 6 Fucheng Road, Beijing, 100048, People’s Republic of China, Tel +86-10-66939651, Email [email protected]
Methods: Related studies on PD and ferroptosis were searched in Web of Science Core Collection (WOSCC) from inception to 2023. VOSviewer, CiteSpace, RStudio, and Scimago Graphica were employed as bibliometric analysis tools to generate network maps about the collaborations between authors, countries, and institutions and to visualize the co-occurrence and trends of co-cited references and keywords.
Results: A total of 160 original articles and reviews related to PD and ferroptosis were retrieved, produced by from 958 authors from 162 institutions. Devos David was the most prolific author, with 9 articles. China and the University of Melbourne had leading positions in publication volume with 84 and 12 publications, respectively. Current hot topics focus on excavating potential new targets for treating PD based on ferroptosis by gaining insight into specific molecular mechanisms, including iron metabolism disorders, lipid peroxidation, and imbalanced antioxidant regulation. Clinical studies aimed at treating PD by targeting ferroptosis remain in their preliminary stages.
Conclusion: A continued increase was shown in the literature within the related field over the past decade. The current study suggested active collaborations among authors, countries, and institutions. Research into the pathogenesis and treatment of PD based on ferroptosis has remained a prominent topic in the field in recent years, indicating that ferroptosis-targeted therapy is a potential approach to halting the progression of PD.
Keywords: Parkinson’s disease, ferroptosis, bibliometric analysis, iron accumulation, alpha-synuclein, mitochondrial dysfunction, oxidative stress, lipid peroxidation
Introduction
Parkinson’s disease (PD) is the second most common central neurodegenerative disorder globally,1 with the fastest-growing prevalence and disability rate.2 By 2040, more than 12 million PD patients are predicted worldwide.3 Cardinal motor symptoms, including bradykinesia, rigidity, and tremor, are of crucial importance in the accurate diagnosis of PD, and non-motor manifestations such as constipation, depression, anosmia, cognitive decline, and sleep disorders are prominent during the disease course.4 The etiology and pathogenesis of PD remain unclear, while compelling evidence suggests that complex interactions between genetic, environmental, and age factors determine the risk and progression of PD,5 and various mechanisms, such as oxidative stress (OS), neuroinflammation, and mitochondrial dysfunction, are intimately involved in pathophysiological development. The gradual degeneration of dopaminergic neurons in the substantia nigra (SN), along with abnormally aggregated α-synuclein (α-syn) forming Lewy bodies, are the pathological hallmarks of PD.6 Although the current dopamine-based pharmacological therapies can alleviate motor symptoms in early-stage patients, there is a lack of effective neuroprotective treatment methods to prevent PD from progressively deteriorating. Therefore, investigating the underlying mechanisms of PD to explore novel targets and develop the next generation of therapies is urgently needed.
Ferroptosis, a newly proposed cell death pathway characterized by iron-dependent reactive oxygen species (ROS) accumulation and lipid peroxidation of polyunsaturated fatty acids (PUFAs) present in plasma membranes, has been found to be a key player in the pathomechanism of PD and has gained the spotlight.7 Recent studies have increasingly indicated that the principal features or triggering factors of ferroptosis, including iron ion imbalance, reduced glutathione levels, lipid peroxidation, DJ-1 depletion, and coenzyme Q10 (CoQ 10) decline, are consistent with the pathological features observed in PD,8–14 suggesting a strong link between ferroptosis and the development of PD. Moreover, ferroptosis is a novel potential therapeutic target for PD, as ferroptosis inhibitors or iron chelators considerably attenuate the death of dopaminergic neurons and impede PD progression. Thus, studies into the aetiopathogenesis and therapeutic strategy of PD based on ferroptosis have attracted widespread attention, with its extensive research potential and value.
The past decade has witnessed a consistent rise in the amount of related research in this field. However, bibliometric analysis and visualization methodologies have rarely been used in this study area. CiteSpace, a Java-based information data processing tool that can analyze and visualize trends and patterns of related literature through metrics, co-occurrence analysis, and clustering analysis, is one of the most widely deployed bibliometric software in various research fields.15 Therefore, in this study, we conducted a comprehensive analysis of relevant literature accessed from the WOSCC database mainly based on CiteSpace software, aiming to explore the current status, hot topics and potential trends of ferroptosis in PD mechanism research.
Materials and Methods
Data Sources and Search Strategy
The original literature data were extracted from the WOSCC database, with the time span for retrieval extending from extending from the database’s inception to May 23, 2023. The search query employed was TS=(Parkinson or Parkinson’s disease) AND TS=(Ferroptosis). The recorded contents comprised the quantity of papers and citations, H-index, publication year, countries/regions, affiliations, authors, journals, cited references, and keywords. A total of 167 articles were retrieved. After deduplication, screening, and reading, 160 articles were ultimately selected based on the predetermined inclusion and exclusion criteria (Figure 1).
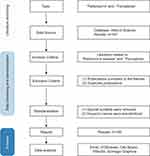 |
Figure 1 Detailed process for literature screening. |
Inclusion Criteria
(1) Literature related to ferroptosis and Parkinson’s disease;
(2) Published in English;
(3) The types of literature included clinical trial studies, in vitro experimental studies, in vivo experimental studies, public database analysis studies, reviews, etc. ;
(4) Complete bibliographic information (including title, country, authors, keywords, source).
Exclusion Criteria
(1) Duplicated publications;
(2) Publications unrelated to the themes.
Data Conversion and Standardization
After completing the screening process, the literature was exported in both Refworks and plain text formats. All special symbols were removed, and keywords were standardized. For example, “Parkinsons disease” was merged into “Parkinson’s disease”, and “Parkinson’s-disease” was confirmed as “Parkinson’s disease”. Format conversion of the retrieved literature was performed using the Data Import/Export feature in CiteSpace software.
Analysis Tools and Methods
CiteSpace (Version 6.1. R3), VOSviewer (Version 1.6.18), and RStudio software (Version R-4.2.2) were employed as the main visualized analysis tools for statistical computing and graphics. R, a highly extensible language or environment capable of automating statistical computing and graphics, served to visually present journal literature, countries, cited authors, and keyword clouds. VOSviewer software assisted in constructing maps of core authors and keywords. Moreover, CiteSpace software is mainly adopted for analyzing keywords, research institutes and cited literature. Keyword integration was conducted, and synonyms were merged. Software-based keyword categorization or classification relies on mainstream bibliometric algorithms. The size of nodes in the graph depicts the frequency of occurrence, while the connecting lines represent co-occurrence relationships, and centrality is a metric for assessing node significance in the visual network. LLR algorithm analysis, a clustering label extraction algorithm provided by CiteSpace, was implemented for keyword clustering, where the silhouette average silhouette value (S value) and modularity (Q value) indicate the efficiency of clustering. Generally, an S value above 0.5 suggests reasonable clustering, and a Q value above 0.3 represents a significant clustering structure. In the visual analysis of authors, the evaluation was performed based on Price’s law formula (Mp=0.749*√Npmax, Npmax= the maximum number of papers published by the most productive authors), in which core authors have more than Mp publications.
Results
Analysis of Literature by Year of Publication and Journal Source
The analysis of the volume of publications concerning ferroptosis in the PD domain demonstrated an overall upward trend, despite the initial low base number (Figure 2A). The first article appeared in 2013, and the highest peak of publications occurred in 2022, with 40 articles. The publication trend is divided into two phases: from 2013 to 2018 as the initial phase, with an annual publication volume consistently below 10 articles; from 2018 to the present as the stable growth phase, where the trend shows a steady increase, indicating growing interest from scholars in this field.
The articles were published in a total of 105 journals, among which Free Radical Biology and Medicine had the most frequent publications, with 13 articles, as displayed in Figure 2B. Based on Bradford’s law, the following 12 journals were identified as core journals in this field: Free Radical Biol. Med., Oxid. Med. Cell. Longevity, Antioxidants, Mol. Neurobiol., Neuroscience, Ageing Res. Rev., Cells, Front. Cell. Neurosci., Front. Pharmacol., Neurobiol. Dis., Acta Pharmacol. Sin., and Antioxid. Redox Signaling (Figure 2C). J Biol Chem, with 479 citations, held the topmost position in the field of Parkinson’s disease and ferroptosis research (Figure 2D). Furthermore, the publication volume for Free Radical Biol. Med. and Oxid. Med. Cell. Longevity experienced noticeable growth after 2018 and 2021, respectively (Figure 2E).
The dual-map overlay of journals illustrated the citation relationships between citing and cited journals, with the left side representing the cluster of citing journals and the right side representing the cluster of cited journals. The orange path in Figure 2F depicts that journals from the domains of Molecular Biology and Genetics possess the highest likelihood of being cited by journals in the fields of Molecular Biology and Immunology.
Country/Regional Contributions to Global Publications
Figure 3A shows the literature from 35 countries that have published studies related to ferroptosis and PD. Typically, this sort of information is depicted by maps or bubble charts. The top ten countries in terms of the number of publications were China (84 articles), USA (27 articles), Australia (16 articles), France (12 articles), England (11 articles), India (8 articles), Germany (7 articles), Japan (7 articles), Sweden (5 articles), and Italy (4 articles). The country with the most citations was USA (3546 articles), followed by China and Australia (Figure 3B). In Figure 3C and D, which illustrate the collaborative relationships among various countries in the research on ferroptosis and PD, the thickness of the connecting line between any two countries is directly proportional to the extent of their cooperation in the respective research field. Based on this, we can infer that China had a closer working relationship with other countries, especially in cross-continental collaborations. Single Country Publications (SCP) in Figure 3E indicates that all the authors of an article come from the same country, while Multiple Country Publications (MCP) indicates that the article’s authors are from multiple countries. It is inferred that both China and Australia boast the largest number of co-authors from foreign nations within this field, while internal collaborations in China still accounted for a larger proportion.
In summary, this result reveals the global cooperation patterns and trends related to ferroptosis and PD. Such information could aid researchers in ascertaining which countries wield greater influence in this field, as well as which nations collaborate more frequently. Furthermore, it could assist researchers in identifying appropriate collaboration partners and gaining insight into the worldwide research landscape.
Author and Affiliations Analysis
According to the visualization chart of 160 screened publications, a total of 958 authors performed related studies. The top 10 authors ranked by the output of publications are displayed in Figure 4A, and their annual publication variations are displayed in Figure 4B. The highest quantity of publications (9 articles) belongs to Devos, David from Université Lille Nord de France. The author with the most cited documents was Bush AI from the University of Melbourne, with 152 articles, as shown in Figure 4C. Mp = 1.98 was determined based on Price’s law formula. Accordingly, authors with ≥2 publications were designated core authors, and there existed 84 core authors in total. In the co-authors network (Figure 4E), nodes stand for authors, whose publication count is indicated by node size. The number of connecting lines illustrates the degree of cooperation between authors, and different colours denote various cooperation groups. The results indicated the formation of 4 closely collaborating academic groups in this field, including Zhu ML, Devos D, Cao J, and Huang DY. Their research focused on experimental studies of Sorting Nexin 5,16,17 lipid metabolism,18,19 DJ-1 proteins,20 and iron chelators21 in regulating ferroptosis in PD.
