Back to Journals » Clinical Interventions in Aging » Volume 17
Managing Musculoskeletal and Kidney Aging: A Call for Holistic Insights
Authors Cailleaux PE , Cohen-Solal M
Received 24 January 2022
Accepted for publication 20 April 2022
Published 4 May 2022 Volume 2022:17 Pages 717—732
DOI https://doi.org/10.2147/CIA.S357501
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Nandu Goswami
Pierre-Emmanuel Cailleaux, Martine Cohen-Solal
Inserm UMR-S 1132 Bioscar, Université Paris Cité - Hôpital Lariboisiere, Paris, F-75010, France
Correspondence: Martine Cohen-Solal, Inserm UMR-S 1132 (Université Paris Cité), Hôpital Lariboisière, 2 rue Ambroise Paré, Paris, F-75010, France, Tel/Fax +33-1-49-95-63-58, Email [email protected]
Abstract: Aging represents a major concern, with a two-fold increase in individuals > 65 years old by 2040. Older patients experience multiple declines in condition, with overlapping concerns. Fractures, frailty and falls remain underestimated events in routine practice. They are shared by numerous conditions and diseases, such as osteoporosis, sarcopenia and undernutrition, which mostly feature low evolution and are silent. In this review, we focused on musculoskeletal decline in older individuals who also have chronic kidney disease (CKD), which promotes fractures and falls. We aimed to highlight the need for a global approach for musculoskeletal and kidney aging. Although strategies limiting falls remain controversial, the need for an early diagnosis can limit these declines and allow for specific treatment of bone fragility in addition to non-pharmacological approaches. The emergence of senolytic agents offers new hope for preventing musculoskeletal disorders. This scoping review describes these overlapping silent diseases, provides evidence for their global understanding and management, and sheds light on new therapeutic directions.
Keywords: older individuals, osteoporosis, sarcopenia, kidney disease, senolytics
Introduction
Dependence represents the threshold of tertiary prevention that we aim to avoid. Many injuries leading to dependence are affected by aging, and the effects of some could be minimized, if early managed. Early prevention remains a challenge in poorly symptomatic patients with concomitant silent multiple declines. Therefore, the “F-issues” (fragility fractures, falls and frailty), should be considered as red flags in an asymptomatic aging patient. Hip fracture represents a burden, with a yearly 25% mortality rate, especially in the elderly. In Europe, the number of death due to fracture events in 2019 was around 250.000, when the number of new fragility fractures was estimated at 4.3 million, comprising 826,708 hip fractures (19%) and 662,544 vertebral fractures (16%).1 The majority of fragility fractures are related to osteoporosis after the age of 50. Falls are the main trigger for fracture in older individuals, and a relevant marker shared with musculoskeletal frailty such as muscle loss and undernutrition.
Although kidney function is not systematically assessed in musculoskeletal frailty, chronic kidney disease (CKD) exposes to increased risk of fracture. Age-standardized incidence of hip fracture among patients with non-dialysis-requiring CKD is estimated 1.81/1000 persons, 2-times higher than in normal kidney function patients.2 In addition, CKD also exposes to increased risk of falling, frailty and sarcopenia, and these increases with age. Here, we addressed the crosstalk between these declining functions in older people and reported the assessment tools and the combined management of musculoskeletal failure in patients with altered renal function. We aimed to highlight the existing gap in this specific field. Although this work was designed as a narrative review, our research methodology is available at the bottom of the text.
State of the Art in Aging of Either Musculoskeletal System or Kidney Function
Aging Muscle: Is This Frailty?
Frailty is defined as a phenotype leading to morbidity and dependence. Frailty is reversible and could be prevented by an early intervention. As dependence increases with age, frailty is found in 10.7% of individuals >65-yo and in 25% to 50% of those >85-yo. Frailty is associated with increased risk of falls and fractures.3 The assessment of frailty includes different approaches, such as the Fried’s model in which five indicators overlap with the sarcopenia assessment.4
Sarcopenia is a global muscle disease that precipitates the loss of muscular mass, altering muscle function (strength and power) and physical performance. The diagnosis of sarcopenia remains a challenge, given the extensive number of tools available for the definition. The expert group of the European Society for Clinical and Economic Aspects of Osteoporosis and Musculoskeletal Diseases advises the use of grip strength to measure muscle strength, and 4-m gait speed or the Short Physical Performance Battery, to measure physical performance in daily practice.5 In addition, dual energy X-ray absorptiometry (DEXA) provides the distribution of fat mass and appendicular lean mass. However, recent studies demonstrated the lean mass is a poor marker for fracture prediction after adjustment of femoral neck bone mineral density (BMD).6 Thus, in the more recent definitions of sarcopenia, lean mass has been associated with measures of physical function/performance/strength. The European Group on Sarcopenia in Older People (EWGSOP) definition (with gait speed, DEXA, grip strength, chair stand) seemed the most predictive of all definitions in predicting fracture independent of BMD, falls and Fracture Risk Assessment Tool (FRAX®) in the MrOS cohort study.7
The prevalence of sarcopenia increases with age and compromises health in 1% to 29% of older people in community living (10% in acute medicine unit) and in 14% to 33% of those in nursing homes.8 The decline in muscular strength occurs earlier and is more severe than the loss of muscular mass (3% per decade after age 70 vs 1–2% after 50).9
Malnutrition is also common in older people and is a major risk factor for frailty and sarcopenia. The prevalence of protein-uptake malnutrition depends on many factors and differs between acute-care or community patients, although the diagnosis relies on the definition and the tools used to define malnutrition. Prevalence rates of high malnutrition risk across all countries and screening tools ranged from 8.5% in the community setting to 28.0% for people in hospitals, in a recent analysis including 22 malnutrition screening tools validated in adults aged 65 years or older.10–13 This observation highlights the need for a parallel assessment of both sarcopenia and undernutrition. Although anthropometry indices are used to assess nutritional status in older adults, these are not relevant for muscle mass assessment. Body mass index (BMI) measurement remains the simplest approach, with the 21 kg/m2 threshold considered for malnutrition in older people. The extent of metabolic undernutrition is assessed with serum albumin level, according to the last recommendations. However, calf circumference remains an alternative in muscle mass evaluation when no other diagnostic methods are available.14
As far as treatment is concerned in undernutrition, avoiding a restrictive diet in older people is recommended first, as is prescribing a nutritional oral complement when needed. Together, loss in muscle strength, function and performance are associated with severe prevalent adverse health conditions in older people. Frailty, undernutrition and sarcopenia are reliable markers associated with several outcomes such as falls, fractures and death.15
Ageing Bone Issues
Most fractures occur after a fall, the risk increasing with age. Bone strength includes both bone density (BMD) and bone quality (microarchitecture and turnover). In post-menopausal women, the association with BMD and the fracture risk easily allows to predict fracture risk. Fractures, especially hip fractures, are a public health challenge because of their cost and mortality. Most patients who experience a hip fracture are older than 80 years.16 The impact of hip fracture on quality of life and life expectancy in older people is similar to that for other chronic diseases.17 The advances in Fracture Liaison Service and reeducation therapies can improve the consequences of fracture and re-fracture risk.18 However, about 75% to 80% of hip fractured patients, whom are mainly older, never received any treatment for their bone fragility within the year after the fracture. In the oldest old individuals, the risk of death competes with the risk of fracture.19 Thus, life expectancy represents a key factor for decision-making in this population, especially in patients with major disabilities.
