Back to Journals » Clinical Interventions in Aging » Volume 18
Management of Glucose-Lowering Therapy in Older Adults with Type 2 Diabetes: Challenges and Opportunities
Authors Doucet J, Gourdy P, Meyer L, Benabdelmoumene N, Bourdel-Marchasson I
Received 28 June 2023
Accepted for publication 12 September 2023
Published 9 October 2023 Volume 2023:18 Pages 1687—1703
DOI https://doi.org/10.2147/CIA.S423122
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Maddalena Illario
Jean Doucet,1 Pierre Gourdy,2,3 Laurent Meyer,4 Nabil Benabdelmoumene,5 Isabelle Bourdel-Marchasson6,7
1Department of Polyvalent Internal Medicine, Saint Julien Hospital, Rouen University Hospital, Rouen, France; 2Department of Diabetology, Toulouse University Hospital, Toulouse, France; 3Institute of Metabolic and Cardiovascular Diseases, UMR1297 INSERM/UT3, Toulouse University, Toulouse, France; 4Department of Endocrinology, Diabetes and Nutrition, University Hospital of Strasbourg, Strasbourg, France; 5Department of Internal Medicine and Geriatrics, University Hospital of Marseille, Marseille, France; 6CNRS, CRMSB, UMR 5536, University of Bordeaux, Bordeaux, France; 7University Hospital of Bordeaux, Bordeaux, France
Correspondence: Jean Doucet, Email [email protected]
Abstract: The population of older adults (≥ 65 years) with type 2 diabetes mellitus (T2DM) is diverse, encompassing individuals with varying functional capabilities, living arrangements, concomitant medical conditions, and life expectancies. Hence, their categorization into different patient profiles (ie, good health, intermediate health, poor health) may aid in clinical decision-making when establishing glycemic goals and pharmacological treatment strategies. Further granularity in assessing each patient profile through interdisciplinary collaboration may also add precision to therapeutic and monitoring decisions. In this review, we discuss with a multidisciplinary approach how to deliver the best benefit from advanced diabetes therapies and technologies to older adults with T2DM according to each patient profile. There remain however several areas that deserve further research in older adults with T2DM, including the efficacy and safety of continuous glucose monitoring and automated insulin delivery systems, the switch to once-weekly insulin, the effectiveness of multidisciplinary care models, and the use of supported telemedicine and remote blood glucose monitoring in the oldest-old (≥ 85 years) who particularly require the assistance of others.
Keywords: type 2 diabetes, older adults, patient profiles, management
Introduction
Aging, a natural phenomenon, is typically assessed based on an individual’s chronological age. Individuals who are 65 years of age or older are considered “elderly”.1–4 However, with the increase of people’s life expectancy worldwide and the improving health and functional status in older people, there has been a redefinition of old age. Some authors define the age from 65 to 74 years as pre-old age, and 75 years or over as old age.1–3 Some studies further classify adults between the ages of 75 and 84 or 89 years as old or middle-old, and those aged at least 85 or 90 years as oldest-old.3,4
As aging progresses, and irrespective of the adopted definition of old age, pathophysiologic changes occur in various organs, including the liver, kidneys, heart, and musculoskeletal system (ie, bone and muscle loss), and the risk of chronic illnesses increases.4 Among these chronic illnesses, type 2 diabetes mellitus (T2DM) is a widely prevalent health condition among the older population.5 It is estimated that almost one-quarter of people with T2DM are aged 70 years or over.6 By altering glucose metabolism, T2DM leads to accelerated biological age, which specifically measures the physiological breakdown of cells and organs. Hence, considering biological age, in addition to chronological age, may also provide a tool to accurately assess healthspan in T2DM.7
Older adults with T2DM constitute a diverse group of patients characterized by varying functional capabilities, living arrangements, concomitant medical conditions, and life expectancies.8 Moreover, there is broad variation in functional status and health-related quality of life within every age subgroup (ie, from pre-old to oldest-old), with individuals having good and poor health at both ends of the age spectrum.8,9 Hence, older adults with T2DM can be fit individuals living independently in the community, or functionally dependent residents of nursing homes with multiple comorbidities, with in-between a whole spectrum of intermediate health states at risk of deterioration (frail). Older adults can also have either newly diagnosed T2DM or long-standing T2DM with onset in middle or early age.10 In addition, older adults with diabetes can fall into any of the five clusters that were proposed by Ahlqvist et al: severe autoimmune diabetes, severe insulin-deficient diabetes, severe insulin-resistant diabetes, mild obesity-related diabetes, and mild age-related diabetes.11 Consequently, the pharmacological management of T2DM in older adults can be challenging for clinicians, warranting frequent evaluation of medical, psychological, functional, and social domains.
T2DM management in older adults should aim to achieve a good quality of life and avoid diabetes-related complications as well as treatment side effects, mainly hypoglycemia.5,12 Importantly, T2DM management should aim to promote healthy aging, defined as the preservation of functional ability that enables individuals to fulfill their needs and actively participate in society.13 Unfortunately, older adults are vastly underrepresented in clinical trials, particularly frail or dependent individuals, resulting in a lack of sufficient evidence-based data to inform clinical decision-making in the population of older patients with T2DM.8 In light of recent guidelines on the management of T2DM in older adults,5,14,15 we discuss in this review how to deliver, with a multidisciplinary approach, the best benefit from advanced diabetes therapies and technologies to older adults with T2DM according to each patient profile.
Methods
A systematic literature search was performed on MEDLINE (PubMed) to identify clinical studies (either observational or interventional), qualitative studies, systematic reviews/meta-analyses, literature reviews, position statements, expert consensus, and guidelines describing the management of T2DM in older adults. The used keywords were: (“diabetes” OR “diabetic”) AND (“older” OR “elderly”) AND (“management” OR “care”). Only studies published in English within the last 10 years (2013–2023) were included in the search. We also manually screened the reference lists of the included studies.