Figure 4D, a CiteSpace-based institutional co-occurrence map, contains 162 nodes and 378 lines. Each institution is symbolized by a node, with a larger node signifying higher productivity of the institution. Lines connecting the nodes indicate institutional cooperation, and their color and thickness represent the duration and intensity of the cooperation. Betweenness centrality (BC) represents the importance of the node in the network. The top 10 institutions out of a total of 162 institutions conducting ferroptosis in PD by volume and centrality are listed in Table 1. The University of Melbourne had the highest publication volume with 12 articles, followed by Florey Institute of Neuroscience & Mental Health and Research Libraries UK (RLUK), while the top three institutions in terms of centrality were RLUK, Institut National de la Sante et de la Recherche Medicale (Inserm) and University of Melbourne. University of Melbourne, the Florey Institute of Neuroscience & Mental Health, Inserm and Centre National de la Recherche Scientifique (CNRS), among others, were found to possess closer co-operations. These findings demonstrated that the majority of institutions were universities from various nations, with a higher rate of collaboration observed among institutions in Europe and Oceania.
 |
Table 1 Top 10 Institutions by Output (Left) and Centrality (Right) |
Co-Cited References Analysis
Highly cited papers assist in detecting pioneering research within a research field and are used within the WOS to identify the most influential research papers. The top 1% of papers by cited frequency were listed in Figure 5A. Citation burst refers to a sharp frequency increase in the citations of a specific article during a particular time period and serves to unearth the latest high-interest research topics in related fields. The 20 cited references with the highest citation bursts are presented in Figure 5B, where the blue line depicts the interval of time with citation emergence, and the red line indicates the period of time when a citation burst of a reference was detected.22 Cocitation refers to that two or more articles are cited by one or more papers at the same time, and the two articles are considered to be a cocitation relationship. As a result, 16 highly cited papers (Figure 5A), 20 references with the strongest citation burst (Figure 5B), and 32 papers with co-citation frequency not less than 20 (Figure 5C) were extracted, which we refer to as a, b, and c for convenience. Through Venn analysis (Figure 5D), it was found that 1 paper is in a, b, and c simultaneously, which was published by Do Van B on NEUROBIOL DIS in 2016 and is the first to emphasize the role of ferroptosis in PD,23 making it one of the most influential papers. 6 papers in both a and c; 14 papers in both b and c. The detailed information, in particular the main highlights and contributions of each paper, is listed in Table 2.
 |
Table 2 The Detailed Information of the 16 Highly Cited Papers, 20 References with the Strongest Citation Burst, and 33 Papers with Co-Citation Frequency Not Less Than 20 |
The thematic coupling analysis of the co-cited literature (Figure 5E) identified a total of five clusters: the blue cluster contained most of the articles on the mechanisms of ferroptosis;38,65 the red cluster mainly focused on ferroptosis-targeted therapies;23,39 the green cluster concentrated on iron metabolism in neurodegenerative pathologies;41 the yellow cluster included articles with content primarily related to mitochondrial dysfunction;45,66 and the purple cluster featured researches on molecular therapeutics of PD.19,67
Clustering analysis of co-cited references proves to be an effective method for uncovering the cutting-edge topics of the field.68 Further clustering results for these references are displayed in Figure 5F, and in Figure 5G, each cluster label is matched by the corresponding timeline. Clusters #0 and #1 indicated that ACSL4 inhibitors (ferroptosis inhibitors) and lipofuscin remain the ongoing focal points of research to date. Clusters #0 ACSL4 inhibitors, #1 lipofuscin, #3 ROS regulation, #9 chloroquine, and #10 ceruloplasmin emerge as significant therapeutic targets; Clusters #2 cancer and #4 cognitive dysfunction are other domains having dependencies; Clusters #5 neuronal cells, #6 transcription, #7 cell death, and #8 necrosis are related to the corresponding mechanisms under investigation.
Keywords Analysis
Keywords Co-Occurrence Analysis
The analysis of keywords portrays the professional focus of an article or an author, providing an overall synopsis of the corresponding research pattern.69 High-frequency keywords signify a widely explored topic of investigation, and high-centrality keywords highlight the significance and impact of the related research content within this particular field.70
As shown in Figure 6A-C, 50 keywords appeared more than 5 times in total. Remarkably, in addition to ferroptosis and PD, OS/Reactive oxygen species (ROS), iron/iron metabolism, lipid peroxidation/polyunsaturated fatty acids, glutathione peroxidase 4 (GPX4)/n-acetylcysteine (NAC), α-syn, mitochondrial dysfunction, nuclear factor E2-related factor 2 (Nrf2), and mouse/in vivo/in vitro models were all ranked. Combined keyword co-occurrence analysis (Figure 6D) reveals a research emphasis on in vivo experiments, mechanism of action and targeted therapy. Among the animal models implemented in the field, mouse models, particularly 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) models, were the most frequently employed. Additional animal models included the 6-OHDA model, rotenone model, α-Syn model, LPS model, and PINK1 knockout model, among others. In terms of mechanistic studies, the primary focus was on the molecular regulation or signaling pathways that play a role in regulating apoptosis, OS, lipid peroxidation, iron metabolism, and mitochondrial function, thereby affecting cellular ferroptosis in PD. Commonly used drugs and drug targets in experimental studies based on cellular, mouse and other models included glutathione (GSH), GPX4, Nrf2, protein kinase C (PKC) inhibitors, lipoxygenase (LOX) inhibitors, and iron chelators.
Keyword Citation Burst Analysis
By analyzing the emergent keywords, it is feasible to identify the focal themes of research in the field over time and to predict research trends. As illustrated in Figure 7A, with the blue line denoting the time interval and the red line signifying the duration during which the keyword bursts were detected, the keywords demonstrating the strongest emergence intensity were mitochondrial dysfunction and iron metabolism. Furthermore, iron accumulation, cognitive disorders, and antioxidants remain research hotspots to date.
 |
Figure 7 Visualization Map of Keyword bursts and time zone Analysis. (A) Keyword Emergence. (B) Keyword timeline. (C) Keyword Time Zone. (D) Trends in thematic terms. |
Combining the keyword time zone map, timeline map and topic trend, as shown in Figure 7B-D, the evolution of research hotspots in this field is divided into 3 time phases: (1) from 2013–2016, only research-related basic terms, such as Parkinson’s disease, iron, mouse model, OS and apoptosis, appeared in the keywords, indicating that academics had started researching in the field, but the heat is lacking; (2) from 2017–2020, keywords including in vivo experiments, targeting GPX4, and mitochondrial dysfunction garnered significant attention, leading to an apparent boom in research on antioxidant effects; and (3) from 2021–2023, the α-Syn model, double-blind trials, and iron accumulation have successively become research hotspots, indicating a shift towards clinical trials of targeted therapies. Based on this, it is predicted that antioxidant therapies, clinical trials, and specific molecular targets will be the hotspots of future research in the field of ferroptosis and PD after 2023.
Discussion
This paper employs software tools such as CiteSpace, VOSviewer, and the RStudio, to summarize and collate a total of 160 publications related to ferroptosis in PD from the WOSCC database, with records up to May 23, 2023. By creating knowledge maps, the paper provides a visual representation of the publication status, journal sources, research authors, institutional collaboration networks, related keywords, and citation situations in this field for the ten years since the first relevant article was published in 2013. Ferroptosis, a novel modality of cell death, has received widespread attention and has shown a steady growth trend in publication volume over the past decade. Currently, the United States, China, and Australia are leading in this research field. Specifically, the United States has the most published and most cited journals (Free Radical Biology and Medicine, J Biol Chem), making it the most cited country, with the second-highest overall number of publications. China leads in the total number of publications, with citation counts second only to the United States. The University of Melbourne is the institution with the most publications, and Bush AI from this university is the author with the most citations and the highest influence.
The types of publications in this field mainly include in vitro and in vivo experiments, reviews, and information from public databases, with reviews accounting for approximately half of the publications. These reviews tended to describe the molecular pathways between cellular ferroptosis and PD along with potential treatment strategies based on these pathways, reflecting the current focus of researchers on developing a scientific basis and potential new targets for treating PD based on ferroptosis. Current relevant experimental researches primarily aim to explore the scientific validity and feasibility of various compounds, especially natural small molecule compounds, as potential inhibitors of PD-related ferroptosis. Keyword analysis has indicated that current research hotspots are the mechanisms of iron metabolism disorders, lipid peroxidation, and imbalanced antioxidant regulation, involving specific targets such as GPX4, NRF2, and ACSL4. It should be highlighted that clinical research aimed at treating PD by targeting ferroptosis is an important direction and ultimate goal for future investigation and remains in its preliminary stages.
The Correlation Between Ferroptosis and the Pathological Mechanism of PD
The results of keyword co-occurrence and emergence analysis showed that the keywords iron accumulation, α-syn, mitochondrial dysfunction, OS, lipid peroxidation and antioxidant had a high co-occurrence frequency and emergence intensity, indicating that the exploration of the ferroptosis pathway in PD is the hotspot and trend of current research, which is conducive to the search for a new therapeutic target targeting ferroptosis.
Iron Accumulation
In the central nervous system, the ability of iron to circulate in its oxidized state and to form ligand bonds makes it an important cofactor for numerous enzymes involved in a variety of fundamental physiological processes. However, overloaded iron breaks down peroxides by triggering the Fenton reaction, resulting in the production of high levels of hydroxyl radicals that cause oxidative damage to cells. Iron also acts as a LOX enzyme family cofactor, enabling the formation of lipid peroxides and playing a central role in cellular ferroptosis.71,72 Several studies have shown that cellular iron overload leads to the accumulation of lipofuscin, intralysosomal aggregates mainly composed of lipids and oxidized proteins, and the amount of iron contained in lipofuscin plays a crucial role in its cytotoxicity.73,74 The iron in lipofuscin catalyzes the formation of free radicals, jeopardizes the stability of the lysosome, triggers apoptosis and induces mitochondrial dysfunction.75–78 Shi L et al found that lipofuscin accumulated significantly in the SN neurons and the accumulation levels were positively correlated with iron content, DA neuron loss and PD scores, suggesting that MPTP-induced oxidative stress, marked by lipofuscin, leads to the accumulation of iron, which in turn creates a feed-forward loop that exacerbates the toxicity of MPTP.79 Meanwhile, excessive iron accumulation in the SN represents a key pathological characteristic of PD. Iron is deposited selectively in certain brain regions, including the SN, caudate nucleus and pallidum, as humans age, and this phenomenon is particularly severe in PD patients,80 with iron levels positively correlating with the PD process and its associated motor dysfunction.81,82 Inductively coupled plasma spectroscopy,83 magnetic resonance imaging,84 laser microprobe mass spectrometry,85 susceptibility-weighted imaging,86,87 enhanced T2-weighted angiography,88 and single photon emission tomography of the dopamine transporter89 are all complementary diagnostic tools that confirmed a link between dopaminergic striatal impairment and nigrostriatal iron deposition in PD patients. Ferroptosis was found to precede apoptosis in dopaminergic cells treated with the iron fortifier ferric ammonium citrate (FAC).42 Abnormalities in iron metabolism, including the down-regulation of iron transport protein (FPN),90 up-regulation of divalent metal transfer protein 1 (DMT1),91 and reduced plasma cuprocyanin activity of iron oxidase, have also been reported in animal models and PD patients.92,93 Furthermore, iron chelation therapies have demonstrated the capability to attenuate motor deficits and cognitive impairment in tau-knockout and MPTP-induced PD mouse models.26,94,95 The iron chelator DFO rescued FAC-induced iron overload and MPP+-induced ferroptosis in nerve growth factor (NGF) treated PC12 cells.21 Additionally, a pilot clinical study evidenced enhanced GPX activity in cerebrospinal fluid and improvements in motor symptoms in PD patients by iron chelation therapy.26 This evidence also reinforced the pathological link between iron-dependent cell death and PD at a therapeutic level.