Although the mean life expectancy at birth in Europe is 82.6 years for women and 76.7 years for men, residual life expectancy above age 85 and 95 is about 6.97 and 3.19 years, respectively, for both genders. Thus, the question of preventing osteoporotic fractures remains crucial.
When investigating bone fragility in older people, the usual tools for young adults do not reach the same level of validation. Major factors remain the history of fracture or the history of fall. Although the FRAX® now adjusts the result in very old patients for the competing hazard of death, the FRAX® remains a 10-year prediction of fracture risk.
Biological assays are performed for ruling out malignant bone diseases, a metabolic bone disorder and monitoring contraindications to therapy. Bone biomarkers help to determine the levels of bone remodeling and the monitoring/adherence to therapy (always after a reasonable time delay after a fracture). The International Osteoporosis Foundation (IOF) only recommends serum P1NP and CTX assays because they are modified by therapy.20
Measurements of Vitamin-D levels are recommended because Vitamin-D level must be in the normal range to initiate a therapy. Supplementation also had a significant effect on femoral neck BMD,21 with no major effect on incident fracture prevention after age 70.22 The last results were confirmed in a meta-analysis that only analyzed vitamin D without calcium supplementation.23 Vitamin-D supplementation could have numerous positive effects, in particular on muscle and falls, in which the effect appears to be dose-dependent24 and not found in the same Bolland work.
BMD measurement in this population also has several limitations. BMD does not accurately reflect the situation for patients with prevalent major fracture. Therefore, reference curves and thresholds for the diagnosis are questionable. The low reproducibility in positioning and frequent presence of bilateral hip prosthesis, lumbar spine osteoarthritis, unknown vertebral fracture, spine deformity and aortic calcification25 are frequent issues limiting BMD interpretation and reproducibility (Figure 1). Lateral imaging of the spine reinforce BMD measurement interpretation, providing information on silent vertebral fracture (Figure 1A), the level of aortic vascular calcification and the presence of osteoarthritis (Figure 1B and C).
Osteoporosis drugs have proved their efficiency in patients with >1 year life expectancy.26 With age >70 and vascular risk, selective estrogen receptor modulators are a non-relevant option in this population. Therefore, discussion of osteoporosis drugs in older people will involve bisphosphonates, teriparatide and denosumab. Recently, the International Conference on Frailty and Sarcopenia Research Task Force developed targets for research on osteoporosis in frail older adults.27 According to these recommendations, optimal treatment for osteoporosis in older people may require combined or sequential therapies. Romosozumab followed by denosumab reduced the risk of fracture in postmenopausal women in the recent FRAME phase-2 study. This combination should have been a helpful sequence,28 although currently not approved in women above 75 with a history of ischemic heart failure.
The tolerance of any osteoporosis therapy is good in older individuals, with mild and reversible adverse effects. In older patients, oral drugs should be evaluated in the light of the lower bioavailability of oral treatment, slower metabolic rate, concomitant deficiencies and treatments. The remnant effect of parenteral bisphosphonates calls for their use when life expectancy is reduced and the risk of poor adherence is increased. Reevaluation is relevant within 3 years if no new fracture or risk factor has occurred. Overall, because of the diagnostic tools and treatment available, osteoporosis is one of the best characterized diseases to achieve musculoskeletal prevention in older people, in which osteoporosis is prevalent.
Kidney Function and Assessment in Older Individuals
Kidney function declines with age. The loss of function is the result of kidney aging and lifelong pathologic injuries. A progressive decline of glomerular filtration rate (GFR) of about 1 mL/min/year represents normal ageing of kidney function above age 40. Although the incidence of renal impairment is still increasing,29 most adults with CKD never reach end-stage renal disease.30,31 After the age of 80, the incidence of CKD increases by 10-fold as compared with 18 to 50 years. In addition, the longitudinal SCOPE study assessed the profile of comorbidity conditions in 2252 subjects aged above 75 years across Europe. They showed that CKD was the most frequent conditions and was rarely observed without any other co-occurring disease. Besides the cardiovascular diseases that were predominant, CKD was highly associated with osteoporosis and hip fracture.32 The score of physical performance was reduced in subjects with severe CKD, suggesting that multi-organ decline.
Numerous frequent comorbidities, such as cognitive impairment, probably because it is a vascular component, are related to renal impairment in older people.33 In a diabetes mellitus cohort, patients aged >80 with dementia had an increased mortality in case of renal impairment.34 Nonetheless, in patients with CKD stage 3, the 10-year cumulative incidence of dialysis and transplantation (0.04) contrasts with the mortality incidence (0.51, mainly due to cardiovascular diseases).35 This fact highlights that the phenotype of older CKD patients is not similar to younger patients.
Thus, this observation could be related to a difference in the assessment of renal function in older versus younger people. However, older people with CKD stage 3A without proteinuria have no additional risk of mortality as compared with similar age-class individuals with estimated GFR>60 mL/min/1.73 m2.36 This underlined the probable overestimation of CKD stage 3 in older individuals, in which a few CKD stage 3A patients met the 2-parameters assessment recommended by the Kidney Disease: Improving Global Outcomes (KDIGO) guidelines.
Other limitations in the CKD assessment in older individuals are identified. The measurement of GFR using isotopic or iohexol injection is optimal but is limited to dedicated physiology departments. In a routine setting, serum creatinine level remains widely used as a surrogate marker for renal function estimation. To date, no strong evidence is available to prefer other biomarkers (cystatin-C …) in old patients. However, in acute care, creatinine in older patients do not correctly reflect the renal function. Furthermore, creatinine levels are determined by muscular production, which is a major limitation in this population. Moreover, technical conditions also limit the assessment of the 24-hr urinary creatinine level.
The most widely used tools for estimating GFR remains creatinine-based equations (4-variable Modification of Diet in Renal Disease [MDRD], CKD Epidemiology Collaboration [CKD-EPI] and Cockcroft and Gault [CG] equations). Values for patients above age 90 years are not correctly represented in each of these reference populations, which raises concerns about their validity. Of all methods, MDRD equations produce the most accurate results for an association with the gold-standard.37
Besides kidney failure, older patients are exposed to iatrogenic issues. We will not get into it here. In the musculoskeletal approach, attention should either be paid to drugs with a negative impact on bone long-term corticosteroid, heparin, vitamin K antagonist38 or with a positive impact (bisphosphonates, teriparatide, thiazide diuretics, statins, etc.). Also, it is important to balance the individual risk on drugs that expose to falls such as antihypertensive therapy, especially for renin-angiotensin antagonists, which protect kidney function.39
Need for Novel Comprehensive Approach in Concomitant Declines of Musculoskeletal and Kidney Function
Frailty and Sarcopenia in CKD
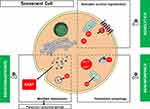
Musculoskeletal frailty involves several interacting systems that converge toward fracture occurrence (Figure 2), including kidney failure that increased with aging. The prevalence of frailty in CKD patients is estimated at about 14%, which increases their mortality risk by 2.5 points. Sarcopenia is mostly prevalent in late CKD stages, whatever the definition,40 and with an altered handgrip,41 as confirmed by results from the NHANES study.42 Although sarcopenia progressively increases with renal impairment in CKD, only some evidence links muscular function to fracture risk in CKD,43 with numerous hypotheses studied for their potential role in change in myostatin metabolism.
