Back to Journals » Journal of Asthma and Allergy » Volume 16
Management of Chronic Rhinosinusitis with Nasal Polyps (CRSwNP) in the Pan-Arab Region: Consensus Recommendations from a Multidisciplinary Expert Working Group
Authors Marglani O, Al Abri R , Al Ahmad M, Alsaleh S , Abuzakouk M, Kamel R
Received 22 March 2023
Accepted for publication 14 September 2023
Published 29 September 2023 Volume 2023:16 Pages 1055—1063
DOI https://doi.org/10.2147/JAA.S413610
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Luis Garcia-Marcos
Osama Marglani,1,2 Rashid Al Abri,3 Mona Al Ahmad,4 Saad Alsaleh,5 Mohamed Abuzakouk,6 Reda Kamel7
1Department of Ophthalmology, and Otolaryngology, Head and Neck Surgery, Faculty of Medicine, Umm Al-Qura University, Makkah, Saudi Arabia; 2Department of Surgery, King Faisal Specialist Hospital & Research Center, Jeddah, Saudi Arabia; 3Sultan Qaboos University Hospital, Muscat, Oman; 4Al-Rashed Allergy Centre, Kuwait, State of Kuwait; 5Rhinology and Endoscopic Skull Base Surgery Division, Otolaryngology – Head and Neck Surgery Department, King Saud University, Riyadh, Saudi Arabia; 6Department of Allergy and Immunology, Respiratory Institute, Cleveland Clinic Abu Dhabi, Abu Dhabi, United Arab Emirates; 7Department of Otorhinolaryngology, Cairo University, Cairo, Egypt
Correspondence: Osama Marglani, Department of Surgery, King Faisal Specialist Hospital & Research Center, Jeddah, 23431, Saudi Arabia, Email [email protected]
Abstract: Chronic rhinosinusitis with nasal polyps (CRSwNP) is a chronic and often debilitating inflammatory condition of the nasal and paranasal tissues. An expert panel of specialists from the Gulf region (the Kingdom of Saudi Arabia, Kuwait, Oman and the United Arab Emirates) and from Egypt gathered to evaluate existing guidance and develop regional guidance on the management of CRSwNP through a consensus approach. The present article presents the main observations and recommendations from this panel. CRSwNP diagnosis requires the presence of bilateral, endoscopically visualized polyps in the middle meatus (via nasal endoscopy or CT). In most patients, CRSwNP is mediated through predominantly type 2 inflammatory processes and is often observed in patients with asthma and other allergic disease. While many patients respond to medical treatment (principally topical irrigation and intranasal corticosteroids, and adjunctive short-term use of systemic corticosteroids), clinical management of CRSwNP is challenging, and a multidisciplinary approach for complete evaluation and treatment is recommended. Patients with more severe/uncontrolled disease (despite adequate medical therapies) require a complete endoscopic sinus surgery (ESS), although outcomes can be unsatisfactory, and further revision surgery is common. Biological therapies targeting underlying inflammatory processes offer additional, effective treatment options for those patients with persistent symptoms despite complete ESS, and also in those patients where surgery may be contraindicated.
Keywords: chronic rhinosinusitis with nasal polyposis, CRSwNP, type 2 inflammation, biologics
Introduction
Chronic rhinosinusitis (CRS) is a common and often debilitating condition, with a substantial impact upon patients’ quality of life (QoL).1 CRS can be characterized into two broad phenotypes on the basis of the presence or absence of nasal polyps.2,3 Patients with CRS with nasal polyps (CRSwNP) account for up to 20% of all CRS patients.4,5 In CRSwNP, disease is mediated predominantly through type 2 inflammatory processes in the majority of patients,6 and asthma or other inflammatory diseases (such as NSAID/aspirin-exacerbated respiratory disease, or atopic dermatitis) frequently co-exist.7
Clinical management of CRSwNP can be challenging. Conventional treatment approaches include nasal irrigation, topical intranasal (INS) or oral corticosteroids (OCS).2,3 However, a substantial proportion of patients with persistent or uncontrolled disease despite such treatments usually require endoscopic sinus surgery (ESS) to remove disease tissue and restore anatomical patency of the affected sinuses.8,9 Even then, subsequent revision surgery may be required.10,11 More recently, the development and introduction of biologics targeting the underlying type-2 inflammatory processes involved in CRSwNP provides additional treatment options for those patients with persistent disease despite conventional therapy.
