Back to Journals » Journal of Pain Research » Volume 11
Management of pain in patients with temporomandibular disorder (TMD): challenges and solutions
Authors Gil-Martínez A, Paris-Alemany A , López-de-Uralde-Villanueva I, La Touche R
Received 3 October 2017
Accepted for publication 4 January 2018
Published 16 March 2018 Volume 2018:11 Pages 571—587
DOI https://doi.org/10.2147/JPR.S127950
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Katherine Hanlon
Alfonso Gil-Martínez,1–3 Alba Paris-Alemany,1–4 Ibai López-de-Uralde-Villanueva,1–3 Roy La Touche1–4
1Department of Physiotherapy, 2Motion in Brains Research Group, Instituto de Neurociencias y Ciencias del Movimiento, Centro Superior de Estudios Universitarios La Salle, Universidad Autónoma de Madrid, 3Hospital La Paz Institute for Health Research, IdiPAZ, 4Institute of Neuroscience and Craniofacial Pain (INDCRAN), Madrid, Spain
Abstract: Thanks to advances in neuroscience, biopsychosocial models for diagnostics and treatment (including physical, psychological, and pharmacological therapies) currently have more clinical support and scientific growth. At present, a conservative treatment approach prevails over surgery, given it is less aggressive and usually results in satisfactory clinical outcomes in mild–moderate temporomandibular disorder (TMD). The aim of this review is to evaluate the recent evidence, identify challenges, and propose solutions from a clinical point of view for patients with craniofacial pain and TMD. The treatment we propose is structured in a multimodal approach based on a biobehavioral approach that includes medical, physiotherapeutic, psychological, and dental treatments. We also propose a new biobehavioral model regarding pain perception and motor behavior for the diagnosis and treatment of patients with painful TMD.
Keywords: biobehavioral, review, temporomandibular disorders, biobehavioral orofacial pain, multimodal approach, motor behavior, disability
Introduction
According to health sciences definitions, temporomandibular disorder (TMD) comprises a variety of conditions affecting the anatomy and functional characteristics of the TM joint (TMJ). Factors contributing to TMD complexity are related to dentition, clenching, and other related systems that frequently provoke symptoms of muscular, articular, and periarticular pain.1
Orofacial pain is defined as a pain manifested in the face or oral cavity, including such disorders as TMD, which are a major cause of nonodontogenic orofacial pain.2,3 TMD has considerable prevalence, with significant impact on physical and psychosocial factors.2 Its prevalence has been reported to be between 3.7% and 12%, and is three to five times more frequent in women.4 TMD also contributes to a high proportion of socioeconomic costs, which are usually associated with comorbidities, such as depression and other psychological factors.5–7 Also, the loss of work and work productivity is a major issue to consider in TMD patients being treated early on, and it requires significant public education.
Before 2000
Although before the 1980s, malocclusion and other related factors were considered fundamental and key causes of TMD, during this decade authors began to publish critical articles on these subjects.8 In current clinical practice, orthodontic treatments are still used to treat TMD; however, it was established in the 1990s that the role of malocclusion in TMD is very limited or nonexistent,9 and thus these disorders should not be treated with orthodontics.10
Decade 2000 to 2010
During the 2000–2010 decade, invasive treatments and surgical options for TMD came into use. However, by the end of this decade, clinical experience and several studies included in systematic reviews, such as Guo et al, reported a lack of evidence supporting the use of arthrocentesis or arthroscopy for TMD treatment.11
From 2010
Although the cognitive and behavioral profiles of patients with TMD have been debated for some years,11 it was in the present decade that health professionals began to propose behavior-based therapies12 as a promising treatment related to cost-effectiveness.13 This paradigm has since been changing and developing a wider focus, leaving behind the biomedical structural model. Thanks to advances in neuroscience,14,15 biopsychosocial models for diagnostics and treatment (including physical, psychological, and pharmacological therapies) currently have more clinical support and scientific growth.16,17
Classification and research diagnostic criteria for temporomandibular disorders
International classifications have been updated in recent decades, thus adapting to new clinical diagnoses and research. The etiology of TMD is multifactorial, which is due to related functional, structural, and psychological factors.18–20 In regard to the clinical presentation of TMD, one of the most frequent symptoms is pain. Pain can affect such areas as ears, eyes, and/or throat, producing neck pain, facial pain, and headaches.21 Among the physical factors, inflammatory problems, such as traumatic secondary synovitis, infection, and irritation, can be found. TMD can also be associated with disk dysfunction, with or without reduction.22
The Research Diagnostic Criteria for TMD (RDC/TMD) have been one of the most commonly used and recognized classifications by the international scientific community for diagnosis, evaluation, and categorization of TMD to date. Their importance is highlighted by the fact that they have been translated into various languages.23,24 The RDC/TMD are based on a biobehavioral model of pain, including two main axes: physical signs and symptoms (axis I) and psychological and disability factors (axis II). Included in axis I are painful myofascial disorders, disk subluxation, or luxation and arthritis or arthrosis,25 given painful myofascial disorders are included in the most frequent diagnoses observed in the literature.25 The most recent published version of this classification was in 2014, named Diagnostic Criteria for TMD (DC/TMD),26 and aimed to improve the sensibility and specificity of the previous RDC/TMD through more comprehensive instruments for axis I and axis II that can be used by researchers and clinicians.