One of the aims in care for sarcopenia remains to limit falls. The increase in risk of fall is well documented in CKD,44,45 in particular in end-stage renal disease46,47 and in patients >65 years old. Above age 65, falls are more frequent and often generate complications, especially when GFR is <45 mL/min, with the incidence of falls about 38.3 vs 21.7/1000 person-years with GFR>60. Risk of falls is also increased with GFR<60mL/min associated with osteoporosis, especially corticosteroid-induced osteoporosis.48
Whether specific causes of falls in CKD have not been elucidated; some studies considered that diabetes or uremic-related neuropathy could be good candidates.43 In older individuals, in whom falls are multifactorial, risk of falls is increased by two-fold with polymedication, BMI <18.5 and low GFR. Dementia also increases this risk by 1.21 points.49
Some specific quantitative gait abnormalities have been identified in CKD patients (slower gait speed, shorter stride length, reduced time in the swing phase and increased time in the double support stance phase), adjusted for age and sex associated with fall risk.50 Although no specific program is available for these patients, the objective of limiting falls should focus on detecting frailty, improving modifiable factors (visual acuity, multiple medications, and home environment) and focusing on strengthening, gait, and balance in exercise programs. Nutrition management could appear as a realistic way of improving sarcopenia, but no drug or no specific biomarker are yet recommended. However, IGF-1 level, which are low in undernutrition, may have a positive impact on both bone and muscle. Such as serum myostatin, in which level increases along with CKD and inhibits muscular mass.51
Clinical Assessment of Bone Disorders in CKD
In CKD patients, a high prevalence of fracture is reported along with a high mortality rate related to the fracture.52 Osteoporosis in CKD patients is related to diabetes mellitus and hypertension, whose incidence increases with age. However, a recent meta-analysis reported that even about 24% of CKD cases could be related to diabetes mellitus, the condition increasing the fracture risk independent of kidney function.53 Different entities have been identified in bone mineral and metabolism disorders related to CKD (CKD-mineral and bone disorders [CKD-MBD]) to better understand the participation of renal impairment. The KDIGO work-group defined CKD-MBD as one or a combination of three manifestations. Manifestations can be disorders of bone and mineral metabolism and/or extra-skeletal calcification, and/or renal osteodystrophy defined as altered bone morphology associated with CKD (turnover, mineralization, volume), assessed by histology.
The risk of hip fracture increases by 2- to 14-fold in CKD patients as compared with the general population. This risk appears at the early stages versus non-CKD and increases with the CKD severity.54 Hip fracture incidence increase in parallel with CKD progression.55 Fracture risk is increased among older individuals with CKD,48 which illustrates that age is a major risk factor for fracture,17 although renal impairment and osteoporosis have an independent or additive impact on fracture risk.56 In contrast, older people with osteoporosis frequently have renal impairment with age.57
However, these progressions differ by sex. With menopause, bone mass declines faster in women between age 50 to 70 than men,58 whereas in general, decline in renal function is fastest in men. Nonetheless, CKD seems to remain more common among women, with an estimated prevalence of 11–13%. Regardless, Big data analyses do not individualize a homogenous male: female distribution of CKD across the world, according to countries with different economic states.59 GFR increase its decline after age 70 in osteoporosis women, as seen in the post-hoc analysis of the Horizon-PFT study.60 The overlap between CKD and osteoporosis can be illustrated with the NHANES-III cohort, in which 60% of women with osteoporosis also had CKD stage 3 and 23% stage 4.
In patients with CKD stage 1–2 with osteoporosis and/or high risk of fracture or in those with CKD stage 3 with PTH concentration in the normal range, the KDIGO recommends managing osteoporosis as for the general population. However, CKD does not systematically imply associated CKD-MBD, whereas 85% of women with osteoporosis show altered renal function.
The FRAX® is used for assessment in CKD especially when enhanced with BMD and trabecular bone score results.61 Nevertheless, the FRAX® does not include key factors for CKD-MBD or GFR evaluation, number of fractures and falls.62
Low BMD correctly predicts risk of hip fracture in older people with CKD.63 With CKD stages 1 to 3, BMD can predict risk of fracture, which confirms that people at early stages of CKD with no evidence of CKD-MBD can be considered as having osteoporosis. Conversely, a CKD stage 3–4 was discovered in 84% of older women with osteoporosis. However, BMD in late stages remains controversial in estimating risk of fracture. Thus, the 2009 KDIGO recommendations were updated in 2017 to favor assessing BMD in patients with CKD stage 3a-5 if the result would affect treatment decisions but also to identify patients at high risk of fracture. With longitudinal follow-up in patients with CKD stage 3–5 and with measured GFR and BMD follow-up, a slight bone loss occurs at the radius only, a cortical bone.64 However, because the levels of bone loss remain unknown, the frequency of serial BMD assessments remains to be determined. Therefore, the European Dialysis and Transplant Association and the IOF integrate the evaluation of fracture risk as a target in the management of CKD-MBD.65 In older patients with 11 years of follow-up, a 1-SD change at the femoral neck increased by two-fold the risk of fracture in CKD as compared with no CKD. In end-stage renal disease, BMD is lower, especially at cortical bone sites,66 so this site may be more relevant in CKD patients in whom aortic calcifications are more prevalent, thus possibly distorting lumbar spine BMD.
Investigating Bone Biochemical Parameters in CKD
The last KDIGO recommendations highlighted that other priorities are competing with CKD-MBD management in people aged 75 to 78 with a 2.5-year follow-up, thus justifying the need for screening older people with a global assessment.67 CKD patients have numerous comorbidities and there is a trend to not test patients beyond age 85.
The most frequent conditions in CKD-MBD are: secondary hyperparathyroidism, hyperphosphatemia, hypocalcemia and low calcitriol level.
- In CKD patients with hyperparathyroidism, serum PTH level increases in parallel with CKD progression.68 Other causes such as vitamin D deficiency hyperparathyroidism should be explored to avoid concluding renal secondary hyperparathyroidism. KDIGO guidelines also recommend managing hypocalcemia, hyperphosphatemia and vitamin D deficiency in CKD stage 3 to 5 with hyperparathyroidism.69 BMD should be interpreted with those bone parameters: low BMD in CKD seems to predict fracture better if associated with normal-range PTH level.70
- Although serum phosphate levels are expected to be normal in osteoporosis, they could remain useful to assess bone fragility in older CKD individuals. According to the MrOS and the Rotterdam cohorts, increased phosphate levels could be related to fracture risk along with CKD,71 even after adjustment for PTH and FGF-23.
