Back to Journals » Open Access Emergency Medicine » Volume 11
Management Strategies Of Idiopathic Anaphylaxis In The Emergency Room: Current Perspectives
Authors Le M, Gabrielli S, De Schryver S, Ben-Shoshan M
Received 21 July 2019
Accepted for publication 4 October 2019
Published 1 November 2019 Volume 2019:11 Pages 249—263
DOI https://doi.org/10.2147/OAEM.S200342
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Hans-Christoph Pape
Michelle Le, Sofianne Gabrielli, Sarah De Schryver, Moshe Ben-Shoshan
Division of Allergy, Immunology and Dermatology, Department of Pediatrics, McGill University Health Center, Montreal, QC, Canada
Correspondence: Michelle Le
Division of Pediatric Allergy, Clinical Immunology and Dermatology, Department of Pediatrics, McGill University Health Center, 1001 Boul. Decarie, Montreal, Quebec H4A 3J1, Canada
Tel +1 514-412-4400 (23151)
Fax +1 514-412-4390
Email [email protected]
Background: Idiopathic anaphylaxis (IA) is a diagnosis of exclusion and represents a major diagnostic and management challenge. There are no current guidelines for diagnosis and management of IA. We aim to present a systematic review of the literature on adult and pediatric IA.
Methods: We conducted a systematic review of original articles published in the past 22 years regarding diagnosis and management strategies of adult and pediatric IA.
Results: The current proposed diagnostic approach and treatment regimens are based on a few small studies. Future large-scale studies are required. IA is a diagnosis of exclusion and should be made only after extensive evaluation excludes potential anaphylaxis triggers as well as non-allergic conditions with a similar presentation. There is currently no diagnostic consensus for IA. Furthermore, the current proposed treatment regimens are limited and rely on prophylactic treatment with antihistamines and prednisone for patients with frequent episodes. However, daily treatment with systemic steroids has well-recognized serious adverse effects. More recently, the use of biologics was suggested to benefit patients with IA, although the optimal management protocol is not yet established.
Conclusion: Future studies are needed to optimize diagnosis and treatment strategies in adult and pediatric cases of IA. Omalizumab may be a promising novel therapeutic option for adult and pediatric IA.
Keywords: anaphylaxis, diagnosis, management, treatment
Introduction
Idiopathic anaphylaxis (IA) is a diagnosis of exclusion after known causes for anaphylaxis and other diseases that mimic anaphylaxis have been ruled out1. Known causes of anaphylaxis include mainly foods, medications and venom. IA was first described in 1978 by Bacal et al.2 Currently, there are four reported main phenotypes accounting for anaphylaxis: type I (IgE mediated related to food allergens mainly), cytokine released (associated with monoclonal antibodies/chemotherapy), mixed (associated with chemotherapy/monoclonal antibodies) and complement mediated (associated with contrast material, dialysis membranes, glycosaminoglycans and chondroitin sulfate).3 The pathogenesis in cases of IA, however, has not yet been well established. From previously published studies, it can be inferred that IA may cause a substantial decline in quality of life. Previous investigations on the quality of life in children with food allergies and their respective caregivers suggest that stress and anxiety associated with continuous allergen avoidance and the looming threat of anaphylaxis were associated with significantly impaired food allergy quality of life (FAQOL).4,5 Although no studies have been conducted to investigate the quality of life in patients with IA, given the lack of knowledge of the anaphylaxis trigger, it can be inferred that the anxiety experienced by these patients may be further elevated, and their quality of life may be further impaired than those with known allergies. The exact prevalence of IA is not currently known but has been estimated to be between 20,000 and 47,000 cases in the United States.6 IA is reported to affect 30% to 60% of cases of anaphylaxis in adults and 10% of pediatric cases.7 Given that there are no identifiable triggers of IA, there are substantial challenges in the diagnosis and management of these cases.