Neurophysiology of trigeminal sensory system
The trigeminal nerve, or cranial V nerve, is considered a mixed-function nerve (sensory, motor, and autonomic)27 and a major cranial nerve.28 This nerve is termed “trigeminal” due to its three main branches: the ophthalmic nerve (V1), the maxillary nerve (V2), and the mandibular nerve (V3). Sensitive axons of the trigeminal nerve innervate the majority of cranial and facial tissues, except the posterior area of the cranium, the mandibular angle, part of the external auditory canal and pavilion, and part of the pharynx.29
Although the primary innervation patterns and the nerve signals are similar throughout the body, the craniofacial region has some particularities. Craniofacial innervation depends on several anatomic and functional structures of primary afferent neurons emanating from the trigeminal ganglion (and other cranial nerves). There are certain differences in the neurons and the dorsal root ganglia of the spinal cord. For example, the relationship between myelinated afferent fibers (A) and unmyelinated afferent fibers (C) is closer in the trigeminal nerve than in the spinal nerves. Spinal nerves present relatively few C fibers.30 This situation could generate a higher mean velocity of conduction in trigeminal areas. In addition, cranial region distances to the neuronal body are shorter than in the rest of the body.31
On the other hand, cranial peripheral nerves have fewer efferent sympathetic axons than spinal nerves. Some authors have postulated that this peculiarity could have relative influence on painful states maintained by the sympathetic nervous system itself in the trigeminal region.32 Other sympathetic differences between the trigeminal area and the rest of the body are the intracranial and cutaneous blood vessels. In the trigeminal area, these vessels receive both parasympathetic and sympathetic innervation; however, in the segmental levels parasympathetic innervation is infrequent or nonexistent.33
Physiopathology of TMD
Trigeminal primary afferent fibers are present in the craniofacial tissues as free nerve endings functioning as nociceptors that can activate through mechanical, thermal, or chemical stimuli. Their activation can result in the excitation of small-diameter and slow fibers (αδ or C).34–36 Some neurochemical components (eg, substance P, 5-HT, prostaglandins, and bradykinins) are involved in this peripheral activation by nociceptive stimulation. These substances are present in the peripheral sensitization process, and could thus enhance nerve sensitivity after minimum injury. This sensitization of nociceptive endings is a peripheral mechanism that as an alert system helps to protect injured tissues from repeated stimuli.37,38
The fact that “nociceptive-specific” and “wide dynamic range” neurons located in the trigeminal subnucleus caudalis are excited by nociceptive stimuli (in both cases) and nonnociceptive stimuli (in wide dynamic range) should be taken into account.36 The majority of these neurons can also be excited by other inputs from the meninges, vessels, oral tissues, TMJ, and masticatory muscles.25,27,29 These inputs have widely convergent patterns, explaining a poor and deep pain location, as well as the diffusion of referred pain, which is a typical condition in TMJ pain and its associated muscles.36,38,39 The aim of this review is to evaluate the recent evidence, identify challenges, and propose solutions from a clinical point of view for patients with craniofacial pain and TMD.