Oxidative Stress
OS is one of the major factors in the progressive loss of dopaminergic neurons in PD. OS arises from an imbalance in the redox state, necessitating both an accumulation of ROS products in the cell and a dysregulation of antioxidant protection mechanisms. Excessive amounts of free Fe2+ and H2O2 present in the cytoplasm can generate OH− radicals via the Fenton reaction, and OH− is one of the most unstable ROS products. Meanwhile, an antioxidant system operates within the cell to reduce ROS activity products, thereby maintaining redox balance and defending against cell death caused by OS reactions. GPX4 is a crucial GSH-dependent antioxidant enzyme, and decreased levels of GSH10 and GPX496,97 were observed in the brain tissues of PD patients. A recent blood-based PD holomethylation association study98 showed that hypermethylation of the promoter region of the SLC7A11 (system xc-) gene was associated with the risk of PD. System xc-, an antiporter for cellular uptake of cystine, has a function directly related to intracellular GSH levels. The classic ferroptosis inducer (FIN) erastin triggers ferroptosis by depleting GSH using system xc- as the molecular target, while another type of FIN, like RSL3, acts by directly inhibiting GPX4. The antioxidant enzyme DJ-1 maintains cysteine and GSH biosynthesis by safeguarding the transsulphur pathway, and loss-of-function mutations in the DJ-1 gene have been evidenced to be associated with autosomal recessive early-onset PD.99 Meanwhile, DJ-1 depletion enhances cellular susceptibility to ferroptosis,100 and DJ-1 overexpression markedly inhibits erastin-induced ferroptosis.20 Nrf2 is a transcription factor with antioxidant effects, offering protection against oxidative damage by activating the gene and protein expression of detoxification enzymes and antioxidant proteins. Activation and nuclear translocation of Nrf2 ameliorates pathological phenotypic changes and motor deficits in rat and Drosophila PD models101 and attenuates neuronal damage induced by MPP+, 6-OHDA, etc.37,102 Furthermore, Nrf2 deficiency leads to the up-regulation of iron death markers in PD-associated brain regions, and a vicious cycle of α-syn overexpression and NRF2 inhibition results in increased ferroptosis sensitivity of neuronal cells.103 Coenzyme Q10 (CoQ10) and its reduced state (CoQ10-H2) are potent mitochondrial and lipid antioxidants and are considered to be the second endogenous mechanism for inhibiting ferroptosis, functioning independently of GPX4.12,104 Additionally, reduced CoQ10 has been observed in PD patients and animal models, and CoQ10 supplementation has been found to attenuate PD-associated degenerative changes.105–107
Lipid Peroxidation
Lipid metabolism and cellular lipid structure are major determinants of ferroptosis susceptibility in cells. Evidence from in vitro and in vivo PD models induced by commonly used neurotoxins, including MPTP, rotenone, paraquat (PQ) and 6-OHDA, has shown that abnormalities in lipid metabolism play a key role in driving ferroptosis.23 Brain autopsy analyses have shown reduced levels of PUFAs and increased levels of malondialdehyde (MDA), a lipid peroxidation (LPO) product, and plasma lipid hydroperoxides (LH) in the SN of PD patients.108,109 According to relative reports, the levels of LH, MDA and 1-hydroxynonenal (4-HNE) in the peripheral blood of PD patients increased with the clinical progression of PD, with MDA and 4-HNE having a higher prognostic value.110 It was also found that membrane lipids doped with activated PUFAs (eg, phospholipids) are peroxidative substrates necessary for ferroptosis, a process requiring the involvement of specific enzymes.18,57 Acetyl coenzyme A (CoA) synthetase long-chain family member 4 (ACSL4) and lysophosphatidylcholine acyltransferase 3 (LPCAT3) participate in the activation and incorporation of PUFAs into membrane-localized lipids and were the earliest identified ferroptosis-promoting gene products whose inactivation was proven to render cells resistant to RSL3- and ML162-induced ferroptosis.33,56 ACSL4 expression was increased in the SN of MPTP-treated PD mice and PD patients, and ACSL4 inhibition specifically blocked lipid ROS elevation and ameliorated the PD phenotype.111 It has been suggested that β-hydroxybutyrate and the probiotic strain L. lactis MG1363-pMG36e-GLP-1 have the potential to modulate lipid peroxidation-driven ferroptosis by targeting ACSL4, thereby benefiting the treatment of PD.112,113 Additionally, phospholipase iPLA2β (PLA2G6) was shown to inhibit ROS-induced ferroptosis by scavenging oxidized PUFAs tails in phospholipids,114,115 and its inactivation or deficiency leads to PD-related motor deficits.116
α-Synuclein Aggregation
Abnormal aggregation of α-syn to form Lewy bodies (LB) is one of the hallmark pathological features of PD. Various studies have demonstrated that α-syn plays a role in regulating iron and lipid metabolism, reinforcing the link between PD, as a synucleinopathy, and ferroptosis. Iron deposition in the PD midbrain has been shown to colocalize with α-syn,117,118 and redox-active iron and α-syn were found together in the midbrain LB.119 Both Fe2+ and Fe3+ exhibit robust binding affinities toward α-synuclein, eliciting conformational shifts that lead to the subsequent oligomerization of α-synuclein upon interaction with iron.120–123 Iron-regulatory proteins (IRPs) inhibit the translation of α-syn mRNA by binding to the sequence of iron-responsive elements (IREs),124,125 a process reversed by iron accumulation.126 Evidence has also shown that Fe2+ inhibits autophagosome-lysosome fusion, thereby increasing α-synu spreading between dopaminergic neurons.119 Furthermore, α-synuclein is considered an iron reductase whose overexpression can elevate Fe2+ levels and augment its catalyzed Fenton reaction.127 Meanwhile, conditions like OS and excess Fe2+ promote the further formation of toxic α-syn oligomers or protofibrils,128–130 creating a vicious cycle between iron and α-synuclein.131 α-Syn can also directly regulate cellular iron intake by promoting the uptake of transferrin-bound iron (Tf-Fe)132 and reducing the degradation of divalent metal transporter 1 (DMT1).133
Studies have shown that iron tends to cause increased neural damage and motor defects in PD Drosophila and mouse models overexpressing mutant α-syn.134,135 Various iron chelators have demonstrated the ability to decrease pathological α-syn and ameliorate motor function in in vivo experiments.136,137 Angelova et al discovered that α-syn oligomers can bind to cell membranes and induce ferroptosis by driving lipid peroxidation.40 Furthermore, research findings indicate that α-syn plays a role in metabolizing essential membrane PUFAs like arachidonic acid (AA),6,138 including increasing its membrane fluidity and regulating vesicle assembly and synaptic transmission.109,138,139 A recent study utilizing transmission electron microscopy (TEM) and optical microscopy imaging technologies suggested that the immunoreactivity of α-syn in LB is closely linked to high levels of membrane lipids, fragmented organelles, and vesicles.140 All these results support ferroptosis as a key pathological mechanism in α-synucleinopathy.
Mitochondrial Dysfunction
Mitochondrial dysfunction has long been recognized as a significant initiating factor for the loss of dopaminergic neurons, with its pathological link to PD traced back to 1982.141 Numerous PD risk genes have been found to be related to vital mitochondrial functions such as biogenesis, fusion/fission, transport, and autophagy, including ATP13A2 (PARK9), PRKN (PARK2), PINK1 (PARK6), PLA2G6 (PARK14), VPS13C (PARK23), VPS35 (PARK17), DJ-1 (PARK7), FBXO7 (PARK15), LRRK2 (PARK8), and SNCA (PARK1),142–149 supporting the importance of mitochondria in PD from a genetic perspective. Distinct morphological alterations characterizing ferroptosis include mitochondrial shrinkage, increased membrane density, crista diminution, and membrane potential dissipation, implying the onset of mitochondrial dysfunction.150,151 The electron transport chain (ETC) located on the mitochondrial inner membrane generates superoxide and H2O2 due to electron leakage,152,153 while inhibition of ETC complexes I to IV reduces ROS accumulation and ferroptosis, indicating the involvement of the ETC in ferroptosis.154 Mitochondria harbour iron homeostasis-regulating molecules like Mitoferrin (FtMt), Cysteine Desulfurase (NFS1), and Heme Oxygenase-1 (Hmox1). Overexpression of FtMt can increase storage for excess iron, mitigate oxidative damage, and inhibit erastin-induced ferroptosis,155 along with a significant up-regulation of FtMt immunoreactivity in dopaminergic neurons in PD patients.156 While inhibited NFS1 and activated Hmox1 have been found to cause mitochondrial iron overload, generating excessive ROS and triggering mitochondrial membrane potential (MMP) depolarization,157 ultimately promoting ferroptosis.158 Mitochondrial DHODH and CoQ10 inhibit ferroptosis induced by cysteine starvation and glutathione depletion,159,160 highlighting the role of mitochondria in ferroptosis. Studies have found that the expression of voltage-dependent anion channel (VDAC), a major component of the mitochondrial outer membrane, increases in PD cell models induced by rotenone,161 MPP+,162 and 6-OHDA.163 Inhibition of VDAC1 oligomerization can prevent erastin-induced cell death,164 pointing to the noteworthy role of mitochondria in the pathological mechanism of ferroptosis in PD.
Relevant Experimental Models
From the results of keyword analysis, it is clear that in vivo, in vitro, model, mouse model, and MPTP all have a high frequency of occurrence. To gain insight into the pathophysiological mechanisms of PD and evaluate the efficacy of PD-targeted drugs, sophisticated models have been developed using a series of chemicals typified by PQ, MPTP/MPP+, rotenone, and 6-OHDA (Figure 8), mimicking and replicating the neurodegenerative process of PD.165 The main modeling subjects include mice, rats, Drosophila melanogaster, PC12 cells, SH-SY5Y cells, and LUHMES cells, among others. Designing an appropriate experimental model that aligns with research objectives and modeling characteristics can help harvest compelling indicative results.166,167 Importantly, the current study indicates that PD-inducing chemicals lead to the onset of cellular ferroptosis in various PD models.
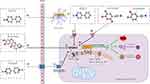 |
Figure 8 Chemicals used to create PD models. |
PQ-induced dopaminergic neurotoxicity stems from elevations in cellular OS and neuroinflammation, leading to α-syn aggregation and lipid peroxidation,168–173 as well as mitochondrial dysfunction and impairment.174 PQ in combination with maneb has been demonstrated to induce ferroptosis in SH-SY5Y cells, which can be inhibited by ferrostatin-1, liproxstatin-1, or deferoxamine.175 This model better reflects the characteristics of PD-like progression, especially early pathological changes,176 and may serve as a candidate model for the mechanism of ferroptosis.