Clinical decision-making is helped by comprehensive evidence-based specialist guidelines. Important guidance includes the European Position Paper on Rhinosinusitis and Nasal Polyps (EPOS 2020),2,3 the consensus-based recommendations from the European Forum for Research and Education in Allergy and Airway Diseases (EUFOREA),12,13 and the International Consensus Statement on Allergy and Rhinology: Rhinosinusitis (ICAR-RS-2021).14 Although clearly useful, such guidance may not fully reflect treatment patterns and patient needs that exist at a regional level, including countries within the pan-Arab Region. Development of guidance from a regional perspective provides additional value and may be more aligned with clinical resources and treatment patterns, physician experience, and patient needs and preferences.
Previous initiatives that focus on such a regional perspective for Gulf Cooperation Council (GCC) countries have been published. This includes a position paper from the Saudi Otorhinolaryngology Society, principally endorsing the recommendations made by EPOS 2020 regarding use of biologics in CRSwNP.15 More recently, guidance on use of biologics in CRSwNP from an expert panel of otorhinolaryngology (ORL) and respiratory physicians from the broader Gulf region has also recently been published.16 Building upon these previous initiatives, an expert panel was convened to re-evaluate existing guidelines such as EPOS 2020 and their application to the current management of CRSwNP within the pan-Arab region. The present article presents the chief observations and recommendations from this panel.
Methodology
The multidisciplinary expert panel comprised specialists in ORL and respiratory allergy from the Kingdom of Saudi Arabia (KSA [SA-S, OM]), Kuwait (MA-A), Oman (RA-A), the United Arab Emirates (MA), and Egypt (RK). Panel participants were selected on the basis of publication record, clinical experience and expertise in treating CRSwNP, and participation in similar guidance activities. This consensus exercise was conducted through review of publicly available literature and did not involve specific human participants or any identifiable data. As such, institutional review board approval and informed consent were not required.
Two online meetings were held in October and December 2021. The first meeting comprised a general discussion on CRSwNP disease assessment and treatment patterns within the pan-Arab region. The second meeting focused on current Guidelines for the management of CRSwNP, chiefly those reported within the most recent EPOS 2020 guidelines,2,3 with discussion on the utility of these for CRSwNP management within the pan-Arab region and whether some modifications should be made to address loco-regional needs. From these meetings, a number of discussion points and recommendation proposals were then developed, with these then reviewed by the panel using an interactive online platform (Within3; https://www.within3.com). This platform provided the opportunity for communication and dialog within the panel to explain their decision-making. In this process (conducted between January 31 and February 14, 2022), each panel member indicated their agreement or disagreement to each discussion point or proposed statement in turn, and indicated any necessary changes, and provided their reasoning when applicable. The aim of this process was not to generate specific formal recommendation statements (or to report on any consensus agreement values) nor to evaluate and compare existing practices within pan-Arab countries with those in other regions. Our overall aims and objectives were to present our views as to the best approach to management of CRSwNP within pan-Arab countries, within the framework of existing EPOS 2020 guidance. Our hope is that this can inform and assist clinicians within specialized and primary care in optimizing outcomes for patients with CRSwNP in the region.
All discussion points, specific feedback, and panelist communication were collected, and presented as a structured series of explanatory text, recommendations (and supportive rationale). This formed the basis of an early draft of the present manuscript. All panelists then reviewed and commented on successive manuscript drafts, with approval of the final version considered as final agreement for the present guidance on the management of CRSwNP in the pan-Arab region.