Management of TMD
A suitable therapeutic approach for TMD should be aimed at alleviating the main signs and symptoms of this condition.40 The most relevant signs of TMD are the presence of joint sounds (clicking and crepitation), reduced mouth opening, and disrupted jaw movements.21,41 However, pain is the primary problem of this pathology, and it is typically the reason these patients request medical care.17,42 Also, it is likely the reason that most studies have been aimed at evaluating the effectiveness of various intervention measures related to pain as the main variable.43
Conservative treatments for TMD include medication, physiotherapy, occlusal splints, self-management strategies, and interventions based on cognitive behavioral approaches.44–49 At present, a conservative treatment approach prevails over surgery, given it is less aggressive and usually results in satisfactory clinical outcomes in mild–moderate TMD.48,50–52 In fact, the evidence for the greatest effectiveness of surgical versus conservative intervention to reduce short-term pain in arthrogenic TMD is controversial and inconclusive.53–55 Indications for the application of each of the interventions, as well as their potential effects for the treatment of patients with TMD, are described in the following sections.
Oral and topical pharmacotherapy
The pharmacological treatment of the patients with TMD is usually empirical. Although several medications are typically prescribed for the treatment of TMD, many lack evidence for this specific pathology;56 however, they have proven their effects in other musculoskeletal conditions. The most commonly used drugs include nonsteroidal anti-inflammatory drugs (NSAIDs), corticoids, analgesics, muscle relaxants, anxiolytics, opiates, tricyclic antidepressants (TCAs), gabapentin, and lidocaine patches.57–60 Some of these medications are used to treat the joint pain of the TMD, and others are more effective for treating muscle pain.
NSAIDs have proven their effect in reducing pain in the TMJ. One of the most frequently used NSAIDs is sodium diclofenac, which can reduce joint pain at a dose of 50 mg twice/thrice daily.49,61 Another NSAID used is naproxen sodium, which has been demonstrated to reduce joint pain compared with placebo. Significant differences have been shown after 3 weeks of treatment (500 mg twice a day), and a significant improvement in clinical signs and symptoms of TMD was obtained compared with celecoxib and placebo.62 Piroxicam 20 mg once a day for 10 days results in greater TMJ pain reduction at 30-day follow-up.63 Another substance not very well known but well tolerated is palmitoylethanolamide (300–1,200 mg daily up to 120 days),64 which appears to have an analgesic and anti-inflammatory effect in patients with TMD.65,66
These results suggest that long-term treatment is needed to obtain the maximal effects of all these drugs, which sometimes become evident only after several weeks of treatment. NSAIDs have-well known adverse effects, however, such as exacerbation of hypertension, gastrointestinal effects ranging from dyspepsia to ulceration, and worsening of renal function, which makes analyzing the clinical situation of each patient extremely important to establish the best individual treatment.
A different approach to NSAID intake for avoiding its systemic absorption is its topical administration in creams or ointments over the TMJ to reduce pain. The application of four doses a day of topical diclofenac combined with dimethyl sulfoxide to improve its absorption is recommended.67 Topical diclofenac has been suggested to achieve local concentrations significant enough to inhibit proinflammatory prostaglandin E2 production and also competitively inhibit the NMDA subtype of the glutamate receptor found in TMJ nociceptors.68
For the treatment of muscle pain in myofascial TMD muscle, such relaxants as diazepam and cyclobenzaprine are commonly used.61,69 Diazepam has shown better effects than ibuprofen for chronic orofacial muscle pain71 and the same effects as placebo for reducing TMD pain.71 A recent meta-analysis concluded that cyclobenzaprine had a positive effect on TMD muscle pain in the short term61 through its effect over local spasms and associated acute pain; it was even more effective than clonazepam in improving jaw pain upon awakening.72 The NSAID sodium diclofenac, both by itself and in combination with acetaminophen, carisoprodol, and caffeine, has been proven to have a more rapid positive effect on masticatory muscle pain compared with placebo.73
TCAs have been proposed by some authors for myofascial masticatory chronic pain, particularly amitriptyline and nortriptyline, as first-line treatments for myofascial pain with referral, with low doses of 10–35 mg per day.74,75 Others propose a second-line treatment of gabapentin for nonresponders or for those who do not tolerate TCAs.75,76
Injected pharmacotherapy
In a recent review, results supported the use of injections of the corticosteroid β-methasone or sodium hyaluronate for TMJ pain.53,61 The corticosteroid might have an anti-inflammatory effect on the joint, and the hyaluronate could improve the joint’s lubrication, but both could also help to dilute local inflammatory substances. Inferior or double TMJ-space injection is recommended over the superior-space injection technique.77