Patient Profiles and Glycemic Goal-Setting
T2DM management in older adults is complicated by multiple factors, which can be additive (Figure 1). Approximately 60% of older adults with diabetes have a minimum of one comorbid chronic condition, while up to 40% have four or more comorbid conditions.16 The population of older adults with T2DM is at an increased risk of traditional microvascular and macrovascular T2DM-related complications, in particular chronic kidney disease (CKD) and cardiovascular disease.17,18 Moreover, a U-shaped relationship between hemoglobin A1c (HbA1c) and all-cause mortality has been established in older adults. Mortality rate is higher at threshold HbA1c values below 6.5% (48 mmol/mol) and above 8.5–11% (69–97 mmol/mol).19,20 In the French GERODIAB prospective, observational study which enrolled 987 patients with T2DM aged 70 years or more and followed over 5 years, increased HbA1c levels (≥8.6% or ≥70 mmol/mol) were significantly associated with increased mortality, even in individuals with preserved functional abilities.19
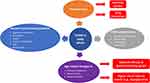 |
Figure 1 Challenges in the management of type 2 diabetes mellitus (T2DM) in older adults. |
Older adults with T2DM are also at a greater risk than other older adults for several adverse conditions, such as sarcopenia, bone loss, frailty, urinary incontinence, depression, cognitive impairment, and falls resulting in injury.5 In addition, older adults with T2DM have an increased risk of malnutrition as a result of changes in taste and smell, dysphagia, inadequate dental health, gastrointestinal problems, anorexia, cognitive impairment, and/or depression.15 Older adults are as well increasingly susceptible to hypoglycemia due to the gradual age-related decrease in β-adrenergic receptor function, particularly if they are treated with insulin and/or insulin secretagogues (ie, sulfonylureas and glinides).10 Dementia and cognitive impairment further put older adults with T2DM at higher risk of severe hypoglycemia due to their inability to identify or report hypoglycemia symptoms to caregivers.21 In turn, caregivers may not recognize hypoglycemia in the population of older adults with T2DM, given the lack of adrenergic alarm symptoms, such as sweating, palpitations, and tremors.12,21
Overall, given the high heterogeneity of the population of older adults with T2DM and the heterogeneity of the individual disease course, profiling patients is essential for better personalized management in order to avoid harm and maximize treatment benefits. Accordingly, several scientific societies, including the American Diabetes Association (ADA),5 the Endocrine Society,15 and the International Diabetes Federation (IDF),14 have categorized older adults into three patient groups (ie, good health, intermediate health, and poor health) based on their functional status, cognitive status, comorbidities, and life expectancy to optimize glycemic goal-setting (Table 1). Glycemic target recommendations provided by these different scientific societies are overall consistent.5,14,15 In short, older adults with good health, who are functionally and cognitively intact and can be expected to live long enough (eg, >10 years), should receive diabetes care achieving glycemic goals developed for younger adults (HbA1c <7.0–7.5% [53–58 mmol/mol]).5,14 The HbA1c target is raised to <8.0–8.5% (64–69 mmol/L) in older adults with intermediate health, who are functionally dependent or have multiple coexisting chronic illnesses or cognitive impairment or dementia. For patients with poor health, who have an end-stage chronic illness and are receiving palliative and/or end-of-life care, reliance on HbA1c should be avoided and glucose control decisions should prioritize the prevention of both hypoglycemia and symptomatic hyperglycemia (Figure 2).5,14 Interestingly, a recent national physician survey from the United States found that physicians tend to select more aggressive HbA1c targets than recommended for older adults with intermediate or poor health.22 This finding highlights the need to increase clinicians’ comfort with guideline-recommended HbA1c targets for older patients with T2DM.22
 |
Table 1 Glycemic Targets in Older Adults (≥65 Years) with Type 2 Diabetes |
 |
Figure 2 Hemoglobin A1c (HbA1c) goals according to patient profiles of older adults with type 2 diabetes. |
Although patient categorization by society guidelines can facilitate clinical decision-making in older adults with T2DM, it also omits certain geriatric considerations of interest, such as the patient’s history and risk of hypoglycemia as well as diabetes duration. In a cross-sectional analysis performed in 1288 adults with diabetes aged ≥65 years using data from the National Health and Nutrition Examination Survey, over 50% of older adults had an HbA1c level <7.0% (53 mmol/mol) while being treated with hypoglycemia-associated drugs (insulin and/or sulfonylureas), regardless of their functional status.23 Having a safe lower limit for HbA1c level may thus be helpful in patients treated with insulin and insulin secretagogues.
Clinical practice guidelines also do not take into account T2DM duration when establishing glycemic goals in older adults.5,14,15 However, HbA1c levels significantly rise with the duration of diabetes.24 Compared to older adults with new-onset T2DM, those with long-standing T2DM tend to develop more diabetic complications (eg, CKD and retinopathy) and are at a higher risk of hypoglycemia, as they are more likely to be treated with insulin and for a longer duration.25 Of note, those who newly develop T2DM at an old age are more likely to have near normal fasting glucose levels but are more prone to experience postprandial hyperglycemia.25
Overall, we believe that for each individual patient, both lower and upper bounds of HbA1c should be set to balance risks and benefits of glucose‐lowering treatment, with the main therapeutic objective of avoiding symptomatic hyperglycemia and hypoglycemia. We also believe that target HbA1c range should be regularly reevaluated on an individual basis, with the aim to deliver comprehensive diabetes care rather than a dichotomous model of care (ie, good or poor glycemic control). It is however important to mention that HbA1c measurement may not be accurate in many conditions that are frequently encountered in old age. These include anemia and other conditions that may alter red blood cell turnover, such as advanced CKD and hemodialysis, gastrointestinal bleeding, valvular heart disease, recent blood loss or transfusion, or erythropoietin therapy.5,15 Hence, in these situations, self-monitoring of blood glucose (SMBG) using a fingerstick and/or continuous glucose monitoring (CGM) measurement should be systematically proposed as an alternative to HbA1c to guide therapeutic decisions.15
Non-Pharmacological Management of T2DM in Older Adults
Lifestyle modifications play an initial role in the effective management of T2DM (Figure 3).5 Multi-component lifestyle interventions, such as physical activity and adopting optimal nutrition practices that include sufficient protein intake, can potentially decrease mobility impairments, improve fitness, and reduce HbA1c levels and systolic blood pressure among older adults with T2DM.26–28
Malnutrition and physical inactivity have also been found to be common risk factors for cognitive impairment and frailty.29 Therefore, a healthy dietary pattern consisting of optimal energy intake (of approximately 30 kcal/kg of body weight/day), high intake of protein (of 1.2–1.5 g/kg of body weight/day), vegetables, and fish, including vitamin D and omega-3 fatty acids, and low intake of meat, refined grains, sugar, and snacks could be an important dietary strategy to prevent frailty, cognitive decline, and mortality in older people with T2DM.29 Similarly, clinical practice guidelines by the ADA encourage regular exercise, including aerobic activity, weight-bearing exercise, and/or resistance training, in all older adults with T2DM who can safely engage in such activities.5 Indeed, not only does exercise improve insulin resistance, inflammation, and oxidative stress associated with impaired cognition, but it has also been shown to reduce glycemic variability in individuals with obesity as well as T2DM or impaired glucose tolerance.28–30
Overall, diet and physical activity represent the first step in the management of diabetes. Hence, making informed choices regarding what older adults with T2DM eat and how they stay active can have a profound impact on glycemic control and overall health.
Pharmacological Management of T2DM in Older Adults
Both patient-specific and drug-specific characteristics determine which glucose-lowering agents are appropriate for an older individual with T2DM. These include comorbidities such as atherosclerotic cardiovascular disease (ASCVD), CKD, and heart failure; hypoglycemia risk; cognitive function; functional status; effect of glucose-lowering agents on body weight and their associated safety profile; individualized glycemic goals; patient preferences; and treatment cost.5 Overall, a close follow-up and repeated patient profile assessments and treatment adjustments (for instance in case of treatment non-adherence, hospitalization, or treatment for cancer) are key in the management of older adults with T2DM.