With high lipophilicity, rotenone freely passes through the blood‒brain barrier (BBB) and various cell membranes and exerts its mitochondrial toxicity and mitotic inhibitory effects177,178 to striatal, pallidum and substantia nigra striatal dopaminergic neurons.179,180 Kabiraj et al found that Fer-1 inhibits rotenone-induced SH-SY5Y cell death, attenuating α-syn aggregation and endoplasmic reticulum stress.181 Meanwhile, rotenone-induced rat models of PD effectively exhibited ferroptosis features such as abnormal iron metabolism, lipid peroxidation, GSH depletion, and OS, which can be rescued by DL-3-n-butylphthalide (NBP),182 erucic acid,183 idebenone,184 NAC,185 and resveratrol.186
MPTP has been extensively employed in developing PD models, especially mouse models.166 MPTP models have been proven to induce significant α-syn accumulation and motor impairments,187 with notable loss of dopaminergic neurons and extensive astrocyte hyperplasia.188 The first animal model of PD in which ferroptosis was found was induced by MPTP, and the toxicity of MPTP was inhibited by Fer-1, a specific ferroptosis inhibitor, as reported by multiple sources.23,111,113 Liangqin Shi et al established a stable MPTP-induced monkey model of PD by adopting a long-term gradual regimen and demonstrated that CQ can help to reduce iron concentration and ROS levels in the SN, block p53-mediated cellular ferroptosis, and improve motor and nonmotor deficits in PD monkeys.189 In addition, LUHMES, SH-SY5Y, and PC12 cells also experienced ferroptosis induced by MPP+.23,48,190,191
6-OHDA is a highly oxidisable catecholamine analogue structurally similar to DA. The 6-OHDA model is the first animal model of PD and one of the most commonly applied drug models.192,193 Sun et al demonstrated that 6-OHDA-induced ferroptosis can be observed in both a zebrafish model and an SH-SY5Y cell model and could be attenuated by Fer-1 treatment.93 Huang and Si et al found that Sorting Nexin 5 (SNX5) plays an important role in PD ferroptosis by establishing an in vivo model of rats and an in vitro model of PC-12 using 6-OHDA.16,17 It has also been reported that maprotiline, teicoplanin A (Th A), a polyphenolic compound, and iron chelators can rescue 6-OHDA-induced ferroptosis in various PD models,194–196 providing new insights and potential for investigating pharmacological targets in PD.
PD Drug Research Targeting Ferroptosis
Currently, there are no effective treatments to halt the progression of PD, and interventions regulating ferroptosis present significant therapeutic potential.67 Insights into the pathological mechanism of ferroptosis in PD neurons may help to identify new targets and therapeutic drug approaches to overcome the obstacles in providing safe and effective treatments for PD.
Compounds targeting ferroptosis have been reported to show positive therapeutic effects in several preclinical settings and in patients with PD, and in particular, natural small molecule compounds have become of significant interest, exerting neuroprotective effects through modulation of ferroptosis-mediated neuronal cell death in both in vitro and in vivo experiments (Table 3). Clinical trials of non-dopaminergic PD drugs against ferroptosis are still in their initial stages but present promising prospects.131
 |
Table 3 PD-Therapeutic Compounds Targeting Ferroptosis |
The high-affinity iron chelator deferoxamine has been shown to significantly reduce nigral iron deposition and improve motor symptoms in PD patients in two phase II clinical trials (NCT00943748, NCT01539837),45,62,211 while an ongoing randomized, double-blind, placebo-controlled phase II clinical trial (NCT02728843) assessed the effects of four different doses of deferiprone and placebo on 140 PD patients diagnosed within the last 3 years and being treated with anti-Parkinsonian drugs. Based on the therapeutic efficacy of deferiprone demonstrated in cells, animal models, and early-stage PD patients in previous experiments,45,109,212 the FAIRPARK-II study was conducted as a European multicentre, parallel-group, international clinical trial (NCT02655315), which enrolled a total of 372 patients with early-stage PD who did not receive any dopaminergic therapy but showed that after 36 weeks of treatment, the nigrostriatal iron content decreased more in the deferiprone group than in the placebo group, but the total MDS-UPDRS score, the main index, was worse than that of the placebo group,213 which needs to be further verified by longer treatment duration clinical trials with iron chelators as a dopaminergic supplementation therapy. The CuII complex, Cu(II)ATSM, as a radical scavenger with anti-lipid peroxidation effects,14 and a phase I study (NCT03204929) provided preliminary evidence of its efficacy in patients with early idiopathic PD. The antioxidant intranasal GSH was shown to be beneficial in PD patients in a phase I clinical study (NCT01398748). Two phase II clinical trials (NCT01470027 and NCT02212678) were also conducted on the therapeutic effects of the GSH precursor n-acetylcysteine NAC in patients with PD. The antioxidant CoQ10 has shown efficacy in improving activities of daily living and locomotor symptoms in PD patients in phase II and III clinical trials (NCT01892176, NCT00004731, and NCT00180037),214–216 and CoQ10 is generally used in combination with vitamin E to provide a synergistic antioxidant effect that enhances absorption. Oral administration of EPI-743 (vitamin E-based), a designed p-benzoquinone analogue with a more than 1000-fold increase in antioxidant protection over CoQ10 and idebenone,217 significantly improved motor function in PD patients in a phase IIb study (NCT01923584). However, some clinical trials investigating the aforementioned antioxidant therapies for PD have produced inconclusive or adverse outcomes, underscoring the necessity for more extensive and pertinent studies to furnish objective evidence of their efficacy and to refine drug development and supplement co-administration.218–220
Limitations
This article analyzed and synthesized 160 relevant literatures to forecast future research trends and directions using visualization techniques. The output supports researchers in comprehending related research deemed germane to this field, identifying research hotspots and development trends, characterizing the structure of the field, and locating collaboration opportunities. Nevertheless, this research approach harbours certain inherent limitations. The amount of published literature in this area is limited. This paper primarily comprises pertinent literature obtained from the WOSCC database, which has limitations in both the source and quantity of literature. As a result, some potential information in the data may not be fully reflected. Additionally, certain authors or institutions within the WOSCC database may have differing name formats, resulting in a dispersion of retrieved research results that can ultimately impact analysis outcomes to some degree. Meanwhile, CiteSpace conducts optimal clustering using its pre-set algorithm, but it cannot analyze the literature content. The analyzed data depend on the database statistics, which can struggle to represent the entire research content accurately. Hence, to enhance the comprehensiveness and accuracy of the research results, future studies should expand the scope of the literature search to encompass superior quality basic experimental and real-world research data, as well as strive to employ multiple metrology software programs for comparative analyses. While there are certain limitations to this study, it still offers insights into research trends and focal points within this field, providing valuable references and information for selecting topics and identifying future frontiers in related studies.
Conclusion
This research evaluated the worldwide status of PD and ferroptosis-related research based on an analysis of 160 publications in the WOSCC database from 2013 to 2023. The study employed bibliometric and visual methods using CiteSpace, VOSviewer, and the RStudio. The analysis results indicate that there has been a continued increase in literature within the related field over the past decade. The most prolific countries in terms of article production are China, the USA, and Australia, whereas the journals Free Radical Biol. Med. and J Biol Chem have published the highest number of articles and have been cited extensively, indicating their significant impact within the field. Additionally, our comprehensive analysis of the keywords and cited references has reviewed the research progress on ferroptosis in PD. Our findings demonstrate that the pathogenesis and treatment of PD based on ferroptosis remains a prominent topic in the field in recent years. Despite limitations in the database search scope, sources, and literature, this study provides new insights and directions for future research and topic selection in this field.
Data Sharing Statement
All data used or analyzed during the study were included in this article. Further inquiries about the data are available from the corresponding author on reasonable request.
Author Contributions
All authors made significant contributions to this study, both in terms of conceptualization, study design, data acquisition, analysis and interpretation; and were involved in drafting or revising the article, agreeing on the journal to which the article should be submitted, and ultimately approving the version to be published, and agreeing to take responsibility for all aspects of the work.
Funding
This research was funded by the National Natural Science Foundation of China (Grant No. 82130117).
Disclosure
The authors report no conflicts of interest in this work.
References
1. He T, Lin X, Su A, et al. Mitochondrial dysfunction-targeting therapeutics of natural products in Parkinson’s disease. Front Pharmacol. 2023;14:1117337.
2. Collaborators GBDPs D. Global, regional, and national burden of Parkinson’s disease, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17(11):939–953.
3. Dorsey ER, Sherer T, Okun MS, Bloem BR. The Emerging Evidence of the Parkinson Pandemic. J Parkinsons Dis. 2018;8(s1):S3–S8.
4. Tolosa E, Garrido A, Scholz SW, Poewe W. Challenges in the diagnosis of Parkinson’s disease. Lancet Neurol. 2021;20(5):385–397.
5. Chen YC, Liaw YC, Nfor ON, et al. Epigenetic regulation of Parkinson’s disease risk variant GPNMB cg17274742 methylation by sex and exercise from Taiwan Biobank. Front Aging Neurosci. 2023;15:1235840.
6. Geng Y, Long X, Zhang Y, et al. FTO-targeted siRNA delivery by MSC-derived exosomes synergistically alleviates dopaminergic neuronal death in Parkinson’s disease via m6A-dependent regulation of ATM mRNA. J Transl Med. 2023;21(1):652.
7. Moujalled D, Strasser A, Liddell JR. Molecular mechanisms of cell death in neurological diseases. Cell Death Differ. 2021;28(7):2029–2044.
8. Dexter DT, Wells FR, Agid F, et al. Increased nigral iron content in postmortem parkinsonian brain. Lancet. 1987;2(8569):1219–1220.
9. Dexter D, Carter C, Agid F, et al. Lipid peroxidation as cause of nigral cell death in Parkinson’s disease. Lancet. 1986;2(8507):639–640.
10. Sian J, Dexter DT, Lees AJ, et al. Alterations in glutathione levels in Parkinson’s disease and other neurodegenerative disorders affecting basal ganglia. Ann Neurol. 1994;36(3):348–355.
11. Bonifati V, Rizzu P, van Baren MJ, et al. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science. 2003;299(5604):256–259.
12. Bersuker K, Hendricks JM, Li Z, et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature. 2019;575(7784):688–692.
13. Mahoney-Sanchez L, Bouchaoui H, Ayton S, Devos D, Duce JA, Devedjian JC. Ferroptosis and its potential role in the physiopathology of Parkinson’s Disease. Prog Neurobiol. 2021;196.
14. Wang ZL, Yuan L, Li W, Li JY. Ferroptosis in Parkinson’s disease: glia-neuron crosstalk. Trends Mol Med. 2022;28(4):258–269.
15. Chen C, Song M. Visualizing a field of research: a methodology of systematic scientometric reviews. PLoS One. 2019;14(10):e0223994.
16. Si W, Huang Z, Li X, et al. Super-enhancer-driven Sorting Nexin 5 expression promotes dopaminergic neuronal ferroptosis in Parkinson’s disease models. Biochem Biophys Res Commun. 2021;567:35–41.
17. Huang Z, Han J, Wu P, et al. Sorting Nexin 5 Plays an Important Role in Promoting Ferroptosis in Parkinson’s Disease. Oxid Med Cell Longev. 2022;2022:5463134.