Presently, there are no guidelines on the diagnostic approach of IA, including assessment for underlying diseases and the use of confirmatory tests. Furthermore, guidelines for the appropriate management of IA cases have not yet been established. The current approach to treatment is based on disease frequency; short-term treatment, such as an epinephrine auto-injector, is used for infrequent attacks, while prophylactic treatment with daily H1-antihistamines, glucocorticoids, or omalizumab has been used in patients with more frequent episodes. Frequent episodes are defined as at least two episodes in the preceding two months or at least six episodes in the preceding year. Although several case series have been published regarding IA,8,9 few reviews have focused on the diagnosis and management strategies of IA. In this study, we aim to present a systematic review of the literature published in the past 22 years regarding IA in the adult and pediatric population with a focus on diagnosis and management strategies pertinent for the Emergency Room (ER) physician.
Methods
Original scientific studies pertinent to the clinical diagnosis and management of IA were searched in the PubMed literature database. Search terms “idiopathic anaphylaxis” were used, and the search was limited to articles published between June 1, 1998 and June 1, 2019, that were written in English. The abstracts of the resulting papers were reviewed and those that were relevant to the diagnosis and management of IA were included. In this manuscript, we define IA as an anaphylactic reaction where the diagnostic criteria include at least a negative tryptase test and normal bone marrow aspiration/biopsy.
Results
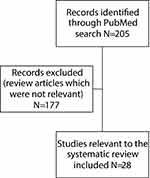
The initial PubMed search yielded 205 articles, which was reduced to 50 articles after applying the aforementioned filters. The resulting search was further narrowed to 28 articles after a more thorough assessment of article abstracts was done to ensure their relevancy to the systematic review (Figure 1).
Diagnosis Of IA
IA is a diagnosis of exclusion and should be made only after extensive evaluation to exclude other potential causes of anaphylaxis and other diseases with similar manifestations, such as mastocytosis. The classification of IA is based on frequency of episodes and clinical manifestations.
The majority of studies included in this review (Table 1) describe an extensive evaluation in adults and children with suspected IA including diagnostic testing for possible triggers of IgE-mediated anaphylaxis such as food, drugs and exercise four hours prior to the reaction. Only 2 of 22 articles describing IA did not specify the diagnostic tests used to determine the diagnosis.6,10 The most common diagnostic tests done were tryptase levels and skin prick test. Six IA studies conducted a bone marrow aspiration of which, four studies reported a concomitant tryptase level to rule out mastocytosis.11–16 Five studies conducted diagnostic tests to rule out neuroendocrine tumors, such as pheochromocytoma and carcinoid tumors.11,12,14,16,17 Oral and exercise challenge tests were conducted less frequently, with only one article reporting an exercise challenge test.18 Specific Immunoglobulin E for α-galactose to exclude the diagnosis of delayed anaphylaxis to red meat were conducted in three of the presented studies.15,19,20 Overall, none of the studies had diagnostic consensus to establish the diagnosis of IA in patients.
 |
 |
 |
 |
 |
 |
Table 1 Summary Of Original Scientific Studies On Adult And Pediatric IA |
In several of the studies presented, additional considerations were made for the method of diagnosis of IA. In a Spanish series by Tejedor and Alonso, 2002,8 insect bites, latex and other rare causes of anaphylaxis such as anaphylaxis attributed to Anisakis simplex or rupture of hydatid cyst were also excluded. Psychiatric disorders were also ruled out in addition to having performed the aforementioned extensive diagnostic evaluation. Of note, in certain case reports, Type I Kounis syndrome has been reported as a concomitant diagnosis of IA.16,21 Kounis syndrome is defined when anaphylaxis causes cardiovascular signs and symptoms. Specifically, Type I Kounis syndrome is described in patients with no evidence of coronary artery disease. In addition, certain studies have reported syndrome of idiopathic urticaria and angioedema (ICUA) with thyroid autoimmunity involved with the diagnosis of IA.22
In the recent case reports by Wolver et al, 201323 and Tripathi et al, 2014,24 the authors suggest that rare disorders may mimic IA, which includes delayed anaphylaxis to red meat. In contrast with immediate food-mediated anaphylaxis, symptoms may occur more than two hours after exposure. Initial clues for the reaction to mammalian oligosaccharide α-gal in the published cases were based on medical history, which indicated ingestion of mammalian products.