Various oral and injectable glucose-lowering agents are available for the treatment of T2DM in older adults (Table 2). When initiating glucose-lowering therapy in the population of older adults with T2DM, the principle of “start low, go slow” applies.31 Overall, most glucose-lowering agents can be safely used for the treatment of T2DM in older persons, with some considerations according to patient profiles (Table 3). In this respect, due to a high risk of hypoglycemia, insulin secretagogues (sulfonylureas or glinides) and/or short-acting insulin should be avoided in older adults with T2DM, or, in the absence of alternative treatment strategies, managed with extreme caution (ie, adoption of low doses, reinforced blood glucose monitoring).32 Alpha-glucosidase inhibitors should also not be administered in older adults with T2DM because of relatively low efficacy and abdominal adverse events (AEs) of bloating, flatulence, and diarrhea.33 In addition, glitazones should be avoided, as they might lead to fluid retention, which could precipitate or exacerbate heart failure. Pioglitazone is also associated with an increased risk of bone fractures, which detracts from its use in frail patients.33
 |
Table 2 Advantages and Disadvantages of Glucose-Lowering Agents in Older Adults (≥65 Years) with Type 2 Diabetes |
 |
Table 3 Treatment Considerations According to Patients’ Health Status |
Patient Profile-Oriented Management
In functionally independent older adults with good health, given their longer remaining life expectancy (>10 years), the same treatment principles should be applied as in younger patients with T2DM, including the use of advanced technologies such as novel insulin delivery systems. Practitioners should nevertheless be mindful of the risk of hypoglycemia in these individuals when adding in glucose-lowering therapies, as the consequences of hypoglycemia may be just as significant in the functionally independent as the functionally dependent older adult with diabetes.
In less fit patients with intermediate or poor health, glucose-lowering treatment regimens (especially insulin) should be kept as simple as possible to reduce polypharmacy and decrease both disease and drug burden as well as complexity of medication regimens.5 A systematic review, of 10 observational cohort and interventional studies conducted in a total of 26,925 older adults (≥65 years) with T2DM and with or without comorbidities such as ASCVD, CKD or dementia, reported no deterioration in HbA1c levels, hypoglycemic episodes, falls, or hospitalizations following deintensification (defined as “complete withdrawal, discontinuation, reducing dosage, conversion, or substitution of at least one glucose-lowering medication”).34 A single-arm, interventional study performed in older adults (≥65 years) with T2DM additionally found that insulin regimen simplification by switching from multiple-dose insulin regimens to once-daily insulin glargine combined or not with non-insulin agents can lower hypoglycemia risk and diabetes-related distress without compromising glycemic control.35 Treatment regimen simplification can also offer practical benefits such as reducing the frequency of administration, minimizing the need for frequent blood glucose checks, and eliminating the requirement for complex calculations like sliding-scale insulin or insulin-carbohydrate ratio calculations.5
Although clinical practice guidelines emphasize the importance of simplified treatment regimens in older adults, such guidance is often uncertain.5,14,15 However, to be mindful of patients’ quality of life, guidelines recommend in functionally dependent older people the avoidance or discontinuation of agents that might cause nausea or gastrointestinal disturbance as well as excess weight loss.5,14 It is important to mention that periodic assessments should be conducted for each individual to evaluate their eligibility for treatment simplification, since simplification may not be appropriate for some patient profiles such as hospitalized individuals, those with severe insulin deficiency, or those at higher risk of occurrence or progression of micro- or macrovascular complications.36
In the particular case of older adults at the end of life receiving palliative medicine or hospice care, individuals are free to decline testing and treatment, and healthcare professionals may consider discontinuing treatment and limiting diagnostic testing, such as reducing the frequency of blood glucose monitoring.5 For dying patients with T2DM, it may be a reasonable approach to discontinue all glucose-lowering medications, as these individuals are unlikely to have any oral intake. If needed, long-acting basal insulin can be implemented, since it provides anabolic benefits.5,14
While individualization of therapy is desirable, availability, cost, and local prescribing regulations are the primary factors that determine therapeutic choice in real-world clinical practice.14
Metformin and Dipeptidyl Peptidase-4 Inhibitors (DPP4is)
In both younger and older adults with T2DM, metformin is unanimously recommended by clinical practice guidelines as the preferred first-line therapy because of its low cost, low potential for hypoglycemia, and its high glycemic efficacy.5,14,15 Nevertheless, metformin should be cautiously used in patients with renal dysfunction (contraindicated in patients with an estimated glomerular filtration rate [eGFR] <30 mL/min/1.73 m2) or impaired hepatic function or heart failure because of the increased risk of lactic acidosis.5,15,21 For patients with contraindications and/or intolerance to metformin, dipeptidyl peptidase-4 inhibitors (DPP4is) can be given as first-line therapy, as they have few AEs and a minimal risk of hypoglycemia, and can be safely used in individuals with CKD. However, their relatively high cost may present a barrier for certain older adults, and DPP4is may not be easily available in many countries.5,14 Metformin is also known to cause gastrointestinal AEs such as nausea and diarrhea, limiting its use in older adults who might be affected by malnutrition and/or reduced oral intake (due to dysphagia for instance).5,21 Healthcare providers could however choose an extended-release formulation of metformin to lower the risk of gastrointestinal intolerance.21
Glucagon-Like Peptide-1 Receptor Agonists (GLP-1RAs) and Sodium-Glucose Co-Transporter-2 Inhibitors (SGLT2is)
Because T2DM slowly worsens over time, adding glucose-lowering medications to first-line therapy may be necessary to achieve adequate glycemic control. However, the optimal sequence for adding drugs after metformin remains uncertain in older adults.15 Sodium-glucose co-transporter-2 inhibitors (SGLT2is) or glucagon-like peptide-1 receptor agonists (GLP-1RAs) are reasonable second agents for older adults with established or at increased risk of ASCVD, given their demonstrated cardiovascular benefits and low hypoglycemia risk.5,14,15
In a meta-analysis of 11 randomized controlled trials (RCTs) in older patients (≥65 years) with T2DM, both GLP-1RAs and SGLT2is significantly reduced major adverse cardiovascular events (MACE) by 14% for GLP-1RAs (hazard ratio [HR], 0.86; 95% confidence interval [CI], 0.80–0.92) and by 13% for SGLT2is (HR, 0.87; 95% CI, 0.74–1.01).37 Of particular relevance to the older population, stroke, a component of MACE, was significantly reduced by GLP-1RAs (HR, 0.82; 95% CI, 0.68–0.98), and SGLT2is significantly reduced heart failure hospitalization (HR, 0.62; 95% CI, 0.51–0.76).37 Growing evidence also suggests that GLP-1RAs have potential neuroprotective effects, which can be useful in the treatment of neurodegenerative diseases, especially Parkinson’s disease and Alzheimer’s disease.38 However, caution should be exercised regarding the use of SGLT2is and GLP-1RAs in individuals older than 80 years, due to the lack of both RCT data and real-world data in this patient population.