18. Bouchaoui H, Mahoney-Sanchez L, Garçon G, et al. ACSL4 and the lipoxygenases 15/15B are pivotal for ferroptosis induced by iron and PUFA dyshomeostasis in dopaminergic neurons. Free Radic Biol Med. 2023;195(8):145–157.
19. Mahoney-Sanchez L, Bouchaoui H, Boussaad I, et al. Alpha synuclein determines ferroptosis sensitivity in dopaminergic neurons via modulation of ether-phospholipid membrane composition. Cell Rep. 2022;40(8):111231.
20. Jiang L, Chen XB, Wu Q, et al. The C terminus of DJ-1 determines its homodimerization, MGO detoxification activity and suppression of ferroptosis. Acta Pharmacol Sin. 2021;42(7):1150–1159.
21. Zeng X, An H, Yu F, et al. Benefits of Iron Chelators in the Treatment of Parkinson’s Disease. Neurochem Res. 2021;46(5):1239–1251.
22. Lin F, Chen Y, Mo W, et al. A bibliometric analysis of autophagy in lung diseases from 2012 to 2021. Front Immunol. 2022;13:1092575.
23. Do Van B, Gouel F, Jonneaux A, et al. Ferroptosis, a newly characterized form of cell death in Parkinson’s disease that is regulated by PKC. Neurobiol Dis. 2016;94:169–178.
24. Dixon SJ, Lemberg KM, Lamprecht MR, et al. Ferroptosis: an Iron-Dependent Form of Nonapoptotic Cell Death. Cell. 2012;149(5):1060–1072.
25. Angeli JPF, Schneider M, Proneth B, et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol. 2014;16(12):567.
26. Devos D, Moreau C, Devedjian JC, et al. Targeting chelatable iron as a therapeutic modality in Parkinson’s disease. Antioxid Redox Signal. 2014;21(2):195–210.
27. Yang WS, Kim KJ, Gaschler MM, Patel M, Shchepinov MS, Stockwell BR. Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc Natl Acad Sci U S A. 2016;113(34):E4966–75.
28. Yang WS, SriRamaratnam R, Welsch ME, et al. Regulation of Ferroptotic Cancer Cell Death by GPX4. Cell. 2014;156(1–2).
29. Skouta R, Dixon SJ, Wang JL, et al. Ferrostatins Inhibit Oxidative Lipid Damage and Cell Death in Diverse Disease Models. J Am Chem Soc. 2014;136(12):4551–4556.
30. Gao MH, Monian P, Quadri N, Ramasamy R, Jiang XJ. Glutaminolysis and Transferrin Regulate Ferroptosis. Molecular Cell. 2015;59(2):298–308.
31. Ayton S, Lei P, Hare DJ, et al. Parkinson’s Disease Iron Deposition Caused by Nitric Oxide-Induced Loss of β-Amyloid Precursor Protein. J Neurosci. 2015;35(8):3591–3597.
32. Jiang L, Kon N, Li TY, et al. Ferroptosis as a p53-mediated activity during tumour suppression. Nature. 2015;520(7545):57–62.
33. Dixon SJ, Winter GE, Musavi LS, et al. Human Haploid Cell Genetics Reveals Roles for Lipid Metabolism Genes in Nonapoptotic Cell Death. ACS Chem Biol. 2015;10(7):1604–1609.
34. Hambright WS, Fonseca RS, Chen LJ, Na R, Ran QT. Ablation of ferroptosis regulator glutathione peroxidase 4 in forebrain neurons promotes cognitive impairment and neurodegeneration. Redox Biol. 2017;12:8–17. doi:10.1016/j.redox.2017.01.021
35. Dixon SJ, Stockwell BR. The role of iron and reactive oxygen species in cell death. Nature Chem Biol. 2014;10(1):9–17. doi:10.1038/nchembio.1416
36. Xie Y, Hou W, Song X, et al. Ferroptosis: process and function. Cell Death Differ. 2016;23(3):369–379.
37. Sun X, Ou Z, Chen R, et al. Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology. 2016;63(1):173–184.
38. Stockwell BR, Friedmann Angeli JP, Bayir H, et al. Ferroptosis: a Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell. 2017;171(2):273–285.
39. Guiney SJ, Adlard PA, Bush AI, Finkelstein DI, Ayton S. Ferroptosis and cell death mechanisms in Parkinson’s disease. Neurochem Int. 2017;104:34–48.
40. Angelova PR, Choi ML, Berezhnov AV, et al. Alpha synuclein aggregation drives ferroptosis: an interplay of iron, calcium and lipid peroxidation. Cell Death Differ. 2020;27(10):2781–2796.
41. Masaldan S, Bush AI, Devos D, Rolland AS, Moreau C. Striking while the iron is hot: iron metabolism and ferroptosis in neurodegeneration. Free Radic Biol Med. 2019;133:221–233.
42. Zhang P, Chen L, Zhao Q, et al. Ferroptosis was more initial in cell death caused by iron overload and its underlying mechanism in Parkinson’s disease. Free Radic Biol Med. 2020;152:227–234.
43. Weiland A, Wang YM, Wu WH, et al. Ferroptosis and Its Role in Diverse Brain Diseases. Mol Neurobiol. 2019;56(7):4880–4893.
44. Tian Y, Lu J, Hao XQ, et al. FTH1 Inhibits Ferroptosis Through Ferritinophagy in the 6-OHDA Model of Parkinson’s Disease. Neurotherapeutics. 2020;17(4):1796–1812.
45. Abdalkader M, Lampinen R, Kanninen KM, Malm TM, Liddell JR. Targeting Nrf2 to Suppress Ferroptosis and Mitochondrial Dysfunction in Neurodegeneration. Front Neurosci. 2018;12:466.
46. Sun YR, He LB, Wang TY, et al. Activation of p62-Keap1-Nrf2 Pathway Protects 6-Hydroxydopamine-Induced Ferroptosis in Dopaminergic Cells. Mol Neurobiol. 2020;57(11):4628–4641.3.
47. Southon A, Szostak K, Acevedo KM, et al. CuII (atsm) inhibits ferroptosis: implications for treatment of neurodegenerative disease. Br J Pharmacol. 2020;177(3):656–667.
48. Ito K, Eguchi Y, Imagawa Y, Akai S, Mochizuki H, Tsujimoto Y. MPP+ induces necrostatin-1- and ferrostatin-1-sensitive necrotic death of neuronal SH-SY5Y cells. Cell Death Discov. 2017;3:17013.
49. Liddell JR, White AR. Nexus between mitochondrial function, iron, copper and glutathione in Parkinson’s disease. Neurochem Int. 2018;117:126–138.
50. Devos D, Cabantchik ZI, Moreau C, et al. Conservative iron chelation for neurodegenerative diseases such as Parkinson’s disease and amyotrophic lateral sclerosis. J Neural Transm. 2020;127(2):189–203.
51. Chen LJ, Hambright WS, Na R, Ran QT. Ablation of the Ferroptosis Inhibitor Glutathione Peroxidase 4 in Neurons Results in Rapid Motor Neuron Degeneration and Paralysis. J Biol Chem. 2015;290(47):28097–28106.
52. Ayton S, Lei P. Nigral Iron Elevation Is an Invariable Feature of Parkinson’s Disease and Is a Sufficient Cause of Neurodegeneration. Biomed Res Int. 2014;2014:581256.
53. Li Q, Han XN, Lan X, et al. Inhibition of neuronal ferroptosis protects hemorrhagic brain. Jci Insight. 2017;2(7):e90777.
54. Hare DJ, Double KL. Iron and dopamine: a toxic couple. Brain. 2016;139(4):1026–1035.
55. Shimada K, Skouta R, Kaplan A, et al. Global survey of cell death mechanisms reveals metabolic regulation of ferroptosis. Nat Chem Biol. 2016;12(7):497–503.
56. Doll S, Proneth B, Tyurina YY, et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat Chem Biol. 2017;13(1):91–98.
57. Kagan VE, Mao G, Qu F, et al. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat Chem Biol. 2017;13(1):81–90.
58. Doll S, Freitas FP, Shah R, et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nature. 2019;575(7784):693–698.
59. Yang WS, Stockwell BR. Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells. Chem Biol. 2008;15(3):234–245.
60. Ward R, Zucca FA, Duyn JH, Crichton RR, Zecca L. The role of iron in brain ageing and neurodegenerative disorders. Lancet Neurol. 2014;13(10):1045–1060.
61. Yang WS, Stockwell BR. Ferrootosis: death by Lipid Peroxidation. Trends Cell Biol. 2016;26(3):165–176.
62. Martin-Bastida A, Ward RJ, Newbould R, et al. Brain iron chelation by deferiprone in a phase 2 randomised double-blinded placebo controlled clinical trial in Parkinson’s disease. Sci Rep. 2017;7(1):1398.
63. Yagoda N, Von Rechenberg M, Zaganjor E, et al. RAS-RAF-MEK-dependent oxidative cell death involving voltage-dependent anion channels. Nature. 2007;447(7146):864–868.
64. Yuan H, Li XM, Zhang XY, Kang R, Tang DL. Identification of ACSL4 as a biomarker and contributor of ferroptosis. Biochem Biophys Res Commun. 2016;478(3):1338–1343.
65. Qiu Y, Cao Y, Cao W, Jia Y, Lu N. The Application of Ferroptosis in Diseases. Pharmacol Res. 2020;159:104919.
66. Angelova PR, Esteras N, Abramov AY. Mitochondria and lipid peroxidation in the mechanism of neurodegeneration: finding ways for prevention. Med Res Rev. 2021;41(2):770–784.
67. Thapa K, Khan H, Kanojia N, Singh TG, Kaur A, Kaur G. Therapeutic Insights on Ferroptosis in Parkinson’s disease. Eur J Pharmacol. 2022;930:175133.
68. Han X, Zhang J, Chen S, Yu W, Zhou Y, Gu X. Mapping the current trends and hotspots of vascular cognitive impairment from 2000-2021: a bibliometric analysis. CNS Neurosci Ther. 2023;29(3):771–782.
69. Li Y, Zheng JJ, Wu X, Gao W, Liu CJ. Postural control of Parkinson’s disease: a visualized analysis based on Citespace knowledge graph. Front Aging Neurosci. 2023;15:1136177.
70. Zhong D, Luo S, Zheng L, Zhang Y, Jin R. Epilepsy Occurrence and Circadian Rhythm: a Bibliometrics Study and Visualization Analysis via CiteSpace. Front Neurol. 2020;11:984.
71. Villalon-Garcia I, Povea-Cabello S, Alvarez-Cordoba M, et al. Vicious cycle of lipid peroxidation and iron accumulation in neurodegeneration. Neural Regen Res. 2023;18(6):1196–1202.
72. Villalon-Garcia I, Alvarez-Cordoba M, Povea-Cabello S, et al. Vitamin E prevents lipid peroxidation and iron accumulation in PLA2G6-Associated Neurodegeneration. Neurobiol Dis. 2022;165:105649.
73. Hohn A, Jung T, Grimm S, Grune T. Lipofuscin-bound iron is a major intracellular source of oxidants: role in senescent cells. Free Radic Biol Med. 2010;48(8):1100–1108.