Regarding botulinum toxin (BTX) injections to the masticatory muscles, a systematic review revealed controversial results for BTX therapy. Of the five studies included, two obtained a significant reduction in pain, one showed equal effects compared with masticatory manual therapy, and two showed no significant differences for BTX compared with placebo.78 More research is needed to assess the possible long-term negative effects of BTX on the infiltrated muscles. Basic research has shown that the size of the muscle recovered, but not the contractile function, after 1 year of BTX injections.79 Also, when comparing BTX with placebo injection for trapezius muscle pain, there were no differences in pain-intensity measurement.80
Surgical interventions
Among the surgical options, two of the most frequently used techniques for internal derangements of the TMJ or degenerative pathology are arthrocentesis based on articular lavage with or without injection of pharmaceuticals and arthroscopy. There are no differences regarding pain and mandibular function when comparing arthroscopy with arthrocentesis;81,82 however, there is a lack of evidence to support arthrocentesis as a better therapeutic intervention than nonsurgical interventions.83,84 For internal derangement of the TMJ, medical management or rehabilitation is recommended over other surgical options;85 patients with symptomatic disk displacement without reduction should be treated with the simplest and least invasive intervention.86 Furthermore, there is growing evidence supporting the benefit of platelet-rich plasma injections over hyaluronate combined with arthrocentesis for TMJ osteoarthritis; however, more clinical trials are needed.87–89
Dental management
Two approaches are usually proposed by odontologists to treat their TMD patients: orthopedic stabilization therapy and occlusal therapy. Splint therapy is frequently used for the first method group; in the second method, orthodontics and occlusal adjustment are commonly used to achieve a definite correct stable occlusion. According to Varga, signs and symptoms of TMD could not be associated with specific types of malocclusion.90 This statement, together with published reports stating insufficient evidence, precludes us from recommending to our patients an orthodontic intervention or occlusal adjustment to treat TMD.91,92
On the other hand, splint therapy is one of the most commonly proposed conservative treatments for TMD pain associated with bruxism and also for internal derangements. It is not clear whether the use of a stabilization splint can be beneficial for reducing pain in TMD,93 given its therapeutic effect remains controversial; however, it appears to have an undeniable placebo effect for pain management.94 A transient effect of reduction in electromyographic activity of the masticatory muscles has been demonstrated, which did not last more than 2 weeks.95,96 Occlusal splints are recommended to prevent dentition damage from tooth grinding.97,98
Physical therapy
Physical therapy plays a prominent role in the treatment of TMD.45,46,99 This therapeutic discipline aims to relieve pain, reduce inflammation, and restore motor function using a wide range of techniques, including manual therapy (eg, joint mobilization/manipulations, soft-tissue mobilization), therapeutic exercise, electrotherapy (eg, low-level laser therapy [LLLT], transcutaneous electrical nerve stimulation [TENS], therapeutic ultrasound, shortwave), dry needling (DN), and acupuncture.45,47s
Manual therapy
Manual therapy for TMD, regardless of its origin, should include joint mobilization and soft-tissue techniques, with the aim of improving function and reducing pain symptoms.52,100 These techniques can trigger neurophysiological mechanisms responsible for pain relief and reduction of muscle activity.101–103
According to the literature, some authors also consider it relevant to apply these types of techniques to the cervical region, especially in the upper cervical spine.52,100 The demonstrated efficacy of the articular upper cervical mobilizations in reducing pain and increasing mandibular range of motion (ROM)52,100 could be due to the neuroanatomical connection between these two segments at the trigeminal–cervical complex36,104 or the biomechanical relationship between the cervical and orofacial regions.105,106
A debate among manual therapists concerns which approach is the best articular technique for treating the cervical spine. Authors have recommended cervical mobilizations, which have been shown to be more effective in reducing orofacial pain over manipulations.52,100,107 They are safer, and produce similar effects at the cervical spine.108–110
Therapeutic exercise
Exercise focused on improving motor control and endurance of masticatory muscles is effective in alleviating the symptoms of patients with TMD.52 However, exercise does not produce greater pain relief than other interventions,52 such as TENS,111 occlusal splints,112–114 patient education,115,116 and acupuncture.117 It is important to keep in mind, however, that the exercises used in randomized controlled trials have been heterogeneous, including stretching, lingual and masticatory relaxation, and coordination exercises, among others.45,52 This aspect, added to the lack of a clear dosage regarding intensity, duration, and frequency, and the low methodological quality of the randomized controlled trials makes it difficult to draw conclusions.45,52 Nevertheless, although the superiority of the exercises cannot be assured, there is a favorable tendency when compared with other active treatments,52 which might justify its use.