Since both GLP-1RAs and SGLT2is promote weight loss, including lean mass loss, practitioners should consider avoiding them in frail older adults or in those who may be at increased risk of anorexia or malnutrition.15 GLP-1RAs are also associated with nausea, vomiting, and diarrhea. To minimize the gastrointestinal AE profile of GLP-1RAs, healthcare providers should initiate GLP-1RAs at a low dosage and gradually increase the dose to reach the maximal tolerated dose.5,21 SGLT2is should also be cautiously used in frail patients due to the risk of plasma volume reduction and potential falls resulting from hypotension.39 The use of SGLT2is in older adults is further restricted by AEs such as dehydration, increased thirst and polyuria, an increased risk of genital and urinary tract infections, and reduced glucose-lowering effectiveness in patients with preexisting CKD.8 Plasma volume status and kidney function (serum creatinine with eGFR) should therefore be assessed prior to starting an SGLT2i and periodically thereafter. In the frail older adult, DPP4is thus remain a convenient add-on treatment alternative option to metformin due to their excellent safety profile.
All GLP-1RAs, with the exception of oral semaglutide, are injectable agents, which require visual, motor, and cognitive skills for appropriate administration.5 There are however once-weekly options, namely exenatide, dulaglutide, and semaglutide, that facilitate adherence and could be a good treatment option in patients with cognitive impairments or depression, particularly if at increased risk of hypoglycemia due to the use of insulin or insulin secretagogues, since they can be administered conveniently by caregivers.39 Indeed, it has been shown that when older patients with T2DM develop cognitive dysfunction, they are significantly less likely to be involved in diabetes self-care and are at increased need of assistance.40
Insulin Therapy
In patients with long-standing T2DM, insulin should be administered as add-on therapy, particularly in case of persistent hyperglycemia on oral glucose-lowering therapies. Interestingly, in adults aged ≥65 years with uncontrolled diabetes, early treatment with insulin glargine resulted in better glycemic control and less hypoglycemia than continuing to titrate oral agents such as insulin secretagogues.41 Insulin therapy in older adults should be tailored according to individualized glycemic targets, level of functional dependency, and supportive care availability.5,42
The most convenient and simple insulin regimen to use in older adults with T2DM is a once-daily injection of one of the long-acting basal insulin analogues (insulin detemir, glargine, or degludec), which are associated with minimal AEs, and more specifically with a lower risk of hypoglycemia than neutral protamine Hagedorn (NPH) insulin.5,14,43 Supporting this therapeutic choice, initiation of insulin glargine or detemir was associated with an approximately 30% lower risk of emergency department visits or hospitalizations for hypoglycemia in adults aged ≥65 years with T2DM, as compared to NPH insulin.44 It is also preferable to administer long-acting basal insulin in the morning, since older adults (≥65 years) with diabetes tend to have a higher incidence of postprandial hyperglycemia compared with fasting hyperglycemia than younger adults (<65 years) at all HbA1c levels.45 To minimize the risk of hypoglycemia, long-acting basal insulin should be titrated gradually, and its dosage should be adjusted on a weekly basis until the desired fasting blood glucose target is reached.21
Recently, once-weekly basal insulin analogues (eg, insulin icodec) have shown glucose-lowering effects and a safety profile similar to those of once-daily basal insulin analogues,46 and could hence become another therapeutic advance that may ease the burden of self-care, once available. However, the efficacy and safety of once-weekly basal insulin analogues in the older patient population still need to be demonstrated.
Of note, due to its anabolic effects, insulin therapy is the preferred glucose-lowering therapy in patients with severe malnutrition.43 The use of complex insulin schemes should however be avoided, if possible, due to the increased risk of overall and severe hypoglycemia associated with prandial insulin injections in the context of older patients with diabetes.35
Newer insulin delivery devices, such as insulin pumps and smart insulin pens, are being more frequently used in older adults with diabetes to enhance their quality of life and lower the risk of hypoglycemia. Indeed, these devices can ease the load of self-care and make older adults feel more secure.47
Smart insulin pens are connected insulin delivery devices that can track insulin dosing through a smart phone app.48 Although some older adults with cognitive decline may encounter challenges when operating insulin pens, particularly when it comes to changing cartridges, Bluetooth-enabled pens can be used to track missed or extra doses. The tracking provided by the devices is useful in patients with cognitive impairment, as it can be used by caregivers, whether aids, nurses, or family members, to remind patients to take their insulin or eat meals on time.47 Indeed, in a small cross-sectional study performed in both younger (≤35 years) and older (≥65 years) adults with diabetes on two or more insulin injections per day, the use of Bluetooth-enabled insulin pens was shown to accurately detect any divergence from insulin prescriptions.49 Further dedicated studies evaluating smart insulin pens in the population of older adults with T2DM are warranted.
Insulin pumps deliver a continuous supply of insulin throughout the day. The majority of insulin pumps use tubing to deliver insulin through a cannula, whereas some pumps, known as patch pumps, attach directly to the skin without the need for tubing. The use of insulin pumps offers convenience to older adults by eliminating the need to carry multiple insulin pens.47 Supporting the use of such devices in selected patients, the OpT2mise RCT compared insulin pump treatment with multiple daily injections of insulin in individuals with T2DM aged up to 75 years who had not responded to a basal-bolus regimen after active insulin titration.50 Insulin pump treatment notably improved glycemic control independently of diabetes duration, body mass index, and cognitive ability, and was associated with a low hypoglycemia risk.50 Despite these positive results, the use of insulin pumps in older adults, but also in younger adults, may be limited by the inability to remember the multiple steps to change tubing and cannula, difficulty to see insulin pump screens due to their small size and the lack of magnification abilities, and the risk of diabetic ketoacidosis in insulin-deficient patients.47
Overall, if the use of an advanced insulin delivery system is validated in an older patient, periodic assessment of cognitive and functional status, as well as overall health, should be performed to continually re-evaluate the benefits and potential burden of this technology. Guidelines for the use of insulin administration systems in older adults with T2DM are still needed, along with educational material for the clinicians caring for them and the caregivers helping them at home.47 There is also a need for solid research data assessing the efficacy and safety of insulin delivery systems in the population of older patients with diabetes.