An interesting case series by Ivkovic-Jurekovich in 20159 describes three patients with a clinical picture suggestive of histamine intolerance associated with IA. Interestingly, all three patients were found to have a positive autologous serum tests (ASST), but a histamine release test was negative and the presence of circulating auto-antibodies against IgE and FcεRIα could not be confirmed. In addition, they determined a very low activity of histamine-inactivating enzyme (DAO) for all three patients, which indicated high histamine intolerance. Histamine intolerance describes a state where an individual has a decreased ability to catabolize endogenous or exogenously administered histamine, leading to histamine-mediated adverse reactions. Diagnosis is based on careful clinical history and identification of intestinal or serum activities of DAO and histamine N-methyltransferase. However, there are no large-scale studies published establishing the sensitivity and specificity of these tests.
Several case reports have identified rare causes of anaphylaxis in patients who were initially diagnosed with IA. Such causes of anaphylaxis include pigeon tick, A. Reflexus,25 lymphocytic hypophysitis in the context of complicated adrenal crises in an asthmatic patient,26 and wheat-dependent exercise-induced anaphylaxis.27 In fact, in a study done by Heaps et al, 2014,28 wheat protein, omega-5-gliadin, and shrimp were the main causes of anaphylaxis in the study’s cohort of patients who were initially diagnosed with IA.
Management Of IA
Classification of the disease as frequent or infrequent IA is necessary to determine the appropriate treatment course. Frequent episodes are defined as at least two episodes in the preceding two months or at least six episodes in the preceding year.29 For patients with frequent IA, all studies in this review report a treatment of continuous prednisone at a dose of 20 to 80 mg,9–11,21,22,29 antihistamines (e.g. cetirizine 10 mg)9,13,14,30 and sympathomimetics (e.g. albuterol).11,29 In asymptomatic patients, prednisone may be gradually tapered every two to four weeks.31 Patients with infrequent episodes of IA are treated with epinephrine, antihistamines and oral steroids during the episode. The same treatment regimens have been recommended for children with pediatric dose adjustments.10,32 The authors in the presented studies report that the current treatment regimens are successful in controlling the disease and induce remission in the majority of patients, although no large-scale studies have been conducted to assess the adequacy of control.
Patients requiring long-term prophylactic treatment with prednisone are at risk for significant side effects.33 Alternative prophylactic management strategies are limited. A few reports suggest that ketotifen, a mast cell stabilizer, is effective in patients with corticosteroid dependent-IA.11,15,29,32,34 Recently, the use of the biologic omalizumab has been suggested to benefit patients with recurrent and frequent episodes of IA. Over one-third of the studies included in this review included omalizumab in the prophylactic treatment of IA among patients in which continuous treatment with prednisone and antihistamines did not prevent further episodes of IA.14 Doses of omalizumab ranged from 225 to 375 mg administered at a frequency of every two, four, or eight weeks. The majority of these studies reported resolution of IA episodes that was maintained over 12 months after stopping omalizumab treatment.12–14,21,26,35–38 Two case reports included in this review have described relapse in two patients after a short period of treatment (three and four months of treatment) and an adverse event (anaphylaxis) in another patient undergoing omalizumab treatment for frequent IA episodes.15,39
Certain treatments targeting histamine’s role in IA were also discussed in the studies presented. In the series of case reports by Ivkovic-Jurekovich, 2015,9 a histamine-free diet was not found to be beneficial in decreasing or resolving episodes of IA. Symptoms did not improve with a histamine-free diet alone in any of the patients studied; however, IA symptoms did improve with the introduction of daily antihistamines among all three patients. One of the three patients presented with frequent IA episodes and was recommended to initiate treatment with continuous prednisone, which the parents refused. Instead, the patient was treated with daily antihistamines alone, after which the patient experienced remission of IA.9
Discussion
Given that IA accounts for up to 30% to 60% of cases of anaphylaxis in adults and 10% of pediatric cases,40–42 it represents a major diagnostic and management challenge as it is a diagnosis of exclusion that lacks diagnostic confirmatory tests and the inability to avoid potential triggers.