Therefore, we consider that therapeutic exercise could obtain superior results to other treatments if a program with motor-control exercises and endurance of the cervical and masticatory muscles is applied. Although some studies included cervical exercises, most were aimed at increasing the ROM (mobility and stretching exercises), but none intended to improve the resistance of cervical spine stabilizers.52 Stabilizing muscles are essential to maintaining good postural control and helping to prevent the adoption of a forward head position.118–120
Manual therapy and exercise
A combined intervention of manual therapy and exercise is effective in alleviating the symptoms of patients with TMD, further enhancing the effects of both interventions in isolation.52 These findings match those observed for cervical pain,121 which can be explained by the summation of the hypoalgesic effects of manual therapy104 along with the benefits of exercise, including improvements in physical condition, and the adoption of an active role by the patient in their treatment.122–125 On the other hand, although it is effective to administer this combined intervention in the cervical region, greater benefits are obtained when applied in both the orofacial and cervical regions.52 Therefore, we consider it fundamental that physiotherapy treatment combine manual therapy with a program of therapeutic exercises aimed at restoring motor control and resistance of the masticatory and cervical musculature to improve the clinical condition of patients with TMD.
Dry needling and acupuncture
Few studies were found that applied dry needling (DN) for TMD.126–129 This intervention is used for treating local and referred pain produced by myofascial trigger points.130 From these few studies, we conclude that DN results in a reduction in pain and improvements in mandibular function of patients with myofascial TMD.126–129 The effects of DN are comparable to the effects obtained by injection of the trigger points with lidocaine and corticosteroid.131
Acupuncture is a good therapeutic modality for short-term pain relief in patients with myofascial TMD, but not in those cases in which there is a limitation of mandibular movement.132,133 At present, the mechanisms responsible for the analgesia produced by acupuncture are unknown, but appear to be based on the spinal and supraspinal release of serotonin,134,135 endogenous opioids,136–138 and other neurotransmitters with anti-inflammatory actions.139,140 The application of acupuncture is preferable by selecting points in the orofacial region, rather than distal to it, because enhanced effects are obtained in this manner,141,142 and not necessarily by selecting the standard acupuncture points.143 These findings can be explained by the participation of peripheral opioid receptors in the analgesic process, given these receptors generate blockage of the painful input locally and unsystemically, and by the noxious stimulus itself independently of where it is applied.143,144 In fact, there are no differences when acupuncture and DN are compared with placebo that includes skin perforation.132,133,145
Electrotherapy
Current evidence does not support the use of electrotherapy for pain relief in patients with TMD.45,53 In particular, various types of electrotherapy, such as pulsed radiofrequency energy, TENS, and LLLT, show no better results than their respective placebos in the treatment of TMD.45,53 However, the application criteria need to be homogenized in order to establish definitive conclusions, especially for the application of LLLT, given contradictory results are observed depending of the type of TMD, as well as the choice of parameters, such as intensity and frequency.146 Regarding functional improvement, LLLT has proven effective in increasing mandibular ROM.146 This effect could be due to a reduction in inflammation by suppressing cyclooxygenase, which would allow greater mobility to the joint.147 However, LLLT’s mechanisms of action are not yet fully understood.