Disease Definitions and Diagnostic Approach
Diagnostic criteria for acute rhinosinusitis, CRS and CRSwNP are based upon clinical symptoms and evidence of sinonasal mucosal disease. For acute rhinosinusitis, diagnosis requires the presence of ≥2 symptoms, one of which should be either nasal blockage, obstruction, congestion, or discharge, and either facial pain/pressure or headache, and/or reduction or loss of smell.2,3 In addition, mucosal disease (presence of polyps, mucopurulent discharge and/or edema or mucosal obstruction within the ostiomeatal complex or sinuses) must be confirmed via endoscopy or CT.2,3 Early referral for specialist evaluation and management of CRS (and CRSwNP) is essential. Although in routine clinical practice, acute rhinosinusitis may be diagnosed on clinical grounds alone, the importance of endoscopic/CT confirmation of CRS (and for CRSwNP) for a more complete disease classification and treatment planning must be emphasized.
In EPOS 2020, CRS is defined as rhinosinusitis lasting for ≥12 weeks.2,3 The cardinal symptoms of CRS are similar to acute disease, as described above.2,3,17,18 The presence of red-flag alarm signs or symptoms (eg, visual upset, severe headache, focal neurological signs) may indicate an important alternative etiology, and requires an immediate specialist referral.2,3 CRSwNP diagnosis requires the presence of bilateral, endoscopically visualized polyps in the middle meatus.2,3 If polyps are absent, patients are considered to have chronic rhinosinusitis without nasal polyps (CRSsNP). When endoscopy or CT shows only unilateral disease, then alternative diagnoses must be considered.2,3
Visualization of nasal polyps via nasal endoscopy or CT is essential for the confirmatory diagnosis of CRSwNP. Nasal endoscopy and CT each have high diagnostic accuracy,3,19 and choice of initial modality may depend on physician specialty and available resources. Endoscopy is generally preferred by ORL specialists, both in initial assessment and throughout follow-up. However, CT scans offer an alternative modality for the initial diagnosis, especially for physicians in other specialties, and is useful in excluding other differential diagnoses. CT scans are often preferred by ORLs in later stages of clinical management (eg, in surgical evaluations).
For either modality, the extent of sinus disease can be assessed with nasal endoscopy and CT using simple, widely available measures.2,12,13 The endoscopic nasal polyp score (NPS) assesses polyp size and distribution within the middle meatus and nasal cavity on each side, to generate a total NPS ranging from 0 to 8, with higher scores indicating more extensive disease.20 CT evaluation is usually via the Lund-Mackay scoring system (LMS), used as a principal outcome in most clinical studies.21 This grades each of the paranasal sinuses, and also the ostiomeatal complex on the basis of no, partial, or complete opacification (scored as 0, 1 or 2 respectively) to generate a total LMS score ranging from 0 to 24.21
Pathogenesis and Disease Classification
Pathophysiologic mechanisms in CRSwNP involve a complex interaction of host and environmental factors. Specific individual patient profiles are heterogeneous. In EPOS 2020, CRSwNP is characterized on the basis of the predominant underlying inflammatory pattern (or endotype) and predominant clinical phenotype.2,3 Endotypes reflect the predominant inflammatory profile (eg, a type 2 dominant inflammatory signature), and clinical phenotypes reflect the patient’s clinical profile (including comorbid disease).6,22 From a phenotypic perspective, one important pattern is the strong association of co-existing asthma (evident in 30–70% of CRSwNP patients), with asthma severity usually greater in CRSwNP patients compared with other forms of CRS.4,23–25 Other comorbidities such as NSAID/aspirin-exacerbated respiratory disease (N-ERD/AERD), allergic rhinitis and atopic dermatitis are also more prevalent in CRSwNP than in CRSsNP.7 Some data indicate that bronchiectasis may also be a frequent comorbidity in some populations.26
This association with other inflammatory disease may in part be a product of the underlying type 2 inflammatory processes (eosinophilic tissue inflammation mediated by type 2 cytokines, eg, interleukin (IL)-IL-4, IL-5, and IL-13 and local/circulating IgE) evident in these conditions and in the majority (up to 80%) of CRSwNP patients.6 This aspect is of particular importance following the availability of novel biologic therapies for CRSwNP targeting these type 2 inflammatory processes.27,28 It should be noted however, that some patients with CRSwNP do not show a type-2 inflammatory endotype, where the predominant inflammatory profile is either type-1 or type-3).29