- Vitamin-D insufficiency must be managed with a targeted level for 25OH-vitamin D above 75 nmol/mL whatever is the aim (osteoporosis, CKD, or falls). In CKD stage 3–4, a normal gait speed is associated with the highest level of 25(OH)-vitamin D.72 When associated with hypocalcemia, calcitriol level can also be assessed to adapt therapy. Some evidence suggests that in inhibiting the Wnt signaling pathway, vitamin D could limit vascular calcification, one of the components of CKD-MBD.73 Because of the effect on muscle via the vitamin D receptor, as for the mitochondrial or non-genomic pathway, vitamin D remain a good candidate for intervention in sarcopenia.74 Vitamin D is also known to lower myostatin level75 and increase levels of insulin-like growth factor 1, sclerostin, osteocalcin and FGF-23 and therefore muscular mass.76
- In addition, serum FGF-23 is a hormone produced by osteocytes that stimulates renal phosphate excretion. FGF-23 level increases from the early stages of CKD and plays a role in the decrease in calcitriol level. FGF-23 is not associated with fracture or with lower BMD after adjustment on GFR.77 Klotho allows for contact between FGF-23 and its receptor. Decreased levels along with CKD lead to a lower number of osteoclasts with higher activity.78
- Finally, urinary calcium levels can also be helpful because fractures seem to occur more frequently in CKD stages 3 and 4 in patients with a history of kidney stone (5.56/100 pa).79
Several other bone biomarkers are available, but their renal elimination limits their interpretation. Among bone remodeling biomarkers, only bone-specific alkaline phosphatase (bs-ALP), TRAPc5b and the trimeric form of P1NP are not impacted by kidney elimination. Bs-ALP is a bone formation biomarker associated with both low80 and high levels of bone turnover. TRAPc5b & P1NP are poorly reported in the aging CKD population.
Therapeutic Approaches in Old CKD Patients
Non-Pharmacological Approaches
Also here, several types of intervention in fall prevention have been developed with poor results.81 However, the need for care in fall prevention remains a key element for patients at high risk of fracture. Muscle stretching and strengthening, especially the pelvic belt and lamb triceps, could be advised. Physical activities such as Tai-Chi should have a positive effect on osteoporosis prevention.82 Specific gait abnormalities in old CKD patients could be targeted with specific interventions, as long as no program is recommended. Other risk factors for falls must be corrected according to the evaluation. Malnutrition is one of them. The recommended protein uptake (1–2g/kg/d) is in competition with nephrology guidelines, stating that uptake must be maintained from 0.8 to 1mg/kg/day. In osteoporotic patients too, nutritional recommendations do not systematically match with CKD-MBD, for which avoiding a high phosphate diet is recommended.
In older people, the targeted 25(OH)-vitamin D is >75 nmol/L. In the DOPPS cohort of CKD patients, all those taking vitamin D were healthier than those who did not.83 Supplementation also affects other aspects (osteoporosis, sarcopenia, falls84) especially in CKD patients for whom supplementation could improve global mobility and muscular function.85 In older people, improving calcium uptake assessment is useful although some evidence suggests increased vascular risk in patients without any calcium insufficiency.86
Pharmacological Approach
In older people, drug prescription is challenged by the prevalence of numerous treatments for their comorbidities, by cognitive impairment limiting drug observance, and by the risk itself. CKD stages 1–3a should be treated like osteoporosis.87 In CKD stage 3b-5, the needs for priority settings on treatment must be discussed with multidisciplinary global insight. The management of confirmed biochemical abnormalities (hyperphosphatemia, hyperparathyroidism and vitamin D deficiency) should be considered before specific fracture prevention therapeutics. Table 1 summarizes data on CKD and age provided by pivotal studies. Although the older individual is the targeted population, Table 1 also illustrates that a global approach with renal function and geriatric musculoskeletal parameter outcomes remain poorly reported. Here, we discuss the main limitations of drugs in CKD patients.
 |
Table 1 Age and Chronic Kidney Disease (CKD) Assessment from Pivotal Studies or Among Them, for Drugs Used in Osteoporosis in Older People |
According to a post-hoc analysis of pivotal studies, CKD-MBD can be treated like osteoporosis until stage 3, including if biochemical parameters remain in the normal range; however, the use of bisphosphonates as any anti-resorptive drug therapy in CKD can paradoxically increase fracture risk by increasing failure in mineralization, but not bone volume. This limitation is related to the prevalence of adynamic bone disease (ABD) in CKD.
Thus, if levels of bone turnover biomarker remain low as in ABD, the use of bisphosphonates can worsen the condition.88 Long-term use of bisphosphonates in CKD can also lead to ABD.89 The kidney excretes bisphosphonates hours after ingestion by passive glomerular filtration or a proximal tubular way, whereby about 27% to 62% of the drug is set on bone. A threshold of 30 mL/min is considered for drug prescription. This threshold resulted from pivotal studies and nephrotoxicity studies of animals.90
- The more the GFR decreases, the more the drug accumulation increases, with verified nephrotoxicity in rapid intravenous infusion of zoledronate or pamidronate,91,92 which justifies the spacing between delivery.93 Nonetheless, the use of oral bisphosphonates such as 5mg risedronate remains safe on the kidney and efficient for BMD94 and has been tested specifically in older individuals.95 Alendronate also increases BMD even with CKD stage 3 and 4, and also increases PTH level at 18 months.96 Alendronate has also been tested on vascular calcifications.
- Teriparatide is a PTH analog. Actually, data are missing in CKD patients with GFR <30 mL/min other than the post-hoc analysis of the 2014 pivotal study.97 Teriparatide could be useful in ABD or parathyroidectomy.
- Denosumab is a monoclonal anti-Rank-ligand antibody whose specificity was tested in patients with 15 to 30 mL/min GFR. Denosumab remains an antiresorptive therapy with the same bone complications as for bisphosphonates. In addition, because of a lack of residual effect when stopped, denosumab must be relayed by another antiresorptive therapy to avoid a cascade of vertebral fractures, which limits the prescription in the same range as for other drugs. Post-hoc analysis of the FREEDOM study of dialysis showed an increase in BMD at 6 months but also in PTH level and prevalence of hypocalcemia.98 In CKD stage 4 with high risk of fracture in younger adults, Hampson suggested considering denosumab or off-label prescription of bisphosphonate.99
Bone turnover must be explored (bone biopsy, biomarkers) to validate the absence of ABD when antiresorptive drugs have no effect. If this option is retained, the recommendation for patients with low BMD and reduced life expectancy is to keep the therapy to the end, making it suitable also in older CKD patients.
Future Directions
Although aging is a common notion, its mechanisms remain incompletely known. In cell biology, aging could be characterized by programmed senescence or an accumulation of lifelong injuries. Cellular senescence is a cell fate involving extensive changes in gene expression and proliferation arrest. Pathways involved are genetic instability, telomere attrition, hormonal cycle influence or immune system decline. This senescence also involves cell cycle deregulation (via p21CDKN1α or p16INK4α), an increase in lysosomal β-galactosidase activity, and apoptosis resistance. Many other pathways have been described, such as oxidative stress pathways and non-enzymatic protein glycation and accumulation of advanced glycation endproducts altering protein function. Moreover, a dynamic process is involved, whereby the senescence phenotype spreads to the surrounding tissue by a specific secretion of senescent cells. Such cells secrete a range of pro-inflammatory cytokines, chemokines and proteases (interleukin 6, CXCL-12, matrix metalloproteinase, etc.), termed the senescence-associated secretory phenotype (SASP), which contributes to local and systemic dysfunction with aging. To date, three types of senotherapies are described: senolytics, senomorphics, and senosuppressors (Figure 3). They inhibit apoptosis resistance and the activation of survival pathways and decrease autophagy, SASP and metabolic aberrations. The use of senotherapeutic agents prevented tissue degeneration and improved longevity in mice models.100 Senotherapeutic agents also provide new perspectives for musculoskeletal aging. Different approaches are efficient for targeting cellular senescence in age-related bone loss in mice, with a transgene or a pharmacological approach. The use of Janus kinase inhibitors on the SASP also provides efficient results with lower bone resorption and maintained or higher bone formation in trabecular and cortical bone, respectively.101 In another SASP model of senescence with senescent cell transplant impairing functional parameters used in clinical practice (maximal walking speed, muscle strength, physical endurance, body weight), the use of senolytics such as dasatinib+quercetin reduced senescent cell burden and decreased pro-inflammatory cytokine secretion, even in human adipose tissue explants. Senolytics prevent and alleviate the senescent cell transplantation-induced physical dysfunction. Clearing senescent cells alleviates physical dysfunction and increases the remaining lifespan in older mice.102 These promising approaches could be a relevant perspective in frailty and dependence prevention in older people.