T2DM Treatment Monitoring in Older Adults
Although there are limited clinical data to guide T2DM treatment monitoring in older adults, it should be tailored to individual glycemic targets, functional status, and patient characteristics (eg, vision, hearing, manual dexterity, comorbidities, and social and financial situation). Guidelines by the Endocrine Society recommend that in patients with diabetes aged ≥65 years who are treated with insulin, frequent SMBG and/or CGM should be performed in addition to HbA1c measurement (Figure 4).15
Even though HbA1c measurement serves as an appropriate means to assess overall glycemic control, it does not aid in identifying hypoglycemia.15 However, this is particularly important in the aging population, since both hypoglycemia and hyperglycemia (dysglycemia) may increase the risk of frailty in older people with T2DM.51 Older adults with T2DM also display distinct glucose patterns, characterized by a relatively higher incidence of postprandial hyperglycemia compared to fasting hyperglycemia.15 CGM can be a practical tool to safely assess glycemic profiles and decreasing glucose variability in older adults, especially for those on multiple daily doses of insulin.5,15 In contrast to SMBG that is a static measurement providing an instantaneous glucose level, CGM is a dynamic measurement that not only provides real-time glucose values but also offers information about fluctuations and trends in glucose levels over time.52
The benefits of CGM have been demonstrated in both older adults with type 1 and type 2 diabetes. In the WISDM RCT including adults aged ≥60 years with type 1 diabetes, CGM for 6 months compared with standard blood glucose monitoring was associated with a reduced time spent in hypoglycemia by approximately 27 minutes per day.53 Similarly, CGM proved to be useful for investigating hypoglycemia and glycemic variability in the IMPERIUM study performed in moderately ill and/or frail older (≥65 years) individuals with inadequately controlled T2DM.54 In the MOBILE RCT comparing CGM to SMBG in adults with T2DM treated with basal insulin without prandial or bolus insulin, improvement in key glycemic outcomes, including time-in-range and less time in hyperglycemia, was observed with CGM compared with SMBG in participants ≥65 years old (mean ± standard deviation age of 69±4 years), and was comparable with the treatment outcomes observed in younger participants.55
A recent analysis of a heterogeneous non-critically ill inpatient population with diabetes on insulin therapy demonstrated good overall accuracy of CGM, even in the presence of comorbidities such as impaired renal function, cardiovascular and respiratory illnesses, and mild-to-moderate anemia.56 Nonetheless, CGM accuracy decreased with hypoglycemia (<70 mg/dL or 3.9 mmol/L) and severe anemia (hemoglobin <7.0 g/dL or 4.3 mmol/L).56 Other factors that can potentially compromise the accuracy of CGM measurements include skin edema that might dilute interstitial fluid glucose, vasoconstrictor drugs that might decrease blood flow to the skin and result in a slower shift of glucose from capillaries to the interstitial fluid compartment, and hypotension that could lead to peripheral vasoconstriction and hypoxemia.57
To date, despite recent guidelines for the use of technology in diabetes, the optimal utilization of CGM in older adults is still not well-established, highlighting the importance of the development of dedicated guidelines for CGM in the older patient population. Moreover, CGM remains underused in routine clinical practice, as a consequence of logistical, educational, and financial barriers.58 Education and behavioral support may equip caregivers with skills to support the use of CGM in older adults with T2DM.58
In the context of end-of-life care, the decision regarding the discontinuation or the continuation of glucose monitoring, as well as the choice between CGM devices and fingerstick blood glucose monitoring, must be tailored to individual preferences. It is also important to consider patient and caregiver preferences, the stage of end-of-life care, and the level of experience of the medical and nursing staff.59 The IDF specifies that the threshold for continuing glucose monitoring in patients receiving end-of-life care should be high and only considered under unique circumstances (eg, beginning of corticosteroids known for their hyperglycemic effects) and when the risk of hypoglycemia is specifically high (eg, in case of important nutritional problems).14 In functionally dependent patients with intermediate health (eg, with multiple comorbidities), the use of CGM is useful to prevent unrecognized hypoglycemia episodes, especially in individuals on insulin regimen or those who have difficulties with fingerstick monitoring.47 In fit patients with good health, glycemic monitoring should be considered as an optional component of self-management, especially when there is an agreed purpose for testing (eg, if intensification of pharmacotherapy, modified eating patterns, use of medications causing hypoglycemia such as insulin or insulin secretagogues).14
During the COVID-19 pandemic, telemedicine has greatly expanded, and the role of CGM in relaying patient data to healthcare providers has been instrumental.60 Indeed, there is now an availability to use CGM transmitters connected to glucose sensors transmitting glucose values through Bluetooth connectivity to selected users who can remotely monitor glucose levels on compatible smart devices. This data-sharing feature can be especially helpful to caregivers of frail older adults who struggle with cognitive decline. It also reduces the workload of healthcare providers and adds to quality of diabetes care, especially when patient access to diabetes care facilities is limited.47,61 Importantly, the stereotype that older adults are not technology-savvy should be avoided, with assessment on an individual basis of the use of CGM and other telemedicine technologies in older adults. Further longitudinal research data on both short-term and long-term outcomes of older adults with diabetes receiving care through telemedicine is needed.
Multidisciplinary Care in Older Adults
A multidisciplinary patient-centered approach, involving an endocrinologist or diabetes care specialist, a geriatrician, a general practitioner, a dietician or nutritionist, a pharmacist, a physical therapist, and in some cases a home nurse, is important to guarantee high-quality diabetes care and to improve older patients’ quality of life.62 Effective multidisciplinary teams are also core to the assessment of geriatric syndromes in older adults with T2DM, given that geriatric syndromes are multifactorial and do not fit into distinct disease categories.63 Moreover, geriatric syndromes such as frailty may be difficult to diagnose in clinical practice, particularly since the trajectories and transitional states of frailty in older adults with diabetes have yet to be firmly established.42,64,65 Acknowledging that frailty is a multifactor clinical syndrome, multidisciplinary teams can facilitate the implementation of frailty screening in an integrated management approach.66 Management of geriatric syndromes in older adults with diabetes should be especially directed by the Comprehensive Geriatric Assessment (CGA), a multidimensional, interdisciplinary evaluation used to assess older patients’ medical, psychological, physical functions and social status.67
It is particularly crucial that healthcare providers engage in in-depth and open discussions with their patients, when evaluating their personalized care, to increase the likelihood of effectively integrating glucose-lowering treatment into the patient’s daily routine.36 Unfortunately, in a qualitative study of 32 adults aged ≥60 years with T2DM and other chronic conditions, older adults perceived a general unwillingness from their healthcare providers to treat their multiple-health conditions and to accommodate to their individual care preferences. The limited support and empathy from the healthcare providers may have been attributed to age discrimination.68 Patient-reported outcome measures can be a useful tool to inform shared decision-making, by gaining insight into a patient’s experience of glucose-lowering treatment and its burden.36
To date, there is a paucity of studies evaluating the effectiveness of a multidisciplinary care model for older adults with T2DM. A five-year, prospective, cohort study, performed in 121,584 Chinese primary care patients with T2DM, evaluated a multidisciplinary, risk-stratified, chronic disease model care and found that compared with usual care, multidisciplinary care led to significantly greater reductions in ASCVD risk by 56.6%, microvascular complications by 11.9%, mortality by 66.1%, specialist attendance by 35.0%, emergency attendance by 41.2%, and hospitalizations by 58.5%.69 Geriatric day hospitals (GDHs), usually englobing multidisciplinary teams of physicians, nurses, dieticians, psychologists, physiotherapists, occupational therapists, and social workers, can also allow for a collaboration between the inpatient and community care for older adults with diabetes. Indeed, a prospective cohort study performed in 469 outpatients admitted to a Canadian GDH demonstrated short- and long-term effectiveness of GDH in helping patients reach their individualized outcome targets.70 Overall, multidisciplinary care models can improve T2DM treatment outcomes and help prevent or reduce T2DM complications. However, in order to optimize their effectiveness, multidisciplinary teams require robust team structures, dependable communication methods, and high patient engagement.71
Summary
Given that older adults with diabetes are a highly heterogeneous patient population with unique care needs, their categorization into different patient profiles (ie, good health, intermediate health, poor health) may aid in clinical decision-making when establishing glycemic goals and treatment strategies. Further granularity in assessing each patient profile through interdisciplinary collaboration may also add precision to therapeutic and monitoring decisions. Thus, management of T2DM in older adults should always be tailored, based on comprehensive geriatric and environmental assessments that include ASCVD risk, functional status, hypoglycemic risk and awareness, renal function, body weight, presence of depression and/or cognitive impairment, history of urinary incontinence and/or falls, duration of diabetes, patient preferences, and available care facilities. There are still several areas that deserve further research in older adults with T2DM, including the efficacy and safety of CGM and automated insulin delivery systems, the switch to once-weekly insulin, and the use of supported telemedicine and remote blood glucose monitoring in the oldest-old (≥85–90 years) who particularly require the assistance of others.