In EPOS 2020, formal classification of CRS is firstly on the basis of disease distribution, as either localized (unilateral) and diffuse (bilateral) disease, and then on the basis of the dominant endotype (eg, the presence/absence of type-2 inflammation) and then the clinical phenotype.2,3 In this approach, diffuse CRS disease with a predominantly type-2 inflammatory endotype includes CRSwNP. However, other conditions with predominantly type-2 inflammatory pathways are also included within this category, including allergic fungal rhinosinusitis (AFRS) and central compartment atopic disease (CCAD), a relatively recently described variant of CRS.2 AFRS can be either unilateral or bilateral, and often also associated with a type I hypersensitivity reaction to fungi (and by the presence of non-invasive fungal hyphae within the sinonasal mucosa) and may also respond to biologic therapies.3,30,31 CCAD is strongly associated with inhalant allergen exposure, with polypoidal mucosal changes in the superior nasal septum, middle and/or superior turbinates.2,32 While both AFRS and CCAD are specific clinical conditions, they do fall within the broader category of CRSwNP, although with the caveat that unilateral disease would not strictly conform with the conventional definition of CRSwNP (reserved for bilateral disease).2,3 Although beyond the scope of the present recommendations, management broadly follows that of CRSwNP.3,30,31
Care Pathways and Diagnostic and Clinical Assessment
Multidisciplinary specialist management involving both ORLs and allergists/immunologists/pulmonologists provides the optimal approach for the comprehensive evaluation of patients with CRSwNP and the subsequent decision-making on use of the most appropriate surgical and medical therapeutic strategies.33,34 While cross-specialty referrals are valuable, use of specialist multidisciplinary clinics is preferred, although such referral pathways and patient access to such services may be limited, especially in more rural settings. Broader implementation and access to formal multidisciplinary clinics is encouraged. This is of particular importance given the prevalence of comorbidity and allergic disease in patients with CRSwNP, and clinicians can benefit from cross-specialty collaboration, sharing knowledge and clinical experience to achieve optimal outcomes in patient care. Although data are limited for the prevalence of co-existent asthma and allergy in CRSwNP in pan-Arab countries, one recent study from KSA reports high prevalence of asthma (38.0%), AERD (9.8%) and allergic disease (28.4%).35 Greater reporting of comorbidities and associated allergies in patients with CRSwNP from other pan-Arab countries is welcome.
EPOS 2022 describes a self-care element to CRS (where patients may receive pharmacist advice and over the counter symptomatic relief medication), and then management within primary care, with specialist referral if CRS symptoms persist after 6–12 weeks. The panel concurs and recommends early referral from primary care for all patients with CRS or suspected CRSwNP (ideally within 6–12 weeks).2,3 This allows earlier endoscopic and/or CT assessment to confirm CRSwNP and exclude other diagnoses, and the complete clinical and laboratory evaluation required to define the associated inflammatory endotype and clinical phenotype (including relevant comorbidities). A complete assessment of comorbidities is essential, and pulmonologist consultation is recommended for CRSwNP patients with comorbid asthma, as this may influence treatment choices and outcomes.13,34
Clinical and laboratory evaluation of CRSwNP follows well-established guidance.2,3,12–14,36 As indicated earlier, either endoscopy or CT may be used to confirm CRSwNP. Routine laboratory investigations include assessment of biomarkers to assess endotype and confirm the presence of type 2 inflammation; typically, total serum IgE and serum eosinophils. Tissue eosinophilia is often performed on surgical specimens and may not necessarily be part of the routine initial work-up. For those patients with co-existing asthma, baseline spirometry and Asthma Control Test (ACT) at initial specialist evaluation is useful (and throughout follow-up).