Conclusions
In the multi-approach course that represent the understanding of aging, this review emphasizes that common clinical concerns (CKD, fall and fractures …) need more than ever a multidisciplinary approach in order to get the big picture. We should remain as simple as possible, and we first recommend assessing residual life expectancy as a major key factor for decision-making. Osteoporosis remains a good model with efficient drugs even on CKD older individuals. It seems important to have in mind the limits of all the assays and measures that could be performed, to limit misinterpretation and excessive inflation of risk of error.
Concise Methodology
In this review, we first performed an electronic search from January 1980 to February 2020 using MEDLINE (PubMed) for original works and expert report. An iterative approach included 2 equations with the following MeSH terms: “Fracture” + “Chronic kidney disease”; “Bone” +” Elderly” +”Chronic Kidney Disease”. The first reviewer (PEC) screened the titles and abstracts according to these keywords criteria. Then, the selection was transferred to the second reviewer (MCS) who refined and confirmed the selection. The two reviewers then performed a more careful reading of the manuscripts and selected the most relevant papers to their aim. We also added guidelines of international and national societies as well as relevant review articles in order to illustrate positions in case scientific data are not available. A narrative synthesis of each organ failure was conducted, aiming to describe the evidence and limitations for the diagnosis for each tissue insufficiency. We then analyzed the literature in the light of concomitant diseases and identify the needs for further research.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
Pierre-Emmanuel Cailleaux declares that he has no competing interest.
Martine Cohen-Solal declares that she has no competing interest.
References
1. Kanis JA, Norton N, Harvey NC, et al. SCOPE 2021: a new scorecard for osteoporosis in Europe. Arch Osteoporos. 2021;16:82. doi:10.1007/s11657-020-00871-9
2. Kim SM, Long J, Montez-Rath M, et al. Hip fracture in patients with non-dialysis-requiring chronic kidney disease. J Bone Miner Res off J Am Soc Bone Miner Res. 2016;31:1803–1809. doi:10.1002/jbmr.2862
3. Tom SE, Adachi JD, Anderson FA, et al. Frailty and fracture, disability, and falls: a multiple country study from the global longitudinal study of osteoporosis in women. J Am Geriatr Soc. 2013;61:327–334. doi:10.1111/jgs.12146
4. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol a Biol Sci Med Sci. 2001;56:M146–156. doi:10.1093/gerona/56.3.M146
5. Beaudart C, Rolland Y, Cruz-Jentoft AJ, et al. Assessment of muscle function and physical performance in daily clinical practice: a position paper endorsed by the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO). Calcif Tissue Int. 2019;105:1–14. doi:10.1007/s00223-019-00545-w
6. Harvey NC, Odén A, Orwoll E, et al. Measures of physical performance and muscle strength as predictors of fracture risk independent of FRAX, falls, and aBMD: a meta-analysis of the osteoporotic fractures in men (MrOS) Study. J Bone Miner Res off J Am Soc Bone Miner Res. 2018;33:2150–2157. doi:10.1002/jbmr.3556
7. Harvey NC, Orwoll E, Kwok T, et al. Sarcopenia definitions as predictors of fracture risk independent of FRAX, falls, and BMD in the osteoporotic fractures in men (MrOs) study: a meta‐analysis. J Bone Miner Res. 2021;36:1235–1244. doi:10.1093/ageing/afy144
8. Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16–31. doi:10.1093/ageing/afy169
9. Hughes VA, Frontera WR, Roubenoff R, et al. Longitudinal changes in body composition in older men and women: role of body weight change and physical activity. Am J Clin Nutr. 2002;76:473–481. doi:10.1093/ajcn/76.2.473
10. Cederholm T, Jensen GL, Correia MITD, et al. GLIM criteria for the diagnosis of malnutrition - A consensus report from the global clinical nutrition community. J Cachexia Sarcopenia Muscle. 2019;10:207–217. doi:10.1002/jcsm.12383
11. Norman K, Haß U, Pirlich M. Malnutrition in older adults—recent advances and remaining challenges. Nutrients. 2021;13:2764. doi:10.3390/nu13082764
12. Crichton M, Craven D, Mackay H, et al. A systematic review, meta-analysis and meta-regression of the prevalence of protein-energy malnutrition: associations with geographical region and sex. Age Ageing. 2019;48:38–48.
13. Leij-Halfwerk S, Verwijs MH, van Houdt S, et al. Prevalence of protein-energy malnutrition risk in European older adults in community, residential and hospital settings, according to 22 malnutrition screening tools validated for use in adults ≥65 years: a systematic review and meta-analysis. Maturitas. 2019;126:80–89.
14. Landi F, Onder G, Russo A, et al. Calf circumference, frailty and physical performance among older adults living in the community. Clin Nutr Edinb Scotl. 2014;33:539–544. doi:10.1016/j.clnu.2013.07.013
15. Cruz-Jentoft AJ, Sayer AA. Sarcopenia. Lancet Lond Engl. 2019;393:2636–2646. doi:10.1016/S0140-6736(19)31138-9
16. Chevalley T, Guilley E, Herrmann FR, et al. Incidence of hip fracture over a 10-year period (1991–2000): reversal of a secular trend. Bone. 2007;40:1284–1289. doi:10.1016/j.bone.2006.12.063
17. Kannegaard PN, van der Mark S, Eiken P, et al. Excess mortality in men compared with women following a hip fracture. National analysis of comedications, comorbidity and survival. Age Ageing. 2010;39:203–209. doi:10.1093/ageing/afp221
18. Lih A, Nandapalan H, Kim M, et al. Targeted intervention reduces refracture rates in patients with incident non-vertebral osteoporotic fractures: a 4-year prospective controlled study. Osteoporos Int J Establ Result Coop Eur Found Osteoporos Natl Osteoporos Found USA. 2011;22:849–858. doi:10.1007/s00198-010-1477-x
19. Berry SD, Ngo L, Samelson EJ, et al. Competing risk of death: an important consideration in studies of older adults. J Am Geriatr Soc. 2010;58:783–787. doi:10.1111/j.1532-5415.2010.02767.x
20. Evenepoel P, Cavalier E, D’Haese PC. Biomarkers predicting bone turnover in the setting of CKD. Curr Osteoporos Rep. 2017;15:178–186. doi:10.1007/s11914-017-0362-3
21. Reid IR, Bolland MJ, Grey A. Effects of vitamin D supplements on bone mineral density: a systematic review and meta-analysis. Lancet Lond Engl. 2014;383:146–155. doi:10.1016/S0140-6736(13)61647-5
22. Grant AM, Avenell A, Campbell MK, et al. Oral vitamin D3 and calcium for secondary prevention of low-trauma fractures in elderly people (Randomised Evaluation of Calcium Or vitamin D, RECORD): a randomised placebo-controlled trial. Lancet Lond Engl. 2005;365:1621–1628.