Acknowledgments
The authors thank Thomas Rohban, MD, and Magalie El Hajj, PharmD, of Partner 4 Health (Paris, France) for providing medical writing support in accordance with Good Publication Practice guidelines.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the review conception, data review, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This article was funded by Novo Nordisk SAS, France.
Disclosure
Jean Doucet has received occasional fees, either personally or institutionally, for the activities of speaking or scientific advising from Novo Nordisk, Eli Lilly, and Boehringer Ingelheim. Pierre Gourdy has received occasional fees, either personally or institutionally, for the activities of speaking, scientific advising, or clinical research from Abbott, Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Gilead, GlaxoSmithKline, Merck Sharp & Dohme, Novo Nordisk, Organon, Pfizer, and Sanofi. Laurent Meyer has received occasional fees, either personally or institutionally, for the activities of speaking, scientific advising, or clinical research from Abbott, AstraZeneca, Boehringer Ingelheim, Dexcom, Eli Lilly, Novo Nordisk, Merck Sharp & Dohme, Pfizer, Servier, Medtronic, and Isis Diabète. Nabil Benabdelmoumene has received occasional fees, either personally or institutionally, from AstraZeneca, Novo Nordisk, Boehringer Ingelheim, and Eli Lilly. Isabelle Bourdel-Marchasson has received occasional fees from Novo-Nordisk for activities of speaking or scientific advising. The authors report no other conflicts of interest in this work.
References
1. Orimo H, Ito H, Suzuki T, Araki A, Hosoi T, Sawabe M. Reviewing the definition of “elderly”. Geriatr Gerontol Int. 2006;6(3):149–158. doi:10.1111/j.1447-0594.2006.00341.x
2. Singh S, Bajorek B. Defining ‘elderly’ in clinical practice guidelines for pharmacotherapy. Pharm Pract. 2014;12(4):489. doi:10.4321/s1886-36552014000400007
3. Ouchi Y, Rakugi H, Arai H, et al. Redefining the elderly as aged 75 years and older: proposal from the joint committee of Japan gerontological Society and the Japan Geriatrics Society. Geriatr Gerontol Int. 2017;17(7):1045–1047. doi:10.1111/ggi.13118
4. Lee SB, Oh JH, Park JH, Choi SP, Wee JH. Differences in youngest-old, middle-old, and oldest-old patients who visit the emergency department. Clin Exp Emerg Med. 2018;5(4):249–255. doi:10.15441/ceem.17.261
5. ElSayed NA, Aleppo G, Aroda VR, et al. 13. Older adults: standards of care in diabetes-2023. Diabetes Care. 2023;46(Suppl 1):S216–229. doi:10.2337/dc23-S013
6. Khan MAB, Hashim MJ, King JK, Govender RD, Mustafa H, Al Kaabi J. Epidemiology of type 2 diabetes - Global burden of disease and forecasted trends. J Epidemiol Glob Health. 2020;10(1):107–111. doi:10.2991/jegh.k.191028.001
7. Cortez BN, Bahour N, Aguayo-Mazzucato C. Biological age in diabetes and precision medicine. Aging. 2022;14(11):4622–4623. doi:10.18632/aging.204123
8. Bradley D, Hsueh W. Type 2 diabetes in the elderly: challenges in a unique patient population. J Geriatr Med Gerontol. 2016;2(2):14. doi:10.23937/2469-5858/1510014
9. Lowsky DJ, Olshansky SJ, Bhattacharya J, Goldman DP. Heterogeneity in healthy aging. J Gerontol a Biol Sci Med Sci. 2014;69(6):640–649. doi:10.1093/gerona/glt162
10. Longo M, Bellastella G, Maiorino MI, Meier JJ, Esposito K, Giugliano D. Diabetes and aging: from treatment goals to pharmacologic therapy. Front Endocrinol. 2019;10:45. doi:10.3389/fendo.2019.00045
11. Ahlqvist E, Storm P, Käräjämäki A, et al. Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol. 2018;6(5):361–369. doi:10.1016/S2213-8587(18)30051-2
12. Sanz-Cánovas J, López-Sampalo A, Cobos-Palacios L, et al. Management of type 2 diabetes mellitus in elderly patients with frailty and/or sarcopenia. Int J Environ Res Public Health. 2022;19(14):8677. doi:10.3390/ijerph19148677
13. Menassa M, Stronks K, Khatmi F, et al. Concepts and definitions of healthy ageing: a systematic review and synthesis of theoretical models. EClinicalMedicine. 2023;56:101821. doi:10.1016/j.eclinm.2022.101821
14. International Diabetes Federation (IDF). IDF global guideline for managing older people with type 2 diabetes; 2017. Available from: https://www.idf.org/e-library/guidelines/78-global-guideline-for-managing-older-people-with-type-2-diabetes.html.