Based on the existing literature, a diagnosis and management algorithm specifically targeting adult and pediatric cases of IA are required. Most papers reviewed did not include an exhaustive diagnostic approach and hence we suggest using the term anaphylaxis of unknown trigger (AUT) rather than IA until the diagnosis of true IA is firmly established.
It is clear from this systematic review that a fundamental diagnostic criteria have not yet been determined for IA. Similar to the recently published British guidelines,43,44 for patients presenting at the emergency room with AUT, we recommend a careful history, corroborated with at least serum tryptase levels one to two hours following the onset of symptoms and another tryptase level at least 24 hrs after. A referral for an allergist/immunologist consultation and prescription of an epinephrine auto-injector is also strongly recommended in the emergency department setting. When assessed by a family medicine physician, pediatrician and/or allergy specialist, the differential diagnosis of anaphylaxis should be taken into account (Table 3). In fact, in recent studies published by Alverez-Perea et al, in 201545 and in 2017,46 authors suggest that the rate of IA is lower than previously described if an appropriate anaphylaxis work up is conducted by allergists/immunologists. In these studies, a trigger for the anaphylactic reaction was identified in 75% of children46 and 20% of adults45 initially presenting with AUT after appropriate investigations were conducted. These results further support the importance of a consultation to an allergist/immunologist for patients who are diagnosed with AUT in the ER.
 |
Table 2 Diagnostic Tests To Consider In A Patient With Possible IA |
 |
Table 3 Differential Diagnosis Of Anaphylaxis |
Following the diagnosis of AUT and after conducting a thorough clinical history and physical exam, we suggest considering additional diagnostic tests done by the specialist (Table 2). Immediate skin testing, in vitro tests for specific IgE and oral challenges under the supervision of an allergist should be conducted for relevant drugs or foods which may help to confirm or exclude food or drug allergy as a possible trigger including sIgE to ω-5 gliadin and alpha-gal in the case of a clinical history suggestive of delayed anaphylaxis to red meat. This rare disorder should be suspected as a culprit in any case of AUT, especially in events occurring three to six hours after eating, particularly when reactions start in the early morning hours. Other potential causes that should be considered are exercise and food-dependent exercise-induced anaphylaxis. Exercise and/or food-dependent exercise challenges should thus be considered in the case of a suggestive history.
Systemic mastocytosis, monoclonal mast cell activation syndrome and mast cell activation syndrome are disorders that may also mimic IA. Elevated serum tryptase levels during attacks are suggestive for IA and patients with elevated tryptase levels both at baseline and during attacks should be evaluated with a bone marrow biopsy to rule out mastocytosis or other clonal mast cell disorders. All patients with suspected IA should have tryptase levels done at baseline and during attacks in order to confirm the presence of anaphylaxis as the difference in these levels rather than the absolute level of tryptase is crucial for the diagnosis of anaphylaxis.47 Bone marrow biopsy should be considered in all cases of frequent AUT with tryptase levels consistent with anaphylaxis even if baseline levels are within the norm given that some studies have suggested that systemic mastocytosis may present with values that are within the norm.48
Pheochromocytoma and carcinoid syndrome are rare disorders in which patients may also present with symptoms similar to anaphylaxis, such as flushing induced by release of vasoactive substances: vanillylmandelic acid in pheochromocytoma, 5-hydroxyindoleacetic acid (5-HIAA) in a 24 hr urine collection, and chromogranin A in blood, which is a recently proposed biomarker for the diagnosis of neuroendocrine tumors.49,50 Detection of these mediators may help exclude or confirm the diagnosis in patients with a suggestive history.