23. Bolland MJ, Grey A, Avenell A. Effects of vitamin D supplementation on musculoskeletal health: a systematic review, meta-analysis, and trial sequential analysis. Lancet Diabetes Endocrinol. 2018;6:847–858. doi:10.1016/S2213-8587(18)30265-1
24. Bischoff-Ferrari HA, Dawson-Hughes B, Staehelin HB, et al. Fall prevention with supplemental and active forms of vitamin D: a meta-analysis of randomised controlled trials. BMJ. 2009;339:b3692. doi:10.1136/bmj.b3692
25. Ott SM. Review article: bone density in patients with chronic kidney disease stages 4–5. Nephrol Carlton Vic. 2009;14:395–403. doi:10.1111/j.1440-1797.2009.01159.x
26. Rizzoli R, Branco J, Brandi M-L, et al. Management of osteoporosis of the oldest old. Osteoporos Int J Establ Result Coop Eur Found Osteoporos Natl Osteoporos Found USA. 2014;25:2507–2529. doi:10.1007/s00198-014-2755-9
27. Rolland Y, Cesari M, Fielding RA, et al. Osteoporosis in frail older adults: recommendations for research from the ICFSR task force 2020. J Frailty Aging. 2021;10:168–175. doi:10.14283/jfa.2021.4
28. Cosman F, Crittenden DB, Ferrari S, et al. FRAME study: the foundation effect of building bone with 1 year of romosozumab leads to continued lower fracture risk after transition to denosumab. J Bone Miner Res off J Am Soc Bone Miner Res. 2018;33:1219–1226. doi:10.1002/jbmr.3427
29. Kirsztajn GM, Suassuna JHR, Bastos MG. Dividing stage 3 of chronic kidney disease (CKD): 3A and 3B. Kidney Int. 2009;76:
30. O’Hare AM, Hotchkiss JR, Kurella Tamura M, et al. Interpreting treatment effects from clinical trials in the context of real-world risk information: end-stage renal disease prevention in older adults. JAMA Intern Med. 2014;174:391–397. doi:10.1001/jamainternmed.2013.13328
31. O’Hare AM, Choi AI, Bertenthal D, et al. Age affects outcomes in chronic kidney disease. J Am Soc Nephrol JASN. 2007;18:2758–2765. doi:10.1681/ASN.2007040422
32. Corsonello A, Fabbietti P, Formiga F, et al. Chronic kidney disease in the context of multimorbidity patterns: the role of physical performance: the screening for CKD among older people across Europe (SCOPE) study. BMC Geriatr. 2020;20:350. doi:10.1186/s12877-020-01696-4
33. Maravic M, Ostertag A, Torres PU, et al. Incidence and risk factors for hip fractures in dialysis patients. Osteoporos Int J Establ Result Coop Eur Found Osteoporos Natl Osteoporos Found USA. 2014;25:159–165. doi:10.1007/s00198-013-2435-1
34. Huang P-H, Chen T-H, Lin Y-S, et al. Chronic kidney disease worsens health outcomes in diabetic patients after hip fracture surgery: an asian nationwide population-based cohort study. J Bone Miner Res off J Am Soc Bone Miner Res. 2019;34:849–858. doi:10.1002/jbmr.3663
35. Eriksen BO, Ingebretsen OC. The progression of chronic kidney disease: a 10-year population-based study of the effects of gender and age. Kidney Int. 2006;69:375–382. doi:10.1038/sj.ki.5000058
36. Jonsson AJ, Lund SH, Eriksen BO, et al. The prevalence of chronic kidney disease in Iceland according to KDIGO criteria and age-adapted estimated glomerular filtration rate thresholds. Kidney Int. 2020;98:1286–1295. doi:10.1016/j.kint.2020.06.017
37. Van Pottelbergh G, Van Heden L, Matheï C, et al. Methods to evaluate renal function in elderly patients: a systematic literature review. Age Ageing. 2010;39:542–548. doi:10.1093/ageing/afq091
38. Knapen MHJ, Schurgers LJ, Vermeer C. Vitamin K2 supplementation improves hip bone geometry and bone strength indices in postmenopausal women. Osteoporos Int J Establ Result Coop Eur Found Osteoporos Natl Osteoporos Found USA. 2007;18:963–972. doi:10.1007/s00198-007-0337-9
39. Wray DW, Nishiyama SK, Harris RA, et al. Angiotensin II in the elderly: impact of angiotensin II type 1 receptor sensitivity on peripheral hemodynamics. Hypertens Dallas Tex. 2008;51:1611–1616. doi:10.1161/HYPERTENSIONAHA.108.111294
40. Dam -T-T, Peters KW, Fragala M, et al. An evidence-based comparison of operational criteria for the presence of sarcopenia. J Gerontol a Biol Sci Med Sci. 2014;69:584–590. doi:10.1093/gerona/glu013
41. Han D-S, Chen Y-M, Lin S-Y, et al. Serum myostatin levels and grip strength in normal subjects and patients on maintenance haemodialysis. Clin Endocrinol (Oxf). 2011;75:857–863. doi:10.1111/j.1365-2265.2011.04120.x
42. Foley RN, Wang C, Ishani A, et al. Kidney function and sarcopenia in the United States general population: NHANES III. Am J Nephrol. 2007;27:279–286. doi:10.1159/000101827
43. West SL, Jamal SA, Lok CE. Tests of neuromuscular function are associated with fractures in patients with chronic kidney disease. Nephrol Dial Transplant off Publ Eur Dial Transpl Assoc - Eur Ren Assoc. 2012;27:2384–2388.
44. Li M, Tomlinson G, Naglie G, et al. Geriatric comorbidities, such as falls, confer an independent mortality risk to elderly dialysis patients. Nephrol Dial Transplant off Publ Eur Dial Transpl Assoc - Eur Ren Assoc. 2008;23:1396–1400.
45. Desmet C, Beguin C, Swine C, et al. Falls in hemodialysis patients: prospective study of incidence, risk factors, and complications. Am J Kidney Dis off J Natl Kidney Found. 2005;45:148–153. doi:10.1053/j.ajkd.2004.09.027
46. Cook WL, Tomlinson G, Donaldson M, et al. Falls and fall-related injuries in older dialysis patients. Clin J Am Soc Nephrol CJASN. 2006;1:1197–1204. doi:10.2215/CJN.01650506
47. Farragher J, Chiu E, Ulutas O, et al. Accidental falls and risk of mortality among older adults on chronic peritoneal dialysis. Clin J Am Soc Nephrol CJASN. 2014;9:1248–1253. doi:10.2215/CJN.11001013
48. Dukas L, Schacht E, Stähelin HB. In elderly men and women treated for osteoporosis a low creatinine clearance of <65 mL/min is a risk factor for falls and fractures. Osteoporos Int J Establ Result Coop Eur Found Osteoporos Natl Osteoporos Found USA. 2005;16:1683–1690.