Cognitive behavioral therapy
Patients with chronic TMD usually present associated psychological factors that should be managed with specific interventions. Cognitive behavioral therapy is one of the treatments proposed to manage patients’ thoughts, behaviors, and/or feelings that might exacerbate pain symptoms. It is a noninvasive therapy and unlikely to have adverse effects.148 The literature reports that cognitive behavioral therapy alone is not better than other interventions, but it is a good complement, especially when adapting the treatment to the psychological characteristics of the patient.149–151
Education and self-management strategies
Education and self-management are useful strategies to include in the management of patients with TMD. When comparing these interventions with occlusal splints, a slight benefit was obtained with education.152 However, when compared with other interventions, such as manual therapy or therapeutic exercise, no additional benefits were observed.115,116,152,153 Nevertheless, it is assumed that education and self-management strategies are good to combine with other techniques, as observed by Wright et al154 and Michelotti et al.152 Most studies performed with education and self-management have included only patients with myofascial TMD, leading to a lack of evidence regarding other types of TMD. There is a need to define better what should be included in patients’ education and which self-management strategies are best according to the various types of TMD and regarding the psychosocial impairments that frequently affect patients with TMD. However, the authors consider that public and patient education could be much promising in patients with TMD, especially those based on neuroscience education, because this approach has been shown to reduce pain, disability, and psychological factors in chronic musculoskeletal disorders.154
Relaxation techniques
Relaxation therapy involves self-regulation techniques aimed at reducing pain-induced stress and muscle tension. Relaxation interventions include such techniques as Jacobson’s relaxation. These techniques can be reinforced by external feedback using electromyography and/or biofeedback systems for training. Relaxation interventions included in a multimodal treatment could have a positive influence on pain intensity and maximal mouth opening, but there is scarce and controversial evidence.148,155
From a biomedical to a biobehavioral approach
The biomedical model has been an approach used widely in research on the etiological factors involved in TMD. This model has been based on functional theories and structural or morphological–pathological theories that attempt to explain TMD through theoretical concepts on dysfunctions of the condyle–disk complex, traumas, degenerative processes, occlusal concepts, and alterations related to masticatory muscles.21 Some theories on the structural and functional biomedical model related to TMD have been useful and some concepts are still valid today, because they consistently explain the disorder from a dysfunctional point of view.
Diagnostic criteria for the classification of TMD based on physical signs and symptoms have had great impact in clinical practice and research, and have provided a standardized means of classification into various subtypes. It is important to highlight that instruments for the classification and evaluation of the psychological components involved in TMD have been included (emotional and cognitive factors);26 however, analysis of the research on TMD reveals most studies that classification related to emotional and cognitive factors intended to define the psychological state and disability associated with patients’ pain has not had much impact on its use for the inclusion criteria or classification of patients.
The basis of the biomedical model is limited when we want to understand in depth the pathophysiology and perpetuation factors related to chronic pain in patients with TMD. A broader view on chronic pain is provided by neuroscientific studies from the last decade. There is strong evidence to suggest that neuroplastic changes and hyperexcitation of the central nervous system (CNS) would be part of those responsible for the central sensitization phenomenon.156 Findings present in patients with TMD with chronic pain, such as generalized mechanical hyperalgesia,157 structural and functional changes at the brain level,14 alteration in pain modulation, comorbidities with other chronic diseases, increased expansion of pain areas, and presence of associated psychological factors would indicate a clinical profile compatible with a central sensitization process.156,158 It is important to mention that cognitive aspects, such as memory and learning, are heavily involved in the encoding of affective/emotional aversive stimuli that feed and perpetuate the sensitization process at the central level.159
Current literature suggests that psychological and psychosocial factors have an important association with the duration of symptoms and their perpetuation in cases of chronic pain.158,160,161 Psychological factors, such as pain catastrophizing,162,163 psychological distress,161,164 fear-avoidance beliefs,165,166 beliefs related to painful perception,167 depressed or anxious mood,168–170 self-efficacy,171 and passive coping,164,172–174 are related to increased pain perception, increased levels of disability, and movement disorders in patients with chronic painful TMD. On the other hand, it has been noted that some psychosocial factors have also been identified as predictors of treatment outcome in patients with TMD.175 We consider that somatic awareness is an important sensory-discriminative factor to be taken into account, since it has been related to an increased risk of suffering a TMD.173,176
The abundant current scientific evidence shows that the mechanistic biomedical model is not sufficient to establish a diagnosis or accurate treatment to manage patients with chronic painful TMD. A change in approach toward a more comprehensive and integral vision is necessary. We propose a diagnostic and therapeutic approach based on a biobehavioral approach. Many authors share this thought,177–179 even suggesting that from an ethical point of view a compulsory change is needed, given the application of unnecessary and irreversible interventions due to traditional management could endanger the patient’s well-being.177,178
The biobehavioral model for the diagnosis and treatment of patients with chronic painful TMD recognizes the importance of psychological factors, such as pain history, current emotional and cognitive status, beliefs, learned behaviors, and coping skills, in interaction with the physiological alterations that determine the pain experience. From the therapeutic point of view, the model allows the patients to acquire the ability to self-manage the pain, allowing an improvement in general functioning.180
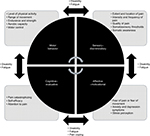
Based on the current clinical and scientific context, we propose a diagnostic and intervention model to address patients with painful TMD based on four dimensions (affective–motivational, sensory–discriminative, cognitive–evaluative, and motor behavior) integrated in a biobehavioral approach (Figure 1). This model has been termed the biobehavioral model of pain perception and motor behavior, and although we have designed it to study any musculoskeletal disorder, in this article we adapt it to TMD. Table 1 presents information about all of these approaches, grade of evidence, and magnitude of effects.