Hereditary angioedema may mimic IA as it presents as recurrent episodes of angioedema as a principal symptom. Laboratory investigations should include serum levels of C4 and C1 inhibitor in selected patients who present with corresponding symptoms of hereditary angioedema (Table 2).
The current proposed treatment regimens are based on small and outdated studies. Daily treatment with systemic steroids, such as prednisone, are associated with an increased risk of long-term side effects, including osteoporosis, adrenal suppression and immunosuppression, in both adults and children.33 We, therefore, recommend a change in the current practice relying on the use of systemic steroids for the treatment of IA. Based on recently published case reports and case series, we suggest the following algorithm for the management of adult and pediatric IA (Figures 2 and 3). All patients should be instructed on the management of acute episodes and should be prescribed an epinephrine auto-injector. In patients with frequent episodes, daily H1-antihistamines at regular doses should be prescribed and the patient should have close clinical follow-up. If there is no improvement after one month, the physician should consider increasing the daily antihistamine dose up to four times the regular dose. If no improvement is demonstrated with the increased dose of daily antihistamines after one to two months, treatment with omalizumab should be considered. Omalizumab is a humanized monoclonal antibody that binds to the FcεRI receptor on IgE. Allergen-specific IgE plays a central role in IgE-mediated anaphylaxis. Cross-linking of the receptor bound IgE on mast cells and basophils by allergens leads to activation of those cells and subsequent release of inflammatory mediators causing anaphylaxis. The exact mechanism of IA remains unknown, but it has been postulated that the high affinity IgE receptors may be cross-linked by autoimmune mechanisms.51 Omalizumab may prevent IgE expression on effector cells and subsequent cross-linking of IgE.
Omalizumab is considered a promising prophylactic therapy for IA in treatment-resistant patients. This therapy has been shown to have both rapid and long-term benefits in multiple case reports.35 The dosage and treatment regimen should be determined on an individual basis. Further large-scale studies are needed to assess the efficacy of omalizumab therapy for IA in both adults and children. Hence, we believe that prophylactic glucocorticoid treatment should be reserved only for cases that are not well controlled with omalizumab.
Conclusion
In conclusion, few studies have evaluated the diagnosis and management strategies of true IA. Future studies are needed to optimize treatment regimens for IA in both children and adults. Health care providers should be aware of the potential differential diagnosis and underlying causes in order to develop an appropriate management strategy.
Omalizumab is a promising therapeutic option for IA as a novel approach to prophylactic treatment. We recommend conducting large-scale studies on the use of omalizumab in IA and a shift in treatment paradigm that will prioritize omalizumab over glucocorticoids as an efficacious and safe second-line prophylactic treatment.
 |
Figure 2 Anaphylaxis management algorithm. |
 |
Figure 3 Frequent and infrequent anaphylaxis treatment algorithm. |
Disclosure
The authors report no conflicts of interest in this work.
References
1. Joint Task Force on Practice Parameters; American Academy of Allergy, Asthma and Immunology; American College of Allergy, Asthma and Immunology; Joint Council of Allergy, Asthma and Immunology. The diagnosis and management of anaphylaxis: an updated practice parameter. J Allergy Clin Immunol. 2005;115(3 Suppl 2):S483–S523. doi:10.1016/j.jaci.2005.01.010