49. Bowling CB, Bromfield SG, Colantonio LD, et al. Association of reduced eGFR and albuminuria with serious fall injuries among older adults. Clin J Am Soc Nephrol CJASN. 2016;11:1236–1243. doi:10.2215/CJN.11111015
50. Sedaghat S, Darweesh SKL, Verlinden VJA, et al. Kidney function, gait pattern and fall in the general population: a cohort study. Nephrol Dial Transplant off Publ Eur Dial Transpl Assoc - Eur Ren Assoc. 2018;33:2165–2172.
51. Verzola D, Procopio V, Sofia A, et al. Apoptosis and myostatin mRNA are upregulated in the skeletal muscle of patients with chronic kidney disease. Kidney Int. 2011;79:773–782.
52. Nitsch D, Mylne A, Roderick PJ, et al. Chronic kidney disease and hip fracture-related mortality in older people in the UK. Nephrol Dial Transplant off Publ Eur Dial Transpl Assoc - Eur Ren Assoc. 2009;24:1539–1544.
53. Wang H, Ba Y, Xing Q, et al. Diabetes mellitus and the risk of fractures at specific sites: a meta-analysis. BMJ Open. 2019;9:e024067. doi:10.1136/bmjopen-2018-024067
54. Ensrud KE, Lui L-Y, Taylor BC, et al. Renal function and risk of hip and vertebral fractures in older women. Arch Intern Med. 2007;167:133–139. doi:10.1001/archinte.167.2.133
55. Naylor KL, McArthur E, Leslie WD, et al. The three-year incidence of fracture in chronic kidney disease. Kidney Int. 2014;86:810–818. doi:10.1038/ki.2013.547
56. Nickolas TL, McMahon DJ, Shane E. Relationship between moderate to severe kidney disease and hip fracture in the United States. J Am Soc Nephrol. 2006;17:3223–3232. doi:10.1681/ASN.2005111194
57. Cesini J, Cheriet S, Breuil V, et al. Osteoporosis: chronic kidney disease in rheumatology practice. Joint Bone Spine. 2012;79(Suppl 2):S104–109. doi:10.1016/S1297-319X(12)70017-9
58. Klawansky S, Komaroff E, Cavanaugh PF, et al. Relationship between age, renal function and bone mineral density in the US population. Osteoporos Int J Establ Result Coop Eur Found Osteoporos Natl Osteoporos Found USA. 2003;14:570–576. doi:10.1007/s00198-003-1435-y
59. Bikbov B, Perico N, Remuzzi G, et al. Disparities in chronic kidney disease prevalence among males and females in 195 countries: analysis of the global burden of disease 2016 study. Nephron. 2018;139:313–318. doi:10.1159/000489897
60. Black DM, Delmas PD, Eastell R, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356:1809–1822. doi:10.1056/NEJMoa067312
61. Naylor KL, Garg AX, Zou G, et al. Comparison of fracture risk prediction among individuals with reduced and normal kidney function. Clin J Am Soc Nephrol CJASN. 2015;10:646–653. doi:10.2215/CJN.06040614
62. Kanis JA, Oden A, Johnell O, et al. The use of clinical risk factors enhances the performance of BMD in the prediction of hip and osteoporotic fractures in men and women. Osteoporos Int J Establ Result Coop Eur Found Osteoporos Natl Osteoporos Found USA. 2007;18:1033–1046. doi:10.1007/s00198-007-0343-y
63. Fried LF, Biggs ML, Shlipak MG, et al. Association of kidney function with incident hip fracture in older adults. J Am Soc Nephrol JASN. 2007;18:282–286. doi:10.1681/ASN.2006050546
64. Cailleaux P-E, Ostertag A, Metzger M, et al. Longitudinal bone loss occurs at the radius in CKD. Kidney Int Rep. 2021;6:1525–1536.
65. Evenepoel P, Cunningham J, Ferrari S, et al. European Consensus Statement on the diagnosis and management of osteoporosis in chronic kidney disease stages G4-G5D. Nephrol Dial Transplant off Publ Eur Dial Transpl Assoc - Eur Ren Assoc. 2021;36:42–59.
66. Rix M, Andreassen H, Eskildsen P, et al. Bone mineral density and biochemical markers of bone turnover in patients with predialysis chronic renal failure. Kidney Int. 1999;56:1084–1093. doi:10.1046/j.1523-1755.1999.00617.x
67. Roetker NS, Peng Y, Ashfaq A, et al. Adherence to kidney disease: improving global outcomes mineral and bone guidelines for monitoring biochemical parameters. Am J Nephrol. 2019;49:225–232. doi:10.1159/000497477
68. Levin A, Bakris GL, Molitch M, et al. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int. 2007;71:31–38. doi:10.1038/sj.ki.5002009
69. Herberth J, Branscum AJ, Mawad H, et al. Intact PTH combined with the PTH ratio for diagnosis of bone turnover in dialysis patients: a diagnostic test study. Am J Kidney Dis off J Natl Kidney Found. 2010;55:897–906. doi:10.1053/j.ajkd.2009.12.041
70. Nickolas TL, Cremers S, Zhang A, et al. Discriminants of prevalent fractures in chronic kidney disease. J Am Soc Nephrol JASN. 2011;22:1560–1572. doi:10.1681/ASN.2010121275
71. Campos-Obando N, Koek WNH, Hooker ER, et al. Serum phosphate is associated with fracture risk: the Rotterdam study and MrOS. J Bone Miner Res off J Am Soc Bone Miner Res. 2017;32:1182–1193. doi:10.1002/jbmr.3094
72. Gordon PL, Doyle JW, Johansen KL. Association of 1,25-dihydroxyvitamin D levels with physical performance and thigh muscle cross-sectional area in chronic kidney disease stage 3 and 4. J Ren Nutr off J Counc Ren Nutr Natl Kidney Found. 2012;22:423–433.
73. He W, Kang YS, Dai C, et al. Blockade of Wnt/β-catenin signaling by paricalcitol ameliorates proteinuria and kidney injury. J Am Soc Nephrol JASN. 2011;22:90–103. doi:10.1681/ASN.2009121236
74. Molina P, Carrero JJ, Bover J, et al. Vitamin D, a modulator of musculoskeletal health in chronic kidney disease. J Cachexia Sarcopenia Muscle. 2017;8:686–701. doi:10.1002/jcsm.12218
75. Elkasrawy MN, Hamrick MW. Myostatin (GDF-8) as a key factor linking muscle mass and bone structure. J Musculoskelet Neuronal Interact. 2010;10:56–63.
76. Mera P, Laue K, Ferron M, et al. Osteocalcin signaling in myofibers is necessary and sufficient for optimum adaptation to exercise. Cell Metab. 2016;23:1078–1092. doi:10.1016/j.cmet.2016.05.004
77. Isakova T, Cai X, Lee J, et al. Associations of FGF23 with change in bone mineral density and fracture risk in older individuals. J Bone Miner Res off J Am Soc Bone Miner Res. 2016;31:742–748. doi:10.1002/jbmr.2750
78. Barker SL, Pastor J, Carranza D, et al. The demonstration of αKlotho deficiency in human chronic kidney disease with a novel synthetic antibody. Nephrol Dial Transplant off Publ Eur Dial Transpl Assoc - Eur Ren Assoc. 2015;30:223–233.