In EPOS 2020, assessment of CRSwNP severity is based upon assessment of disease impact on general QoL,3 where the Sino-Nasal Outcome Test-22 (SNOT-22) is the most widely used tool,37,38 and a cross-cultural version has been validated for Arabic-speaking patients.39 This 22-item patient questionnaire examines severity of physical symptoms (12 questions) and impact on health-related QoL (10 questions); each question asks the patient to rate severity/impact from none through very mild, mild, moderate, severe, and very severe (scored as 0 to 5) to generate a total SNOT-22 score (range 0–110); scores >50 are considered to reflect severe disease impact.37,38 When used as a measure of treatment effects, a reduction of 8–9 points is considered as the minimum clinically important difference (MCID) in the SNOT-22 score.40,41
Patient-reported symptom severity and impact on QoL and sleep or fatigue can also be measured via a visual analog scale (VAS) of 0 to 10 with disease severity classified as mild (VAS 0–3), moderate (>3–7), and severe (>7–10).16 Patient self-assessment of olfactory impairment is often highly subjective; objective assessment can be made using a variety of tests that quantify smell identification, threshold and discrimination. These include the University of Pennsylvania Smell Identification Test (UPSIT), although this has well-recognized cultural biases.3 At present, UPSIT and other tests such as the “Sniffin’ Sticks” test42 are not yet validated for patients within the pan-Arab region, and a standard approach for smell assessment in CRSwNP has yet to be determined.
Treatment Approach
The goals of CRSwNP treatment are to achieve and maintain clinical control (in that following treatment the patient is symptom-free or where symptoms are not impacting QoL).2,3 In the EPOS 2020 guidelines, CRSwNP disease control is characterized on the presence or absence of a wide range of clinical signs and symptoms. These include (1) nasal blockage; (2) mucopurulent rhinorrhea/postnasal drip; (3) facial pain/pressure; (4) smell impairment; (5) sleep disturbance or fatigue; (6) mucosal disease visible on endoscopy; and (7) use or need for rescue medication ie, short-term OCS or antibiotics.2,3 CRSwNP is considered controlled when none of these are present. Where at least one of these findings is present, CRSwNP can be considered as partly controlled, while if three or more of these findings exist, CRSwNP is considered to be uncontrolled.2,3 While this approach has some value, the panels’ view is that in routine clinical care the persistence of smell impairment and also persistence of mucosal disease on endoscopy despite appropriate treatment are perhaps the most important of these signs and symptoms.
Principles of Treatment
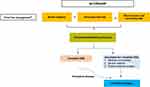
For all patients with CRSwNP the principal first-line treatment options are nasal saline irrigation and use of topical INS, either as sprays or drops, with intermittent use of OCS2,3 (Figure 1). A wide range of INS preparations are available,7,43–45 with evidence for some improvement in CRSwNP symptoms and in QoL (as evident by reductions in SNOT-22 scores).2,3 Although antibiotics are often used, there are limited data for benefit in the treatment of CRSwNP,2,3 and in the panels view routine use is to be avoided, reserving antibiotic use only for more complicated cases or those cases without a confirmed type-2 inflammatory endotype. In the context of acute exacerbations, the authors preference is that antibiotics should be used only when there are clear clinical signs and symptoms of acute infection (eg, clear purulent discharge on endoscopy). While antibiotic choice should be guided by culture and sensitivity, as many cases are due to gram-negative organisms such as Pseudomonas species, empirical use of oral fluoroquinolones may be considered prior to laboratory confirmation.
EPOS 2020 indicates some evidence for short-term improvement in symptoms with OCS (eg, improvement in smell and nasal blockage),2,3,46 and short-term intermittent use (eg, 1–2 courses per year) can be beneficial in patients with poor response or uncontrolled disease.2,3 The panel’s view is that OCS can be used if symptoms persist despite topical therapies, although only for short-term use (eg, for up to 2 weeks) due to recognized risks with prolonged treatment.2,3,47 While many patients may have received some of these therapies before referral, these could be continued under specialist supervision for a short period (eg, up to 6 weeks), and then patients can be re-evaluated and further treatment decisions can be made.