15. LeRoith D, Biessels GJ, Braithwaite SS, et al. Treatment of diabetes in older adults: an Endocrine Society* clinical practice guideline. J Clin Endocrinol Metab. 2019;104(5):1520–1574. doi:10.1210/jc.2019-00198
16. Huang ES. Management of diabetes mellitus in older people with comorbidities. BMJ. 2016;353:i2200. doi:10.1136/bmj.i2200
17. Corriere M, Rooparinesingh N, Kalyani RR. Epidemiology of diabetes and diabetes complications in the elderly: an emerging public health burden. Curr Diab Rep. 2013;13(6):805–813. doi:10.1007/s11892-013-0425-5
18. An J, Nichols GA, Qian L, et al. Prevalence and incidence of microvascular and macrovascular complications over 15 years among patients with incident type 2 diabetes. BMJ Open Diabetes Res Care. 2021;9(1):e001847. doi:10.1136/bmjdrc-2020-001847
19. Doucet J, Verny C, Balkau B, Scheen AJ, Bauduceau B. Haemoglobin A1c and 5-year all-cause mortality in French type 2 diabetic patients aged 70 years and older: the GERODIAB observational cohort. Diabetes Metab. 2018;44(6):465–472. doi:10.1016/j.diabet.2018.05.003
20. Anyanwagu U, Mamza J, Donnelly R, Idris I. Relationship between HbA1c and all-cause mortality in older patients with insulin-treated type 2 diabetes: results of a large UK Cohort Study. Age Ageing. 2019;48(2):235–240. doi:10.1093/ageing/afy178
21. Idrees T, Castro-Revoredo IA, Migdal AL, Moreno EM, Umpierrez GE. Update on the management of diabetes in long-term care facilities. BMJ Open Diabetes Res Care. 2022;10(4):e002705. doi:10.1136/bmjdrc-2021-002705
22. Pilla SJ, Jalalzai R, Tang O, et al. A national physician survey of deintensifying diabetes medications for older adults with type 2 diabetes. Diabetes Care. 2023;46(6):1164–1168. doi:10.2337/dc22-2146
23. Lipska KJ, Ross JS, Miao Y, Shah ND, Lee SJ, Steinman MA. Potential overtreatment of diabetes mellitus in older adults with tight glycemic control. JAMA Intern Med. 2015;175(3):356–362. doi:10.1001/jamainternmed.2014.7345
24. Verma M, Paneri S, Badi P, Raman PG. Effect of increasing duration of diabetes mellitus type 2 on glycated hemoglobin and insulin sensitivity. Indian J Clin Biochem. 2006;21(1):142–146. doi:10.1007/BF02913083
25. Yotsapon T, Sirinate K, Ekgaluck W, et al. Clinical characteristics and outcomes of the oldest old people with type 2 diabetes - perspective from a tertiary diabetes center in Thailand. BMC Endocr Disord. 2016;16(1):30. doi:10.1186/s12902-016-0115-9
26. Wing RR, Bolin P. Look AHEAD Research Group. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369(2):145–154.
27. Pahor M, Guralnik JM, Ambrosius WT, et al. Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA. 2014;311(23):2387–2396. doi:10.1001/jama.2014.5616
28. Dziegielewska-Gesiak S. Metabolic syndrome in an aging society - role of oxidant-antioxidant imbalance and inflammation markers in disentangling atherosclerosis. Clin Interv Aging. 2021;16:1057–1070. doi:10.2147/CIA.S306982
29. Tamura Y, Omura T, Toyoshima K, Araki A. Nutrition management in older adults with diabetes: a review on the importance of shifting prevention strategies from metabolic syndrome to frailty. Nutrients. 2020;12(11):3367. doi:10.3390/nu12113367
30. Farabi SS, Carley DW, Smith D, Quinn L. Impact of exercise on diurnal and nocturnal markers of glycaemic variability and oxidative stress in obese individuals with type 2 diabetes or impaired glucose tolerance. Diab Vasc Dis Res. 2015;12(5):381–385. doi:10.1177/1479164115579003
31. Yakaryılmaz FD, Öztürk ZA. Treatment of type 2 diabetes mellitus in the elderly. World J Diabetes. 2017;8(6):278–285. doi:10.4239/wjd.v8.i6.278
32. Strain WD, Hope SV, Green A, Kar P, Valabhji J, Sinclair AJ. Type 2 diabetes mellitus in older people: a brief statement of key principles of modern day management including the assessment of frailty. A national collaborative stakeholder initiative. Diabet Med. 2018;35(7):838–845. doi:10.1111/dme.13644
33. Bellary S, Kyrou I, Brown JE, Bailey CJ. Type 2 diabetes mellitus in older adults: clinical considerations and management. Nat Rev Endocrinol. 2021;17(9):534–548. doi:10.1038/s41574-021-00512-2
34. Seidu S, Kunutsor SK, Topsever P, Hambling CE, Cos FX, Khunti K. Deintensification in older patients with type 2 diabetes: a systematic review of approaches, rates and outcomes. Diabetes Obes Metab. 2019;21(7):1668–1679. doi:10.1111/dom.13724
35. Munshi MN, Slyne C, Segal AR, Saul N, Lyons C, Weinger K. Simplification of insulin regimen in older adults and risk of hypoglycemia. JAMA Intern Med. 2016;176(7):1023–1025. doi:10.1001/jamainternmed.2016.2288
36. Jude EB, Malecki MT, Gomez Huelgas R, et al. Expert panel guidance and narrative review of treatment simplification of complex insulin regimens to improve outcomes in type 2 diabetes. Diabetes Ther. 2022;13(4):619–634. doi:10.1007/s13300-022-01222-2
37. Karagiannis T, Tsapas A, Athanasiadou E, et al. GLP-1 receptor agonists and SGLT2 inhibitors for older people with type 2 diabetes: a systematic review and meta-analysis. Diabetes Res Clin Pract. 2021;174:108737. doi:10.1016/j.diabres.2021.108737
38. Cheng D, Yang S, Zhao X, Wang G. The role of glucagon-like peptide-1 receptor agonists (GLP-1 RA) in diabetes-related neurodegenerative diseases. Drug Des Devel Ther. 2022;16:665–684. doi:10.2147/DDDT.S348055
39. Seidu S, Cos X, Brunton S, et al. 2022 update to the position statement by primary care diabetes Europe: a disease state approach to the pharmacological management of type 2 diabetes in primary care. Prim Care Diabetes. 2022;16(2):223–244. doi:10.1016/j.pcd.2022.02.002
40. Sinclair AJ, Girling AJ, Bayer AJ. Cognitive dysfunction in older subjects with diabetes mellitus: impact on diabetes self-management and use of care services. All Wales Research into Elderly (AWARE) Study. Diabetes Res Clin Pract. 2000;50(3):203–212. doi:10.1016/S0168-8227(00)00195-9
41. Papa G, Fedele V, Chiavetta A, et al. Therapeutic options for elderly diabetic subjects: open label, randomized clinical trial of insulin glargine added to oral antidiabetic drugs versus increased dosage of oral antidiabetic drugs. Acta Diabetol. 2008;45(1):53–59. doi:10.1007/s00592-007-0023-6
42. Abd.Ghafar MZA, O’Donovan M, Sezgin D, et al. Frailty and diabetes in older adults: overview of current controversies and challenges in clinical practice. Front Clin Diabetes Healthc. 2022;3:895313. doi:10.3389/fcdhc.2022.895313
43. Abdelhafiz AH, Pennells D, Sinclair AJ. A modern approach to glucose-lowering therapy in frail older people with type 2 diabetes mellitus. Expert Rev Endocrinol Metab. 2022;17(2):95–98. doi:10.1080/17446651.2022.2044304