74. Zeidan RS, Han SM, Leeuwenburgh C, Xiao R. Iron homeostasis and organismal aging. Ageing Res Rev. 2021;72:101510.
75. Brunk UT, Terman A. Lipofuscin: mechanisms of age-related accumulation and influence on cell function. Free Radic Biol Med. 2002;33(5):611–619.
76. Moore MN, Sforzini S, Viarengo A, et al. Antagonistic cytoprotective effects of C(60) fullerene nanoparticles in simultaneous exposure to benzo[a]pyrene in a molluscan animal model. Sci Total Environ. 2021;755(Pt 1):142355.
77. Tian Y, Tian Y, Yuan Z, et al. Iron Metabolism in Aging and Age-Related Diseases. Int J Mol Sci. 2022;23(7):3612.
78. Tian R, Abarientos A, Hong J, et al. Genome-wide CRISPRi/a screens in human neurons link lysosomal failure to ferroptosis. Nat Neurosci. 2021;24(7):1020–1034.
79. Shi L, Huang C, Luo Q, et al. The Association of Iron and the Pathologies of Parkinson’s Diseases in MPTP/MPP(+)-Induced Neuronal Degeneration in Non-human Primates and in Cell Culture. Front Aging Neurosci. 2019;11:215.
80. Belaidi AA, Bush AI. Iron neurochemistry in Alzheimer’s disease and Parkinson’s disease: targets for therapeutics. J Neurochem. 2016;139(Suppl 1):179–197.
81. Thomas GEC, Leyland LA, Schrag AE, Lees AJ, Acosta-Cabronero J, Weil RS. Brain iron deposition is linked with cognitive severity in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2020;91(4):418–425.
82. Pesch B, Casjens S, Woitalla D, et al. Impairment of Motor Function Correlates with Neurometabolite and Brain Iron Alterations in Parkinson’s Disease. Cells. 2019;8(2):96.
83. Dexter DT, Wells FR, Lees AJ, et al. Increased nigral iron content and alterations in other metal ions occurring in brain in Parkinson’s disease. J Neurochem. 1989;52(6):1830–1836.
84. Cho SJ, Bae YJ, Kim JM, et al. Iron-sensitive magnetic resonance imaging in Parkinson’s disease: a systematic review and meta-analysis. J Neurol. 2021;268(12):4721–4736.
85. Good PF, Olanow CW, Perl DP. Neuromelanin-containing neurons of the substantia nigra accumulate iron and aluminum in Parkinson’s disease: a LAMMA study. Brain Res. 1992;593(2):343–346.
86. Wang Z, Luo XG, Gao C. Utility of susceptibility-weighted imaging in Parkinson’s disease and atypical Parkinsonian disorders. Transl Neurodegener. 2016;5:17.
87. Wang JY, Zhuang QQ, Zhu LB, et al. Meta-analysis of brain iron levels of Parkinson’s disease patients determined by postmortem and MRI measurements. Sci Rep. 2016;6:36669.
88. Ji S, Zhang S, Mao Z, et al. Quantitative assessment of iron deposition in Parkinson’s disease using enhanced T2 star-weighted angiography. Neurol India. 2016;64(3):428–435.
89. Biondetti E, Santin MD, Valabrègue R, et al. The spatiotemporal changes in dopamine, neuromelanin and iron characterizing Parkinson’s disease. Brain. 2021;144(10):3114–3125.
90. Song N, Wang J, Jiang H, Xie J. Ferroportin 1 but not hephaestin contributes to iron accumulation in a cell model of Parkinson’s disease. Free Radic Biol Med. 2010;48(2):332–341.
91. Jiang H, Song N, Xu H, Zhang S, Wang J, Xie J. Up-regulation of divalent metal transporter 1 in 6-hydroxydopamine intoxication is IRE/IRP dependent. Cell Res. 2010;20(3):345–356.
92. Zhao X, Shao Z, Zhang Y, Liu F, Liu Z, Liu Z. Ceruloplasmin in Parkinson’s disease and the nonmotor symptoms. Brain Behav. 2018;8(6):e00995.
93. Barbariga M, Curnis F, Andolfo A, et al. Ceruloplasmin functional changes in Parkinson’s disease-cerebrospinal fluid. Mol Neurodegener. 2015;10:59.
94. Lei P, Ayton S, Finkelstein DI, et al. Tau deficiency induces parkinsonism with dementia by impairing APP-mediated iron export. Nat Med. 2012;18(2):291–295.
95. Kaur D, Yantiri F, Rajagopalan S, et al. Genetic or pharmacological iron chelation prevents MPTP-induced neurotoxicity in vivo: a novel therapy for Parkinson’s disease. Neuron. 2003;37(6):899–909.
96. Blackinton J, Kumaran R, van der Brug MP, et al. Post-transcriptional regulation of mRNA associated with DJ-1 in sporadic Parkinson disease. Neurosci Lett. 2009;452(1):8–11.
97. Bellinger FP, Bellinger MT, Seale LA, et al. Glutathione Peroxidase 4 is associated with Neuromelanin in Substantia Nigra and Dystrophic Axons in Putamen of Parkinson’s brain. Mol Neurodegener. 2011;6(1):8.
98. Vallerga CL, Zhang F, Fowdar J, et al. Analysis of DNA methylation associates the cystine-glutamate antiporter SLC7A11 with risk of Parkinson’s disease. Nat Commun. 2020;11(1):1238.
99. Liu Y, Ma X, Fujioka H, Liu J, Chen S, Zhu X. DJ-1 regulates the integrity and function of ER-mitochondria association through interaction with IP3R3-Grp75-VDAC1. Proc Natl Acad Sci U S A. 2019;116(50):25322–25328.
100. Cao J, Chen X, Jiang L, et al. DJ-1 suppresses ferroptosis through preserving the activity of S-adenosyl homocysteine hydrolase. Nat Commun. 2020;11(1):1251.
101. Guo Q, Wang B, Wang X, Smith WW, Zhu Y, Liu Z. Activation of Nrf2 in Astrocytes Suppressed PD-Like Phenotypes via Antioxidant and Autophagy Pathways in Rat and Drosophila Models. Cells. 2021;10(8):1850.
102. Xiao H, Lv F, Xu W, Zhang L, Jing P, Cao X. Deprenyl prevents MPP(+)-induced oxidative damage in PC12 cells by the upregulation of Nrf2-mediated NQO1 expression through the activation of PI3K/Akt and Erk. Toxicology. 2011;290(2–3):286–294.
103. Anandhan A, Chen W, Nguyen N, Madhavan L, Dodson M, Zhang DD. α-Syn overexpression, NRF2 suppression, and enhanced ferroptosis create a vicious cycle of neuronal loss in Parkinson’s disease. Free Radic Biol Med. 2022;192:130–140.
104. Zeitler L, Fiore A, Meyer C, et al. Anti-ferroptotic mechanism of IL4i1-mediated amino acid metabolism. Elife. 2021;10:e64806.
105. Costa I, Barbosa DJ, Benfeito S, et al. Molecular mechanisms of ferroptosis and their involvement in brain diseases. Pharmacol Ther. 2023;108373.
106. Cleren C, Yang L, Lorenzo B, et al. Therapeutic effects of coenzyme Q10 (CoQ10) and reduced CoQ10 in the MPTP model of Parkinsonism. J Neurochem. 2008;104(6):1613–1621.
107. Mischley LK, Allen J, Bradley R. Coenzyme Q10 deficiency in patients with Parkinson’s disease. J Neurol Sci. 2012;318(1–2):567.
108. Dexter DT, Carter CJ, Wells FR, et al. Basal lipid peroxidation in substantia nigra is increased in Parkinson’s disease. J Neurochem. 1989;52(2):381–389.
109. Sharon R, Bar-Joseph I, Mirick GE, Serhan CN, Selkoe DJ. Altered fatty acid composition of dopaminergic neurons expressing alpha-synuclein and human brains with alpha-synucleinopathies. J Biol Chem. 2003;278(50):49874–49881.
110. Fedorova TN, Logvinenko AA, Poleshchuk VV. Lipid Peroxidation Products in the Blood Plasma of Patients with Parkinson’s Disease as Possible Biomarkers of Different Stages of the Disease. Neurochemical J. 2019;13(4):391–395.
111. Tang F, Zhou LY, Li P, et al. Inhibition of ACSL4 Alleviates Parkinsonism Phenotypes by Reduction of Lipid Reactive Oxygen Species. Neurotherapeutics. 2023;20(4):1154–1166.
112. Yue M, Wei J, Chen W, Hong D, Chen T, Fang X. Neurotrophic Role of the Next-Generation Probiotic Strain L. lactis MG1363-pMG36e-GLP-1 on Parkinson’s Disease via Inhibiting Ferroptosis. Nutrients. 2022;14(22):4886.
113. Yu X, Yang Y, Zhang B, et al. Ketone Body β-Hydroxybutyric Acid Ameliorates Dopaminergic Neuron Injury Through Modulating Zinc Finger Protein 36/Acyl-CoA Synthetase Long-Chain Family Member Four Signaling Axis-Mediated Ferroptosis. Neuroscience. 2023;509:157–172.
114. Chen D, Chu B, Yang X, et al. iPLA2β-mediated lipid detoxification controls p53-driven ferroptosis independent of GPX4. Nat Commun. 2021;12(1):3644.
115. Beharier O, Tyurin VA, Goff JP, et al. PLA2G6 guards placental trophoblasts against ferroptotic injury. Proc Natl Acad Sci U S A. 2020;117(44):27319–27328.
116. Sun WY, Tyurin VA, Mikulska-Ruminska K, et al. Phospholipase iPLA2β averts ferroptosis by eliminating a redox lipid death signal. Nat Chem Biol. 2021;17(4):465–476. doi:10.1038/s41589-020-00734-x
117. Guo JJ, Yue F, Song DY, et al. Intranasal administration of α-synuclein preformed fibrils triggers microglial iron deposition in the substantia nigra of Macaca fascicularis. Cell Death Dis. 2021;12(1):81.
118. Minakaki G, Krainc D, Burbulla LF. The Convergence of Alpha-Synuclein, Mitochondrial, and Lysosomal Pathways in Vulnerability of Midbrain Dopaminergic Neurons in Parkinson’s Disease. Front Cell Dev Biol. 2020;8:580634.
119. Xiao Y, Chen X, Huang S, et al. Iron promotes α-synuclein aggregation and transmission by inhibiting TFEB-mediated autophagosome-lysosome fusion. J Neurochem. 2018;145(1):34–50.
120. el-Agnaf OM, Irvine GB. Aggregation and neurotoxicity of alpha-synuclein and related peptides. Biochem Soc Trans. 2002;30(4):559–565.
121. Uversky VN, Li J, Fink AL. Metal-triggered structural transformations, aggregation, and fibrillation of human alpha-synuclein. A possible molecular NK between Parkinson’s disease and heavy metal exposure. J Biol Chem. 2001;276(47):44284–44296.
122. Peng Y, Wang C, Xu HH, Liu YN, Zhou F. Binding of alpha-synuclein with Fe(III) and with Fe(II) and biological implications of the resultant complexes. J Inorg Biochem. 2010;104(4):365–370.