2. Bacal E, Patterson R, Zeiss CR. Evaluation of severe (anaphylactic) reactions. Clin Allergy. 1978;8(3):295–304.
3. Castells M. Diagnosis and management of anaphylaxis in precision medicine. J Allergy Clin Immunol. 2017;140(2):321–333. doi:10.1016/j.jaci.2017.06.012
4. Warren CM, Otto AK, Walkner MM, Gupta RS. Quality of life among food allergic patients and their caregivers. Curr Allergy Asthma Rep. 2016;16(5):38. doi:10.1007/s11882-016-0614-9
5. Soller L, Clarke AE, Lyttle A, et al. Comparing quality of life in Canadian children with peanut, sesame, and seafood allergy. J Allergy Clin Immunol Pract. 2019. doi:10.1016/j.jaip.2019.07.006
6. Patterson R, Tripathi A, Saltoun C, Harris KE. Idiopathic anaphylaxis: variants as diagnostic and therapeutic problems. Allergy Asthma Proc. 2000;21(3):141–144. doi:10.2500/108854100778148963
7. Webb LM, Lieberman P. Anaphylaxis: a review of 601 cases. Ann Allergy Asthma Immunol. 2006;97(1):39–43. doi:10.1016/S1081-1206(10)61367-1
8. Tejedor Alonso MA, Sastre DJ, Sanchez-Hernandez JJ, Perez FC, de la Hoz Caballer B. Idiopathic anaphylaxis: a descriptive study of 81 patients in Spain. Ann Allergy Asthma Immunol. 2002;88(3):313–318. doi:10.1016/S1081-1206(10)62014-5
9. Ivkovic-Jurekovic I. Idiopathic anaphylaxis and histamine intolerance. Pediatr Allergy Immunol. 2015;26(7):685–687. doi:10.1111/pai.12438
10. Hogan MB, Kelly MA, Wilson NW. Idiopathic anaphylaxis in children. Ann Allergy Asthma Immunol. 1998;81(2):140–142. doi:10.1016/S1081-1206(10)62800-1
11. Geller M, Geller P. Malignant idiopathic anaphylaxis does exist in Brazil. Ann Allergy Asthma Immunol. 2002;88(6):645. doi:10.1016/S1081-1206(10)61900-X
12. Jones JD, Marney SR
13. Pitt TJ, Cisneros N, Kalicinsky C, Becker AB. Successful treatment of idiopathic anaphylaxis in an adolescent. J Allergy Clin Immunol. 2010;126(2):415–416. author reply 416. doi:10.1016/j.jaci.2010.05.043
14. Demirturk M, Gelincik A, Colakoglu B, Dal M, Buyukozturk S. Promising option in the prevention of idiopathic anaphylaxis: omalizumab. J Dermatol. 2012;39(6):552–554. doi:10.1111/j.1346-8138.2012.01520.x
15. Stone CA
16. Keber T, Makuc J, Sekavčnik G. Type 1 Kounis syndrome in patient with idiopathic anaphylaxis. Case Rep Cardiol. 2017;2017:1089023. doi:10.1155/2017/1089023
17. Shanmugam G, Schwartz LB, Khan DA. Prolonged elevation of serum tryptase in idiopathic anaphylaxis. J Allergy Clin Immunol. 2006;117(4):950–951. doi:10.1016/j.jaci.2005.12.1356
18. Kim S, Yoon SY, Park SY, et al. A case of idiopathic anaphylaxis followed by acute liver injury. Allergy Asthma Immunol Res. 2013;5(4):245–247. doi:10.4168/aair.2013.5.4.245
19. Carter MC, Ruiz-Esteves KN, Workman L, Lieberman P, Platts-Mills TAE, Metcalfe DD. Identification of alpha-gal sensitivity in patients with a diagnosis of idiopathic anaphylaxis. Allergy. 2018;73(5):1131–1134. doi:10.1111/all.13366
20. Shaker M, Yuan I, Kennedy KL, Capucilli P, Spergel JM. Idiopathic anaphylaxis and undiagnosed anorexia nervosa. Ann Allergy Asthma Immunol. 2019;122(2):215–217. doi:10.1016/j.anai.2018.10.017
21. Sandhu M, Casselman J, Peppers B, Tcheurekdjian H, Hostoffer RW. Type 1 Kounis syndrome in a patient with idiopathic anaphylaxis. Allergy Rhinol. 2017;8(2):103–104.
22. Dreyfus DH, Fraser B, Randolph CC. The syndrome of thyroid autoimmunity and idiopathic chronic urticaria and angioedema presenting as anaphylaxis. Allergy Asthma Proc. 2003;24(3):171–174.