At this point, if disease remains uncontrolled then the preferred option is ESS.8,9,48 While criteria for timing and choice of specific surgical options vary, a useful starting point is the criteria developed by an international expert panel to reduce unnecessary surgery,8 where ESS should be considered in those patients with persistent symptomatic disease after at least 8 weeks of medical therapies (INS and short-course OCS) as measured by a post-treatment total SNOT-22 score ≥20.8 EPOS 2020 recommendations follow similar criteria, although these are suggested as minimal threshold criteria with the qualification that not all patients meeting these should necessarily have surgery.2,3 The goals of ESS are to remove all diseased mucosal tissue and to improve anatomical function within the paranasal sinus and nasal cavities; this optimizes access for topical irrigation and steroid delivery.34,48 However, CRSwNP recurrence following ESS is high, and a substantial proportion of patients (ranging up to 25%) may require subsequent revision procedures, especially in those CRSwNP patients with co-existing asthma.10,49,50 While the extent of endoscopic surgical procedures can vary (from partial to complete) the panel’s view is that a complete ESS procedure is preferred over more focal procedures, which is consistent with most published guidance.2,3,14 As reviewed elsewhere,48 most procedures involve complete sinus opening including anterior and posterior ethmoidectomy, with middle meatal antrostomies, sphenoidotomy and frontal opening.
Use of Biologics in CRSwNP
The availability of effective biologics targeting the underlying type-2 inflammatory pathways in CRSwNP now offers additional options for patients with refractory or uncontrolled CRSwNP.29,51 A range of agents are approved for use in CRSwNP. Dupilumab is a human monoclonal IgG4 antibody targeting the IL-4Rα subunit on the IL-4 and IL-13 receptor complexes, with inhibition of IL-4 and IL-13 signaling leading to reduced IgE production and eosinophil recruitment.28,29,52 Omalizumab is a human monoclonal IgG1 antibody that binds the Fc region of circulating IgE, so blocking IgE interaction with mast cells, basophils and B-cells and reducing IgE production, and also by a direct effect on IgE B-cell production.29 Mepolizumab (human monoclonal IgG1 antibody) binds circulating IL-5, with inhibition of IL-5 interaction with the α-chain on the IL-5 receptor on eosinophils, and downstream inhibitory effects on eosinophil maturation, recruitment and survival.29,52
Biologics are now established in current guidelines for the treatment of severe uncontrolled CRSwNP.2,3,12–14,36 Although the positioning of biologics in the treatment of CRSwNP continues to evolve, the EPOS 2020 guidelines principally recommend use of biologics for those patients with CRSwNP refractory to routine medical therapy, and where disease persists despite previous ESS (although biologics can also be considered in those patients where patients may be unfit for ESS).2,3 The panel was in broad agreement that this EPOS 2020 approach is useful for the pan-Arab region.
Specific criteria are that biologics can be considered if at least three of the following five features are present; (1) evidence of type-2 inflammation (tissue eosinophilia ≥10 cells/high-power field, or serum eosinophilia ≥250 cells/µL, or total serum IgE ≥100 IU/mL); (2) need for systemic corticosteroids (≥2 courses OCS per-year, or long-term [>3 months] low-dose steroids) or contraindications for systemic corticosteroids; (3) significantly impaired QoL (eg, with SNOT-22 ≥40); (4) significant loss of smell (anosmia on smell test); (5) with comorbid asthma (requiring regular inhaled corticosteroids).2,3 The panel’s view is that while the specific cut-off thresholds for evidence of type-2 inflammation may not always apply, these remain an important guide to patient selection. Polyp recurrence after complete ESS or reliance on systemic corticosteroids would also be important considerations for use of biologics. At present, the panel makes no recommendations for use of specific agents; choice will be influenced by patient profile, physician experience and agent availability.