123. Golts N, Snyder H, Frasier M, Theisler C, Choi P, Wolozin B. Magnesium inhibits spontaneous and iron-induced aggregation of alpha-synuclein. J Biol Chem. 2002;277(18):16116–16123.
124. Friedlich AL, Tanzi RE, Rogers JT. The 5’-untranslated region of Parkinson’s disease alpha-synuclein messenger RNA contains a predicted iron responsive element. Mol Psychiatry. 2007;12(3):222–223.
125. Febbraro F, Giorgi M, Caldarola S, Loreni F, Romero-Ramos M. α-Synuclein expression is modulated at the translational level by iron. Neuroreport. 2012;23(9):576–580.
126. Cahill CM, Lahiri DK, Huang X, Rogers JT. Amyloid precursor protein and alpha synuclein translation, implications for iron and inflammation in neurodegenerative diseases. Biochim Biophys Acta. 2009;1790(7):615–628.
127. Davies P, Moualla D, Brown DR. Alpha-synuclein is a cellular ferrireductase. PLoS One. 2011;6(1):e15814.
128. Alam P, Bousset L, Melki R, Otzen DE. α-synuclein oligomers and fibrils: a spectrum of species, a spectrum of toxicities. J Neurochemistry. 2019;150(5):522–534.
129. Binolfi A, Rasia RM, Bertoncini CW, et al. Interaction of alpha-synuclein with divalent metal ions reveals key differences: a link between structure, binding specificity and fibrillation enhancement. J Am Chem Soc. 2006;128(30):9893–9901. doi:10.1021/ja0618649
130. Longhena F, Faustini G, Bellucci A. Study of alpha-synuclein fibrillation: state of the art and expectations. Neural Regen Res. 2020;15(1):59–60.
131. Lin KJ, Chen SD, Lin KL, et al. Iron Brain Menace: the Involvement of Ferroptosis in Parkinson Disease. Cells. 2022;11(23):3829.
132. Baksi S, Tripathi AK, Singh N. Alpha-synuclein modulates retinal iron homeostasis by facilitating the uptake of transferrin-bound iron: implications for visual manifestations of Parkinson’s disease. Free Radic Biol Med. 2016;97:292–306.
133. Bi M, Du X, Jiao Q, Liu Z, Jiang H. α-Synuclein Regulates Iron Homeostasis via Preventing Parkin-Mediated DMT1 Ubiquitylation in Parkinson’s Disease Models. ACS Chem Neurosci. 2020;11(11):1682–1691.
134. Zhu ZJ, Wu KC, Yung WH, Qian ZM, Ke Y. Differential interaction between iron and mutant alpha-synuclein causes distinctive Parkinsonian phenotypes in Drosophila. Biochim Biophys Acta. 2016;1862(4):518–525.
135. Jia F, Song N, Wang W, Du X, Chi Y, Jiang H. High Dietary Iron Supplement Induces the Nigrostriatal Dopaminergic Neurons Lesion in Transgenic Mice Expressing Mutant A53T Human Alpha-Synuclein. Front Aging Neurosci. 2018;10:97.
136. Febbraro F, Andersen KJ, Sanchez-Guajardo V, Tentillier N, Romero-Ramos M. Chronic intranasal deferoxamine ameliorates motor defects and pathology in the α-synuclein rAAV Parkinson’s model. Exp Neurol. 2013;247:45–58.
137. Carboni E, Tatenhorst L, Tönges L, et al. Deferiprone Rescues Behavioral Deficits Induced by Mild Iron Exposure in a Mouse Model of Alpha-Synuclein Aggregation. Neuromolecular Med. 2017;19(2–3).
138. Golovko MY, Rosenberger TA, Faergeman NJ, et al. Acyl-CoA synthetase activity links wild-type but not mutant alpha-synuclein to brain arachidonate metabolism. Biochemistry. 2006;45(22):6956–6966.
139. Ouberai MM, Wang J, Swann MJ, et al. α-Synuclein senses lipid packing defects and induces lateral expansion of lipids leading to membrane remodeling. J Biol Chem. 2013;288(29):20883–20895.
140. Shahmoradian SH, Lewis AJ, Genoud C, et al. Lewy pathology in Parkinson’s disease consists of crowded organelles and lipid membranes. Nat Neurosci. 2019;22(7):1099–1109.
141. Ballard PA, Tetrud JW, Langston JW. Permanent human parkinsonism due to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP): seven cases. Neurology. 1985;35(7):949–956.
142. Lin KJ, Wang TJ, Chen SD, et al. Two Birds One Stone: the Neuroprotective Effect of Antidiabetic Agents on Parkinson Disease-Focus on Sodium-Glucose Cotransporter 2 (SGLT2) Inhibitors. Antioxidants (Basel). 2021;10(12):1935.
143. Nguyen M, Wong YC, Ysselstein D, Severino A, Krainc D. Synaptic, Mitochondrial, and Lysosomal Dysfunction in Parkinson’s Disease. Trends Neurosci. 2019;42(2):140–149.
144. Lesage S, Drouet V, Majounie E, et al. Loss of VPS13C Function in Autosomal-Recessive Parkinsonism Causes Mitochondrial Dysfunction and Increases PINK1/Parkin-Dependent Mitophagy. Am J Hum Genet. 2016;98(3):500–513.
145. Gregory A, Westaway SK, Holm IE, et al. Neurodegeneration associated with genetic defects in phospholipase A(2). Neurology. 2008;71(18):1402–1409.
146. Kinghorn KJ, Castillo-Quan JI, Bartolome F, et al. Loss of PLA2G6 leads to elevated mitochondrial lipid peroxidation and mitochondrial dysfunction. Brain. 2015;138(7):1801–1816.
147. Smolders S, Van Broeckhoven C. Genetic perspective on the synergistic connection between vesicular transport, lysosomal and mitochondrial pathways associated with Parkinson’s disease pathogenesis. Acta Neuropathol Commun. 2020;8(1):63.
148. Billingsley KJ, Barbosa IA, Bandrés-Ciga S, et al. Mitochondria function associated genes contribute to Parkinson’s Disease risk and later age at onset. NPJ Parkinsons Dis. 2019;5:8.
149. Wang Y, Liu N, Lu B. Mechanisms and roles of mitophagy in neurodegenerative diseases. CNS Neurosci Ther. 2019;25(7):859–875. doi:10.1111/cns.13140
150. Daher B, Vučetić M, Pouysségur J. Cysteine Depletion, a Key Action to Challenge Cancer Cells to Ferroptotic Cell Death. Front Oncol. 2020;10:723.
151. Chen X, Kang R, Kroemer G, Tang D. Organelle-specific regulation of ferroptosis. Cell Death Differ. 2021;28(10):2843–2856.
152. Mailloux RJ. An Update on Mitochondrial Reactive Oxygen Species Production. Antioxidants (Basel). 2020;9(6):472.
153. Andréasson C, Ott M, Büttner S. Mitochondria orchestrate proteostatic and metabolic stress responses. EMBO Rep. 2019;20(10):e47865.
154. Gan B. Mitochondrial regulation of ferroptosis. J Cell Biol. 2021;220(9):e202105043.
155. Wang YQ, Chang SY, Wu Q, et al. The Protective Role of Mitochondrial Ferritin on Erastin-Induced Ferroptosis. Front Aging Neurosci. 2016;8:308.
156. Tsubaki H, Yanagisawa D, Kageyama Y, Hafiz Abu Baker Z, Mukaisho KI, Tooyama I. Immunohistochemical Analysis of Mitochondrial Ferritin in the Midbrain of Patients with Parkinson’s Disease. Acta Histochem Cytochem. 2023;56(2):21–27.
157. Chan S, Lian Q, Chen MP, et al. Deferiprone inhibits iron overload-induced tissue factor bearing endothelial microparticle generation by inhibition oxidative stress induced mitochondrial injury, and apoptosis. Toxicol Appl Pharmacol. 2018;338:148–158.
158. Alvarez SW, Sviderskiy VO, Terzi EM, et al. NFS1 undergoes positive selection in lung tumours and protects cells from ferroptosis. Nature. 2017;551(7682):639–643.
159. Mao C, Liu X, Zhang Y, et al. DHODH-mediated ferroptosis defence is a targetable vulnerability in cancer. Nature. 2021;593(7860):586–590.
160. Gao M, Yi J, Zhu J, et al. Role of Mitochondria in Ferroptosis. Mol Cell. 2019;73(2):354–363.e3.
161. Xiong Y, Ding H, Xu M, Gao J. Protective effects of asiatic acid on rotenone- or H2O2-induced injury in SH-SY5Y cells. Neurochem Res. 2009;34(4):746–754.
162. Chaudhuri AD, Choi DC, Kabaria S, Tran A, Junn E. MicroRNA-7 Regulates the Function of Mitochondrial Permeability Transition Pore by Targeting VDAC1 Expression. J Biol Chem. 2016;291(12):6483–6493.
163. Magalingam KB, Somanath SD, Ramdas P, Haleagrahara N, Radhakrishnan AK. 6-Hydroxydopamine Induces Neurodegeneration in Terminally Differentiated SH-SY5Y Neuroblastoma Cells via Enrichment of the Nucleosomal Degradation Pathway: a Global Proteomics Approach. J Mol Neurosci. 2022;72(5):1026–1046.
164. Nagakannan P, Islam MI, Karimi-Abdolrezaee S, Eftekharpour E. Inhibition of VDAC1 Protects Against Glutamate-Induced Oxytosis and Mitochondrial Fragmentation in Hippocampal HT22 Cells. Cell Mol Neurobiol. 2019;39(1):73–85.
165. Wen S, Aki T, Unuma K, Uemura K. Chemically Induced Models of Parkinson’s Disease: history and Perspectives for the Involvement of Ferroptosis. Front Cell Neurosci. 2020;14:581191.
166. Mustapha M, Mat Taib CN. MPTP-induced mouse model of Parkinson’s disease: a promising direction of therapeutic strategies. Bosn J Basic Med Sci. 2021;21(4):422–433.
167. Tanner CM, Kamel F, Ross GW, et al. Rotenone, paraquat, and Parkinson’s disease. Environ Health Perspect. 2011;119(6):866–872.
168. Manning-Bog AB, McCormack AL, Li J, Uversky VN, Fink AL, Di Monte DA. The herbicide paraquat causes up-regulation and aggregation of alpha-synuclein in mice: paraquat and alpha-synuclein. J Biol Chem. 2002;277(3):1641–1644.
169. Bus JS, Aust SD, Gibson JE. Paraquat toxicity: proposed mechanism of action involving lipid peroxidation. Environ Health Perspect. 1976;139–146.
170. Fernagut PO, Hutson CB, Fleming SM, et al. Behavioral and histopathological consequences of paraquat intoxication in mice: effects of alpha-synuclein over-expression. Synapse. 2007;61(12):991–1001.
171. Yang WS, Tiffany-Castiglioni E. The bipyridyl herbicide paraquat produces oxidative stress-mediated toxicity in human neuroblastoma SH-SY5Y cells: relevance to the dopaminergic pathogenesis. J Toxicol Environ Heal A. 2005;68(22):1939–1961.
172. Niso-Santano M, González-Polo RA, Bravo-San Pedro JM, et al. Activation of apoptosis signal-regulating kinase 1 is a key factor in paraquat-induced cell death: modulation by the Nrf2/Trx axis. Free Radic Biol Med. 2010;48(10):1370–1381.