23. Wolver SE, Sun DR, Commins SP, Schwartz LB. A peculiar cause of anaphylaxis: no more steak? The journey to discovery of a newly recognized allergy to galactose-alpha-1,3-galactose found in mammalian meat. J Gen Intern Med. 2013;28(2):322–325. doi:10.1007/s11606-012-2144-z
24. Tripathi A, Commins SP, Heymann PW, Platts-Mills TA. Delayed anaphylaxis to red meat masquerading as idiopathic anaphylaxis. J Allergy Clin Immunol Pract. 2014;2(3):259–265. doi:10.1016/j.jaip.2014.02.017
25. Rolla G, Heffler E, Boita M, et al. Pigeon tick bite: a neglected cause of idiopathic nocturnal anaphylaxis. Allergy. 2018;73(4):958–961. doi:10.1111/all.13344
26. Bobolea I, Guillen D, Barranco P, Alvarez-Escola C, Quirce S. Recurrent anaphylactic reactions: an uncommon debut of lymphocytic hypophysitis. Int Arch Allergy Immunol. 2012;159(1):103–106. doi:10.1159/000335231
27. Wong GK, Huissoon AP, Goddard S, Collins DM, Krishna MT. Wheat dependent exercise induced anaphylaxis: is this an appropriate terminology? J Clin Pathol. 2010;63(9):814–817. doi:10.1136/jcp.2010.078808
28. Heaps A, Carter S, Selwood C, et al. The utility of the ISAC allergen array in the investigation of idiopathic anaphylaxis. Clin Exp Immunol. 2014;177(2):483–490. doi:10.1111/cei.12334
29. Patterson R, Hogan MB, Yarnold PR, Harris KE. Idiopathic anaphylaxis. An attempt to estimate the incidence in the United States. Arch Intern Med. 1995;155(8):869–871. doi:10.1001/archinte.155.8.869
30. Tedeschi A, Lorini M, Suli C, Cugno M. Detection of serum histamine-releasing factors in a patient with idiopathic anaphylaxis and multiple drug allergy syndrome. J Invest Allergol Clin Immunol. 2007;17(2):122–125.
31. Greenberger PA. Idiopathic anaphylaxis. Immunol Allergy Clin North Am. 2007;27(2):
32. Ditto AM, Krasnick J, Greenberger PA, Kelly KJ, McGrath K, Patterson R. Pediatric idiopathic anaphylaxis: experience with 22 patients. J Allergy Clin Immunol. 1997;100(3):320–326. doi:10.1016/s0091-6749(97)70244-6
33. Liu D, Ahmet A, Ward L, et al. A practical guide to the monitoring and management of the complications of systemic corticosteroid therapy. Allergy Asthma Clin Immunol. 2013;9(1):30. doi:10.1186/1710-1492-9-30
34. Ditto AM, Harris KE, Krasnick J, Miller MA, Patterson R. Idiopathic anaphylaxis: a series of 335 cases. Ann Allergy Asthma Immunol. 1996;77(4):285–291. doi:10.1016/S1081-1206(10)63322-4
35. Warrier P, Casale TB. Omalizumab in idiopathic anaphylaxis. Ann Allergy Asthma Immunol. 2009;102(3):257–258. doi:10.1016/S1081-1206(10)60091-9
36. Kibsgaard L, Skjold T, Deleuran M, Vestergaard C. Omalizumab induced remission of idiopathic anaphylaxis in a patient suffering from indolent systemic mastocytosis. Acta Derm Venereol. 2014;94(3):363–364. doi:10.2340/00015555-1687
37. Jung HS, Park CH, Park YT, et al. Gynecomastia induced by H1-antihistamine (ebastine) in a patient with idiopathic anaphylaxis. Asia Pac Allergy. 2015;5(3):187–190. doi:10.5415/apallergy.2015.5.3.187
38. Ozdemir O, Bozkurt HB, Elmas B. Omalizumab’s role in the treatment of steroid dependent malignant idiopathic anaphylaxis. Turk Pediatri Arsivi. 2017;52(2):105–107. doi:10.5152/TurkPediatriArs.2017.2262