Assessment of response to biologics also follows established criteria. Guided by earlier criteria developed by EUFOREA,12 EPOS 2020 indicates that 5 objective criteria should be considered; reduced nasal polyp size on endoscopy; reduced need for systemic corticosteroids; improved QoL (eg, as measured using SNOT-22); improved sense of smell; and reduced impact of comorbidities (chiefly in the context of comorbid asthma).2,3 In this approach, treatment response is then graded based on the number of these parameters being met; 0 (no response); 1–2 (poor); 3–4 (moderate); and 5 (excellent), with assessment after 4 months suggested as an appropriate time-point for the evaluation of initial treatment response.2,3 The panel’s view is that these criteria and grading are also useful to evaluate the initial benefits of a chosen biologic within the pan-Arab region. If after 4 months there is little or no response, then in the panels view additional treatment options should be considered (ideally within a multidisciplinary approach). Options at this point could include additional revision surgery (complete ESS) or switching to an alternative biologic (with subsequent re-assessment after 4 months).
Since our original workshops evaluating current guidance on CRSwNP, and the original drafting of the present paper, a joint update to guidance on biologics in CRSwNP from EPOS and EUFOREA has been published.53 The main changes in these updated recommendations include a reduction in serum eosinophilia thresholds (from ≥250 cells/µL to ≥150 cells/µL) as one of the criteria for evidence of type-2 inflammation.53 Grading of treatment response has been simplified; as no response, poor to moderate response (where 1–3 of the criteria highlighted earlier are met), and good to excellent (satisfying 4–5 criteria). For the time frame for assessment of initial response, assessment at 6 months is recommended.53 In our view, such simplification is welcome and initial 6-month assessment can also be appropriate.
Conclusions
Clinical management of CRSwNP can be challenging. In the present paper, we provide a practical approach to guide the clinical management of patients with CRSwNP within the pan-Arab region. While existing guidance on CRSwNP has been developed for use in other settings and regions, our view is that such recommendations are equally valid for CRSwNP management within pan-Arab countries; we do not advocate for regional-specific differences. Most patients will benefit from a multidisciplinary approach to evaluation and clinical management, and establishment of formal multidisciplinary clinics and broader patient access to such services are welcome. While many patients respond to medical treatment (principally focused on topical treatment directed towards inflamed nasal mucosa and adjunctive short-term use of systemic corticosteroids), those with more severe/uncontrolled disease require surgery (eg complete ESS). The introduction of biologics has the potential to transform the current approach to management of patients with persistent CRSwNP. Biologics should be considered for those patients with persistent, uncontrolled symptoms despite complete ESS (or where such surgery may be contraindicated). Treatment response to biologics should be assessed using established criteria (ideally after 4–6 months) and alternative treatments considered if response is poor; either revision surgery or switching to an alternative biologic.
Acknowledgments
The authors would like thank Irena Mandic and Stephen McGrath (VMLY&RHealth/IntraMed International) for their support in facilitating the Expert Panel meetings and subsequent activities. Iain O’Neill (independent medical writer) provided support in manuscript development.
Funding
The logistics of the panel selection, meeting facilitation and subsequent discussions and manuscript development were supported by an unrestricted grant from Sanofi. The sponsor had no influence or involvement in the recommendations developed from the discussions or on the content and viewpoints expressed in this manuscript.
Disclosure
RA-A has received honorarium from Sanofi; SA received lecture and advisory board honoraria from Sanofi and GSK; MA-A received lecture and advisory board honoraria from Sanofi, AstraZeneca, and GSK. The authors report no other conflicts of interest in this work.