44. Bradley MC, Chillarige Y, Lee H, et al. Severe hypoglycemia risk with long-acting insulin analogs vs neutral protamine Hagedorn insulin. JAMA Intern Med. 2021;181(5):598–607. doi:10.1001/jamainternmed.2020.9176
45. Munshi MN, Pandya N, Umpierrez GE, DiGenio A, Zhou R, Riddle MC. Contributions of basal and prandial hyperglycemia to total hyperglycemia in older and younger adults with type 2 diabetes mellitus. J Am Geriatr Soc. 2013;61(4):535–541. doi:10.1111/jgs.12167
46. Rosenstock J, Bajaj HS, Janež A, et al. Once-weekly insulin for type 2 diabetes without previous insulin treatment. N Engl J Med. 2020;383(22):2107–2116. doi:10.1056/NEJMoa2022474
47. Toschi E, Munshi MN. Benefits and challenges of diabetes technology use in older adults. Endocrinol Metab Clin North Am. 2020;49(1):57–67. doi:10.1016/j.ecl.2019.10.001
48. Munshi MN, Meneilly GS, Rodríguez-Mañas L, et al. Diabetes in ageing: pathways for developing the evidence base for clinical guidance. Lancet Diabetes Endocrinol. 2020;8(10):855–867. doi:10.1016/S2213-8587(20)30230-8
49. Munshi MN, Slyne C, Greenberg JM, et al. Nonadherence to insulin therapy detected by bluetooth-enabled pen cap is associated with poor glycemic control. Diabetes Care. 2019;42(6):1129–1131. doi:10.2337/dc18-1631
50. Reznik Y, Cohen O, Aronson R, et al. Insulin pump treatment compared with multiple daily injections for treatment of type 2 diabetes (OpT2mise): a randomised open-label controlled trial. Lancet. 2014;384(9950):1265–1272. doi:10.1016/S0140-6736(14)61037-0
51. Abdelhafiz AH, Sinclair AJ. Metabolic phenotypes explain the relationship between dysglycaemia and frailty in older people with type 2 diabetes. J Diabetes Complications. 2022;36(4):108144. doi:10.1016/j.jdiacomp.2022.108144
52. Heinemann L. Continuous glucose monitoring (CGM) or blood glucose monitoring (BGM): interactions and implications. J Diabetes Sci Technol. 2018;12(4):873–879. doi:10.1177/1932296818768834
53. Pratley RE, Kanapka LG, Rickels MR, et al. Effect of continuous glucose monitoring on hypoglycemia in older adults with type 1 diabetes: a randomized clinical trial. JAMA. 2020;323(23):2397–2406. doi:10.1001/jama.2020.6928
54. Pratley RE, Rosenstock J, Heller SR, et al. Reduced glucose variability with glucose-dependent versus glucose-independent therapies despite similar glucose control and hypoglycemia rates in a randomized, controlled study of older patients with type 2 diabetes mellitus. J Diabetes Sci Technol. 2018;12(6):1184–1191. doi:10.1177/1932296818776993
55. Bao S, Bailey R, Calhoun P, Beck RW. Effectiveness of continuous glucose monitoring in older adults with type 2 diabetes treated with basal insulin. Diabetes Technol Ther. 2022;24(5):299–306. doi:10.1089/dia.2021.0494
56. Davis GM, Spanakis EK, Migdal AL, et al. Accuracy of Dexcom G6 continuous glucose monitoring in non-critically ill hospitalized patients with diabetes. Diabetes Care. 2021;44(7):1641–1646. doi:10.2337/dc20-2856
57. Klonoff DC, Ahn D, Drincic A. Continuous glucose monitoring: a review of the technology and clinical use. Diabetes Res Clin Pract. 2017;133:178–192. doi:10.1016/j.diabres.2017.08.005
58. Prasad-Reddy L, Godina A, Chetty A, Isaacs D. Use of continuous glucose monitoring in older adults: a review of benefits, challenges and future directions. touchREV Endocrinol. 2022;18(2):116–121. doi:10.17925/EE.2022.18.2.116
59. Wang R, Foskey R, Barmanray R, Le B, Fourlanos S. End-of-life care requires caution with use of continuous glucose monitoring. J Palliat Med. 2022;25(3):516–518. doi:10.1089/jpm.2021.0271
60. Jackson MA, Ahmann A, Shah VN. Type 2 diabetes and the use of real-time continuous glucose monitoring. Diabetes Technol Ther. 2021;23(S1):S27–34. doi:10.1089/dia.2021.0007
61. Shehav-Zaltzman G, Segal G, Konvalina N, Tirosh A. Remote glucose monitoring of hospitalized, quarantined patients with diabetes and COVID-19. Diabetes Care. 2020;43(7):e75–76. doi:10.2337/dc20-0696
62. Garcia SP, Madalosso MM, Bottino LG, et al. Optimization of care for adult outpatients with type 2 diabetes through the diabetes self-management multidisciplinary program: a randomized clinical trial. Can J Diabetes. 2022;46(5):449–456.e443. doi:10.1016/j.jcjd.2022.01.006
63. Inouye SK, Studenski S, Tinetti ME, Kuchel GA. Geriatric syndromes: clinical, research, and policy implications of a core geriatric concept. J Am Geriatr Soc. 2007;55(5):780–791. doi:10.1111/j.1532-5415.2007.01156.x
64. Bruyère O, Buckinx F, Beaudart C, et al. How clinical practitioners assess frailty in their daily practice: an international survey. Aging Clin Exp Res. 2017;29(5):905–912. doi:10.1007/s40520-017-0806-8
65. Liu X, Le MK, Lim AYC, et al. Perspectives on frailty screening, management and its implementation among acute care providers in Singapore: a qualitative study. BMC Geriatr. 2022;22(1):58. doi:10.1186/s12877-021-02686-w
66. Nan J, Duan Y, Wu S, et al. Perspectives of older adults, caregivers, healthcare providers on frailty screening in primary care: a systematic review and qualitative meta-synthesis. BMC Geriatr. 2022;22(1):482. doi:10.1186/s12877-022-03173-6
67. Choi JY, Lee JY, Shin J, et al. COMPrehensive geriatric Assessment and multidisciplinary team intervention for hospitalised older adults (COMPASS): a protocol of pragmatic trials within a cohort. BMJ Open. 2022;12(8):e060913. doi:10.1136/bmjopen-2022-060913
68. Beverly EA, Wray LA, Chiu CJ, LaCoe CL. Older adults’ perceived challenges with health care providers treating their type 2 diabetes and comorbid conditions. Clin Diabetes. 2014;32(1):12–17. doi:10.2337/diaclin.32.1.12
69. Wan EYF, Fung CSC, Jiao FF, et al. Five-year effectiveness of the multidisciplinary Risk Assessment and Management Programme-Diabetes Mellitus (RAMP-DM) on diabetes-related complications and health service uses-a population-based and propensity-matched cohort study. Diabetes Care. 2018;41(1):49–59. doi:10.2337/dc17-0426
70. Moorhouse P, Theou O, Fay S, McMillan M, Moffatt H, Rockwood K. Treatment in a geriatric day hospital improve individualized outcome measures using goal attainment scaling. BMC Geriatr. 2017;17(1):9. doi:10.1186/s12877-016-0397-9
71. Tan HQM, Chin YH, Ng CH, et al. Multidisciplinary team approach to diabetes. An outlook on providers’ and patients’ perspectives. Prim Care Diabetes. 2020;14(5):545–551. doi:10.1016/j.pcd.2020.05.012
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