39. Lee J. Successful prevention of recurrent anaphylactic events with anti-immunoglobulin E therapy. Asia Pac Allergy. 2014;4(2):126–128. doi:10.5415/apallergy.2014.4.2.126
40. Le M, Gabrielli S, Clarke A, et al. Emergency management of anaphylaxis due to an unknown trigger: an 8-year follow-up study in Canada. J Allergy Clin Immunol Pract. 2019;7(4):1166–1173.e1161. doi:10.1016/j.jaip.2018.11.015
41. Asai Y, Yanishevsky Y, Clarke A, et al. Rate, triggers, severity and management of anaphylaxis in adults treated in a Canadian emergency department. Int Arch Allergy Immunol. 2014;164(3):246–252. doi:10.1159/000365631
42. Lee AY, Enarson P, Clarke AE, et al. Anaphylaxis across two Canadian pediatric centers: evaluating management disparities. J Asthma Allergy. 2017;10:1–7. doi:10.2147/JAA.S123053
43. Buka RJ, Knibb RC, Crossman RJ, et al. Anaphylaxis and clinical utility of real-world measurement of acute serum tryptase in UK emergency departments. J Allergy Clin Immunol Pract. 2017;5(5):1280–1287.e1282. doi:10.1016/j.jaip.2017.06.021
44. Dzingina M, Stegenga H, Heath M, et al. Assessment and referral after emergency treatment of a suspected anaphylactic episode: summary of NICE guidance. BMJ. 2011;343:d7595. doi:10.1136/bmj.d7595
45. Alvarez-Perea A, Tomas-Perez M, Martinez-Lezcano P, et al. Anaphylaxis in adolescent/adult patients treated in the emergency department: differences between initial impressions and the definitive diagnosis. J Investig Allergol Clin Immunol. 2015;25(4):288–294.
46. Alvarez-Perea A, Ameiro B, Morales C, et al. Anaphylaxis in the pediatric emergency department: analysis of 133 cases after an allergy workup. J Allergy Clin Immunol Pract. 2017;5(5):1256–1263. doi:10.1016/j.jaip.2017.02.011
47. De Schryver S, Halbrich M, Clarke A, et al. Tryptase levels in children presenting with anaphylaxis: temporal trends and associated factors. J Allergy Clin Immunol. 2016;137(4):1138–1142. doi:10.1016/j.jaci.2015.09.001
48. Bonadonna P, Zanotti R, Muller U. Mastocytosis and insect venom allergy. Curr Opin Allergy Clin Immunol. 2010;10(4):347–353. doi:10.1097/ACI.0b013e32833b280c
49. Metcalfe DD. Differential diagnosis of the patient with unexplained flushing/anaphylaxis. Allergy Asthma Proc. 2000;21(1):21–24. doi:10.2500/108854100778249006
50. Ferrari L, Seregni E, Bajetta E, Martinetti A, Bombardieri E. The biological characteristics of chromogranin A and its role as a circulating marker in neuroendocrine tumours. Anticancer Res. 1999;19(4c):3415–3427.
51. Casale TB, Stokes JR. Immunomodulators for allergic respiratory disorders. J Allergy Clin Immunol. 2008;121(2):288–296. quiz 297-288. doi:10.1016/j.jaci.2007.11.040
52. Gelincik A, Ozseker F, Buyukozturk S, Colakoglu B, Dal M, Alper A. Recurrent anaphylaxis due to non-ruptured hepatic hydatid cysts. Int Arch Allergy Immunol. 2007;143(4):296–298. doi:10.1159/000100576
53. Peppers BP, Vatsayan A, Dalal J, Bonfield T, Tcheurekdjian H, Hostoffer R. A case series: association of anaphylaxis with a significant decrease in platelet levels and possible secondary risk of thrombosis. Immun Inflammation Dis. 2018;6(3):377–381. doi:10.1002/iid3.224
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.