79. Han SG, Oh J, Jeon HJ, et al. Kidney stones and risk of osteoporotic fracture in chronic kidney disease. Sci Rep. 2019;9:1929. doi:10.1038/s41598-018-38191-1
80. Lehmann G, Ott U, Kaemmerer D, et al. Bone histomorphometry and biochemical markers of bone turnover in patients with chronic kidney disease Stages 3–5. Clin Nephrol. 2008;70:296–305. doi:10.5414/CNP70296
81. Bhasin S, Gill TM, Reuben DB, et al. A randomized trial of a multifactorial strategy to prevent serious fall injuries. N Engl J Med. 2020;383:129–140. doi:10.1056/NEJMoa2002183
82. Howe TE, Shea B, Dawson LJ, et al. Exercise for preventing and treating osteoporosis in postmenopausal women. Cochrane Musculoskeletal Group, editor. Cochrane Database Syst Rev. 2011. doi:10.1002/14651858.CD000333.pub2
83. Tentori F, Hunt WC, Stidley CA, et al. Mortality risk among hemodialysis patients receiving different vitamin D analogs. Kidney Int. 2006;70:1858–1865. doi:10.1038/sj.ki.5001868
84. Bischoff-Ferrari HA, Orav EJ, Dawson-Hughes B. Effect of cholecalciferol plus calcium on falling in ambulatory older men and women: a 3-year randomized controlled trial. Arch Intern Med. 2006;166:424–430.
85. Taskapan H, Baysal O, Karahan D, et al. Vitamin D and muscle strength, functional ability and balance in peritoneal dialysis patients with vitamin D deficiency. Clin Nephrol. 2011;76:110–116. doi:10.5414/CN107160
86. Zhao J-G, Zeng X-T, Wang J, et al. Association between calcium or Vitamin D supplementation and fracture incidence in community-dwelling older adults: a systematic review and meta-analysis. JAMA. 2017;318:2466–2482. doi:10.1001/jama.2017.19344
87. Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD-MBD). Kidney Int Suppl. 2017;7:1–59. doi:10.1016/j.kisu.2017.04.001
88. Ureña P, Hruby M, Ferreira A, et al. Plasma total versus bone alkaline phosphatase as markers of bone turnover in hemodialysis patients. J Am Soc Nephrol JASN. 1996;7:506–512. doi:10.1681/ASN.V73506
89. Malluche HH, Mawad HW, Monier-Faugere M-C. Renal osteodystrophy in the first decade of the new millennium: analysis of 630 bone biopsies in black and white patients. J Bone Miner Res. 2011;26:1368–1376. doi:10.1002/jbmr.309
90. Pfister T, Atzpodien E, Bohrmann B, et al. Acute renal effects of intravenous bisphosphonates in the rat. Basic Clin Pharmacol Toxicol. 2005;97:374–381. doi:10.1111/j.1742-7843.2005.pto_160.x
91. Markowitz GS, Appel GB, Fine PL, et al. Collapsing focal segmental glomerulosclerosis following treatment with high-dose pamidronate. J Am Soc Nephrol JASN. 2001;12:1164–1172. doi:10.1681/ASN.V1261164
92. Markowitz GS, Fine PL, Stack JI, et al. Toxic acute tubular necrosis following treatment with zoledronate (Zometa). Kidney Int. 2003;64:281–289. doi:10.1046/j.1523-1755.2003.00071.x
93. Swallow EA, Aref MW, Metzger CE, et al. Skeletal levels of bisphosphonate in the setting of chronic kidney disease are independent of remodeling rate and lower with fractionated dosing. Bone. 2019;127:419–426. doi:10.1016/j.bone.2019.07.007
94. Miller PD, Roux C, Boonen S, et al. Safety and efficacy of risedronate in patients with age-related reduced renal function as estimated by the Cockcroft and Gault method: a pooled analysis of nine clinical trials. J Bone Miner Res off J Am Soc Bone Miner Res. 2005;20:2105–2115. doi:10.1359/JBMR.050817
95. McClung MR, Geusens P, Miller PD, et al. Effect of risedronate on the risk of hip fracture in elderly women. Hip Intervention Program Study Group. N Engl J Med. 2001;344:333–340.
96. Toussaint ND, Lau KK, Strauss BJ, et al. Effect of alendronate on vascular calcification in CKD stages 3 and 4: a pilot randomized controlled trial. Am J Kidney Dis off J Natl Kidney Found. 2010;56:57–68. doi:10.1053/j.ajkd.2009.12.039
97. Miller PD, Schwartz EN, Chen P, et al. Teriparatide in postmenopausal women with osteoporosis and mild or moderate renal impairment. Osteoporos Int J Establ Result Coop Eur Found Osteoporos Natl Osteoporos Found USA. 2007;18:59–68. doi:10.1007/s00198-006-0189-8
98. Cummings SR, San Martin J, McClung MR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756–765. doi:10.1056/NEJMoa0809493
99. Hampson G, Elder GJ, Cohen-Solal M, et al. A review and perspective on the assessment, management and prevention of fragility fractures in patients with osteoporosis and chronic kidney disease. Endocrine. 2021;73:509–529. doi:10.1007/s12020-021-02735-9
100. Khosla S, Farr JN, Tchkonia T, et al. The role of cellular senescence in ageing and endocrine disease. Nat Rev Endocrinol. 2020;16:263–275. doi:10.1038/s41574-020-0335-y
101. Farr JN, Xu M, Weivoda MM, et al. Targeting cellular senescence prevents age-related bone loss in mice. Nat Med. 2017;23:1072–1079. doi:10.1038/nm.4385
102. Xu M, Pirtskhalava T, Farr JN, et al. Senolytics improve physical function and increase lifespan in old age. Nat Med. 2018;24:1246–1256. doi:10.1038/s41591-018-0092-9
103. Black DM, Cummings SR, Ensrud KE. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Lancet. 1996;348(9041):
104. Black DM; Delmas, HORIZON Pivotal Fracture Trial. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356(18):
105. Black DM, Thompson DE, Cummings SR. Fracture risk reduction with alendronate in women with osteoporosis: the fracture intervention trial. J Clin Endocrinol Metab. 2000;85(11):7.
106. Boonen S, Black DM, Colón-Emeric CS. Efficacy and safety of a once-yearly intravenous zoledronic acid 5 mg for fracture prevention in elderly postmenopausal women with osteoporosis aged 75 and older. J Am Geriatr Soc. 2010;58(2):
107. Boonen S, McClung MR, Eastell R. Safety and efficacy of risedronate in reducing fracture risk in osteoporotic women aged 80 and older: implications for the use of antiresorptive agents in the old and oldest old. J Am Geriatr Soc. 2004;52(11):
108. Harris ST. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis. A Randomized Controlled Trial. JAMA. 1999;282(14):1344. doi:10.1001/jama.282.14.1344
109. Kendler DL, Marin F, Zerbini CAF. Effects of teriparatide and risedronate on new fractures in post-menopausal women with severe osteoporosis (VERO): a multicentre, double-blind, double-dummy, randomised controlled trial. Lancet. 2018;391(10117):
110. Neer RM, Arnaud C, Mitlak BH. Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344:1434–1441.
111. Orwoll ES, Scheele WH, Paul GA. The effect of teriparatide [human parathyroid hormone (1–34)] therapy on bone density in men with osteoporosis. J Bone Mineral Res. 2003;18(1):
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.