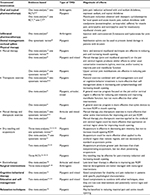  | Table 1 Evidence on treatment options for pain related to TMD Abbreviations: RCT, randomized controlled trial; ROM, range of motion. |
Biobehavioral model of pain perception and motor behavior
A fundamental aspect of our model is the fact that musculoskeletal pain produces changes in motor behavior.181 It has also been observed that pain-related movement disorders are an important cause that influences the impairment of functional capacity and the patient’s quality of life,182 including the possible interaction that cognitive and emotional aspects can have on the relationship between motor behavior and pain perception. Herein, we briefly describe the theoretical aspects that underlie the biobehavioral model of pain perception and musculoskeletal pain.
Motor changes can be explained through the peripheral and central mechanisms related to the CNS.183,184 Experimental studies have found that muscular pain influences motor-control strategies through central mechanisms.185,186 On the other hand, several studies have found functional and structural changes in motor cortical areas of patients with chronic pain.187,188 In this respect, activation of the supplementary motor area in patients with TMD when faced with adverse cognitive or emotional stimuli has been observed.189 That same activation has been found in patients with TMD who have catastrophic helplessness.190 It is important to mention that the supplementary motor area plays an important role in movement planning, and it is theorized that the preactivation of this area found in cases of chronic pain could be due to the preparation of avoidance or anticipatory motor behaviors. We have scientific evidence revealing neurophysiological mechanisms of motor anticipation related to pain perception.191,192 Pain-protection behaviors can include motor activities, such as avoidance of movement and tendencies to touch the affected area of the body.193 It has been proposed that the motor responses involved in the pain experience can be activated when the intensity of the pain rises beyond a certain threshold.194
Emotional factors related to fear of pain play an important role in the degree of protective behaviors triggered by pain.195 Recent research has shown that high levels of fear of pain are associated with being less physically active,196,197 limited range of motion,198,199 physical disability,200 and strategies for adopting alternative movements.201 It should be noted that behaviors associated with psychological distress, interruption of activity, and avoidance of activity are essential components in pain-related disability.202
Motor behavior related to painful experiences can vary according to the case. Some patients with chronic pain use passive motor strategies to avoid pain; however, other patients use active self-regulation strategies to cope with pain.203 Simmonds et al reported that movement dysfunction was not only a consequence of anticipating and minimizing pain. The motor component involved is an even more complicated problem that involves social, environmental, and psychological factors (cognitions and emotions) that can influence motor activity as a complex multidimensional construct.204 Several motor programs have been proposed for the various forms of pain behavior. These can be organized at various levels of the CNS, and can be influenced by social and psychological factors.195
Current evidence holds that in addition to fear of pain, other psychosocial factors might contribute to generating pain-related functional alterations.205–207 In this regard, Sullivan suggested that certain psychological factors, such as pain catastrophizing, fear, and depression can influence pain by reducing the threshold of activation of motor programs related to pain perception.195
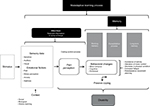
In summary, the biobehavioral model of pain perception and motor behavior presents a specific framework to help us understand the mechanisms involved in chronic painful TMD. Basically, we propose that sustained pain perception produces neuroplastic changes in the CNS that have implications for motor behavior that are directly and indirectly influenced by cognitive and emotional factors. In this model, the motor behavior is an essential element, given its alteration would increase levels of disability, leading to poorer quality of life, and would increase the perception of pain intensity.