173. Cristóvao AC, Choi DH, Baltazar G, Beal MF, Kim YS. The Role of NADPH Oxidase 1-Derived Reactive Oxygen Species in Paraquat-Mediated Dopaminergic Cell Death. Antioxid Redox Sign. 2009;11(9):2105–2118.
174. Castello PR, Drechsel DA, Patel M. Mitochondria are a major source of paraquat-induced reactive oxygen species production in the brain. J Biol Chem. 2007;282(19):14186–14193.
175. Hou LY, Huang RX, Sun FQ, Zhang L, Wang QS. NADPH oxidase regulates paraquat and maneb-induced dopaminergic neurodegeneration through ferroptosis. Toxicology. 2019;417:64–73.
176. Hou LY, Sun FQ, Sun W, Zhang L, Wang QS. Lesion of the Locus Coeruleus Damages Learning and Memory Performance in Paraquat and Maneb-induced Mouse Parkinson’s Disease Model. Neuroscience. 2019;419:129–140.
177. Talpade DJ, Greene JG, Higgins DS, Greenamyre JT. In vivo labeling of mitochondrial complex I (NADH:ubiquinone oxidoreductase) in rat brain using [(3)H]dihydrorotenone. J Neurochem. 2000;75(6):2611–2621.
178. Berry TM, Moustafa AA. A novel treatment strategy to prevent Parkinson’s disease: focus on iron regulatory protein 1 (IRP1). Int J Neurosci. 2023;133(1):67–76.
179. Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat Neurosci. 2000;3(12):1301–1306.
180. Ferrante RJ, Schulz JB, Kowall NW, Beal MF. Systemic administration of rotenone produces selective damage in the striatum and globus pallidus, but not in the substantia nigra. Brain Res. 1997;753(1):157–162.
181. Kabiraj P, Valenzuela CA, Marin JE, et al. The neuroprotective role of ferrostatin-1 under rotenone-induced oxidative stress in dopaminergic neuroblastoma cells. Protein J. 2015;34(5):349–358.
182. Hu CB, Jiang H, Yang Y, et al. DL-3-n-butylphthalide alleviates motor disturbance by suppressing ferroptosis in a rat model of Parkinson’s disease. Neural Regen Res. 2023;18(1):194–199.
183. Avci B, Günaydın C, Külbay M, Kuruca N, Güvenç T, Bilge SS. Neuroprotective effects of sinapic acid involve the iron regulatory role on the rotenone-induced Parkinson’s disease model. Braz J Pharm Sci. 2022;58:e20942.
184. Avci B, Günaydın C, Güvenç T, Yavuz CK, Kuruca N, Bilge SS. Idebenone Ameliorates Rotenone-Induced Parkinson’s Disease in Rats Through Decreasing Lipid Peroxidation. Neurochem Res. 2021;46(3):513–522.
185. Mursaleen L, Noble B, Chan SHY, Somavarapu S, Zariwala MG. N-Acetylcysteine Nanocarriers Protect against Oxidative Stress in a Cellular Model of Parkinson’s Disease. Antioxidants (Basel). 2020;9(7):600.
186. Li H, Shen Y, Xiao H, Sun W. Resveratrol attenuates rotenone-induced inflammation and oxidative stress via STAT1 and Nrf2/Keap1/SLC7A11 pathway in a microglia cell line. Pathol Res Pract. 2021;225:153576.
187. Muñoz-Manchado AB, Villadiego J, Romo-Madero S, et al. Chronic and progressive Parkinson’s disease MPTP model in adult and aged mice. J Neurochem. 2016;136(2):373–387.
188. Zhang QS, Heng Y, Mou Z, Huang JY, Yuan YH, Chen NH. Reassessment of subacute MPTP-treated mice as animal model of Parkinson’s disease. Acta Pharmacol Sin. 2017;38(10):1317–1328.
189. Shi L, Huang C, Luo Q, et al. Clioquinol improves motor and non-motor deficits in MPTP-induced monkey model of Parkinson’s disease through AKT/mTOR pathway. Aging (Albany NY). 2020;12(10):9515–9533.
190. Bai L, Yan F, Deng R, Gu R, Zhang X, Bai J. Thioredoxin-1 Rescues MPP+/MPTP-Induced Ferroptosis by Increasing Glutathione Peroxidase 4. Mol Neurobiol. 2021;58(7):3187–3197.
191. Lin ZH, Liu Y, Xue NJ, et al. Quercetin Protects against MPP+/MPTP-Induced Dopaminergic Neuron Death in Parkinson’s Disease by Inhibiting Ferroptosis. Oxid Med Cell Longev. 2022;2022:7769355.
192. Ungerstedt U, Ljungberg T, Steg G. Behavioral, physiological, and neurochemical changes after 6-hydroxydopamine-induced degeneration of the nigro-striatal dopamine neurons. Mol Neurobiol. 1974;421–426.
193. Bastías-Candia S, Zolezzi JM, Inestrosa NC. Revisiting the Paraquat-Induced Sporadic Parkinson’s Disease-Like Model. Mol Neurobiol. 2019;56(2):1044–1055.
194. Cheng H, Chen Y, Yang HF, Tang X, Zhu WY. Protective Effects of Maprotiline in 6-Hydroxydopamine (6-OHDA)-Induced Ferroptosis in Neuronal Cells. Biomed Nanotechnol. 2022;18(11):2592–2598.
195. Sun Y, He L, Wang W, et al. Activation of Atg7-dependent autophagy by a novel inhibitor of the Keap1-Nrf2 protein-protein interaction from Penthorum chinense Pursh. attenuates 6-hydroxydopamine-induced ferroptosis in zebrafish and dopaminergic neurons. Food Funct. 2022;13(14):7885–7900.
196. Gutbier S, Kyriakou S, Schildknecht S, et al. Design and evaluation of bi-functional iron chelators for protection of dopaminergic neurons from toxicants. Arch Toxicol. 2020;94(9):3105–3123.
197. Wang L, An H, Yu F, et al. The neuroprotective effects of paeoniflorin against MPP+-induced damage to dopaminergic neurons via the Akt/Nrf2/GPX4 pathway. J Chem Neuroanat. 2022;122:102103.
198. Liu L, Yang S, Wang H. α-Lipoic acid alleviates ferroptosis in the MPP+ -induced PC12 cells via activating the PI3K/Akt/Nrf2 pathway. Cell Biol Int. 2021;45(2):422–431.
199. Xi J, Zhang Z, Wang Z, et al. Hinokitiol functions as a ferroptosis inhibitor to confer neuroprotection. Free Radic Biol Med. 2022;190:202–215.
200. Jiang Y, Xie G, Alimujiang A, et al. Protective Effects of Querectin against MPP+-Induced Dopaminergic Neurons Injury via the Nrf2 Signaling Pathway. Front Biosci. 2023;28(3):42.
201. Bellavite P. Neuroprotective Potentials of Flavonoids: experimental Studies and Mechanisms of Action. Antioxidants (Basel). 2023;12(2):280.
202. Song LM, Xiao ZX, Zhang N, et al. Apoferritin improves motor deficits in MPTP-treated mice by regulating brain iron metabolism and ferroptosis. iScience. 2021;24(5):102431.
203. Zilka O, Poon JF, Pratt DA. Radical-Trapping Antioxidant Activity of Copper and Nickel Bis(Thiosemicarbazone) Complexes Underlies Their Potency as Inhibitors of Ferroptotic Cell Death. J Am Chem Soc. 2021;143(45):19043–19057.
204. Tourville A, Viguier S, González-Lizárraga F, et al. Rescue of Dopamine Neurons from Iron-Dependent Ferroptosis by Doxycycline and Demeclocycline and Their Non-Antibiotic Derivatives. Antioxidants (Basel). 2023;12(3):575.
205. Wang Y, Wu S, Li Q, et al. Epigallocatechin-3-gallate: a phytochemical as a promising drug candidate for the treatment of Parkinson’s disease. Front Pharmacol. 2022;13:977521.
206. Chen D, Kanthasamy AG, Reddy MB. EGCG Protects against 6-OHDA-Induced Neurotoxicity in a Cell Culture Model. Parkinsons Dis. 2015;2015:843906.
207. Hirata Y, Yamada C, Ito Y, et al. Novel oxindole derivatives prevent oxidative stress-induced cell death in mouse hippocampal HT22 cells. Neuropharmacology. 2018;135:242–252.
208. Nebie O, Devos D, Vingtdeux V, et al. The neuroprotective activity of heat-treated human platelet lysate biomaterials manufactured from outdated pathogen-reduced (amotosalen/UVA) platelet concentrates. J Biomed Sci. 2019;26(1):89.
209. Gouel F, Do Van B, Chou ML, et al. The protective effect of human platelet lysate in models of neurodegenerative disease: involvement of the Akt and MEK pathways. J Tissue Eng Regen Med. 2017;11(11):3236–3240.
210. Zhao Y, Zhang J, Zhang Y, et al. Proteomic Analysis of Protective Effects of Dl-3-n-Butylphthalide against mpp + -Induced Toxicity via downregulating P53 pathway in N2A Cells. Proteome Sci. 2023;21(1):1.
211. Grolez G, Moreau C, Sablonnière B, et al. Ceruloplasmin activity and iron chelation treatment of patients with Parkinson’s disease. BMC Neurol. 2015;15:74.
212. Cabantchik ZI, Munnich A, Youdim MB, Devos D. Regional siderosis: a new challenge for iron chelation therapy. Front Pharmacol. 2013;4:167. doi:10.3389/fphar.2013.00167
213. Devos D, Labreuche J, Rascol O, et al. Trial of Deferiprone in Parkinson’s Disease. N Engl J Med. 2022;387(22):2045–2055. doi:10.1056/NEJMoa2209254
214. Shults CW, Oakes D, Kieburtz K, et al. Effects of coenzyme Q10 in early Parkinson disease: evidence of slowing of the functional decline. Arch Neurol. 2002;59(10):1541–1550.
215. Seet RC, Lim EC, Tan JJ, et al. Does high-dose coenzyme Q10 improve oxidative damage and clinical outcomes in Parkinson’s disease?. Antioxid Redox Signal. 2014;21(2):211–217.
216. Storch A, Jost WH, Vieregge P, et al. Randomized, double-blind, placebo-controlled trial on symptomatic effects of coenzyme Q(10) in Parkinson disease. Arch Neurol. 2007;64(7):938–944.
217. Enns GM, Kinsman SL, Perlman SL, et al. Initial experience in the treatment of inherited mitochondrial disease with EPI-743. Mol Genet Metab. 2012;105(1):91–102.
218. Mischley LK, Lau RC, Shankland EG, Wilbur TK, Padowski JM. Phase IIb Study of Intranasal Glutathione in Parkinson’s Disease. J Parkinsons Dis. 2017;7(2):289–299.
219. Beal MF, Oakes D; Parkinson Study Group QEI. A randomized clinical trial of high-dosage coenzyme Q10 in early Parkinson disease: no evidence of benefit. JAMA Neurol. 2014;71(5):543–552.
220. Investigators NN-P. A randomized clinical trial of coenzyme Q10 and GPI-1485 in early Parkinson disease. Neurology. 2007;68(1):20–28.
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.