Poor motor behavior can be influenced by fear-avoidance beliefs, a decrease in self-efficacy expectations, catastrophic cognition, and an increase in depressive symptoms. Furthermore, pain-related movement disorders are a means of learning maladaptation that increases the attention to and memory of pain, favoring the perpetuation of the painful experience (Figure 2). Behavioral changes associated with the experience of the perception of maintained pain can cause various movement dysfunctions, mainly when a passive coping strategy is used. The result of this situation is an increase in disability levels (Figure 2).
To perform adequate clinical reasoning and undertake a good diagnostic approach using this model, it is necessary to evaluate the four dimensions and try to discern how they interact with one another, and especially to evaluate what factors are relevant (Figure 1). We recommend performing an assessment that objectifies sensorimotor variables through physical tests and an evaluation of cognitive and emotional factors using self-reports to quantify them. In Figure 1, we suggest the variables that should be evaluated to work with the biobehavioral model of pain perception and motor behavior.
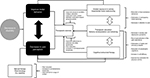
The therapeutic approach we suggest attempts to provide a comprehensive framework for the treatment of patients with chronic painful TMD. The main variable to achieve optimum functional recovery is disability: we propose that if we improve the motor behavior, we will decrease the disability and in turn the painful perception. Therefore, we consider the possibility of a bidirectional relationship through which the treatment that reduces the painful perception can also favor the recovery of motor behavior while decreasing the disability. In order to achieve this change, it is necessary to eliminate erroneous beliefs and negative cognitions that could alter the treatment results. It will also be necessary to use motivational strategies that promote good adherence and compliance with the various types of treatments. It is important to mention that the treatment proposed herein has as a central therapeutic axis the movement to reduce pain and improve function. In relation to this, Luomajoki et al found that treatment with therapeutic exercise to improve motor performance also resulted in an improvement in pain and disability in patients with low-back pain.208 In our model, we also integrate therapeutic strategies, such as therapeutic exercise, that can specifically reduce pain and would make treatment more effective.
Current scientific evidence shows the ability of therapeutic exercise to modulate pain in experimental conditions.209–211 In addition, we have strong scientific clinically relevant evidence that demonstrates the effectiveness of therapeutic exercise in reducing disability and pain intensity in other chronic musculoskeletal conditions.212–217
Multimodal treatment based on a biobehavioral approach
The treatment we propose has a multimodal point of view, but could also be structured in a multidisciplinary way, including the therapeutic interventions of physiotherapists, dentists, psychologists, and physicians. Based on current scientific evidence, we can say that a conservative approach appears to be the best option for the management of chronic painful TMD.
The treatment methods included in the therapeutic approach of this model are structured to achieve three objectives: reduction in pain perception, improvement of motor behavior, and improvement of cognitive and emotional factors related to the experience of pain. To reduce pain intensity, we propose the use of manual therapy, DN, and pharmacology. For improvements in pain and mandibular function, it is relevant to apply a combined intervention of manual therapy and therapeutic exercise directed to the orofacial, craniomandibular, and upper cervical regions. Although it is not yet a sufficiently investigated aspect, we consider that the prescription of generalized exercise in both aerobic and anaerobic modalities could be beneficial for patients with chronic conditions, which could favor the activation of the descending inhibitory system of pain, improve physical condition, and decrease the attentional focus on pain perception. In addition, splints appear to play a prominent role in the protection of dentition.
In order to improve the effectiveness of the aforementioned treatments, it is necessary to apply them in combination with biobehavioral treatments and strategies, in which we would emphasize therapeutic education, cognitive behavioral therapy, experiential motor restructuring, graded exposure to activity, sensory reinterpretation and retraining, counseling, and methods of physiological self-regulation, such as training in relaxation and biofeedback. These treatments should aim to improve adherence to therapeutic exercise and self-management techniques, eradicate counterproductive habits, encourage positive behaviors, reduce catastrophic cognition, reconceptualize erroneous beliefs about pain and movement, reduce fear-avoidance behaviors, improve stress management, and improve the patients’ knowledge of therapeutic exercise benefits. In Figure 3, we present relevant aspects and recommendations to be taken into account for the approach and development of treatment from the biobehavioral model of pain perception and motor behavior. It is also important to mention that the treatment that we propose is applicable to patients with chronic painful TMD. For cases of acute or subacute pain, it is possible that less complex unimodal or bimodal approaches would have good effectiveness.
Disclosure
The authors report no conflicts of interest in this work.
References
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.