References
1. Klonaris D, Doulaptsi M, Karatzanis A, Velegrakis S, Milioni A, Prokopakis E. Assessing quality of life and burden of disease in chronic rhinosinusitis: a review. Rhinol Online. 2019;2:6–13. doi:10.4193/RHINOL/18.067
2. Fokkens WJ, Lund VJ, Hopkins C, et al. European position paper on rhinosinusitis and nasal polyps 2020. Rhinology. 2020;58(S29):1–464. doi:10.4193/Rhin20.600
3. Fokkens WJ, Lund VJ, Hopkins C, et al. Executive summary of EPOS 2020 including integrated care pathways. Rhinology. 2020;58(2):82–111. doi:10.4193/Rhin20.601
4. Laidlaw TM, Mullol J, Woessner KM, Amin N, Mannent LP. Chronic rhinosinusitis with nasal polyps and asthma. J Allergy Clin Immunol Pract. 2021;9(3):1133–1141. doi:10.1016/j.jaip.2020.09.063
5. Hopkins C. Chronic rhinosinusitis with nasal polyps. N Engl J Med. 2019;381(1):55–63. doi:10.1056/NEJMcp1800215
6. Bachert C, Marple B, Hosemann W, Cavaliere C, Wen W, Zhang N. Endotypes of chronic rhinosinusitis with nasal polyps: pathology and possible therapeutic implications. J Allergy Clin Immunol Pract. 2020;8(5):1514–1519. doi:10.1016/j.jaip.2020.03.007
7. Kowalski ML, Agache I, Bavbek S, et al. Diagnosis and management of NSAID-Exacerbated Respiratory Disease (N-ERD)-A EAACI position paper. Allergy. 2019;74(1):28–39. doi:10.1111/all.13599
8. Rudmik L, Soler ZM, Hopkins C, et al. Defining appropriateness criteria for endoscopic sinus surgery during management of uncomplicated adult chronic rhinosinusitis: a RAND/UCLA appropriateness study. Rhinology. 2016;54(2):117–128. doi:10.4193/Rhino16.023
9. Beswick DM, Mace JC, Soler ZM, et al. Appropriateness criteria predict outcomes for sinus surgery and may aid in future patient selection. Laryngoscope. 2018;128(11):2448–2454. doi:10.1002/lary.27227
10. Loftus CA, Soler ZM, Koochakzadeh S, et al. Revision surgery rates in chronic rhinosinusitis with nasal polyps: meta-analysis of risk factors. Int Forum Allergy Rhinol. 2020;10(2):199–207. doi:10.1002/alr.22487
11. Loftus CA, Soler ZM, Desiato VM, et al. Factors impacting revision surgery in patients with chronic rhinosinusitis with nasal polyposis. Int Forum Allergy Rhinol. 2020;10(3):289–302. doi:10.1002/alr.22505
12. Fokkens WJ, Lund V, Bachert C, et al. EUFOREA consensus on biologics for CRSwNP with or without asthma. Allergy. 2019;74(12):2312–2319. doi:10.1111/all.13875
13. Bachert C, Han JK, Wagenmann M, et al. EUFOREA expert board meeting on uncontrolled severe chronic rhinosinusitis with nasal polyps (CRSwNP) and biologics: definitions and management. J Allergy Clin Immunol. 2021;147(1):29–36. doi:10.1016/j.jaci.2020.11.013
14. Orlandi RR, Kingdom TT, Smith TL, et al. International consensus statement on allergy and rhinology: rhinosinusitis 2021. Int Forum Allergy Rhinol. 2021;11(3):213–739. doi:10.1002/alr.22741
15. Alsaleh S, Alqahtani A, Alotaibi N, et al. The use of biologics in chronic rhinosinusitis with polyps: Saudi otorhinolaryngology society position statement. Saudi J Otorhinolaryngol Head Neck Surg. 2020;22(2):93–94. doi:10.4103/SJOH.SJOH_29_20
16. Al-Ahmad M, Alsaleh S, Al-Reefy H, et al. Expert opinion on biological treatment of chronic rhinosinusitis with nasal polyps in the gulf region. J Asthma Allergy. 2022;15:1–12. doi:10.2147/JAA.S321017
17. Dietz de Loos DA, Hopkins C, Fokkens WJ. Symptoms in chronic rhinosinusitis with and without nasal polyps. Laryngoscope. 2013;123(1):57–63. doi:10.1002/lary.23671
18. Passali D, Cingi C, Cambi J, Passali F, Muluk NB, Bellussi ML. A survey on chronic rhinosinusitis: opinions from experts of 50 countries. Eur Arch Otorhinolaryngol. 2016;273(8):2097–2109. doi:10.1007/s00405-015-3880-6
19. Kim DH, Seo Y, Kim KM, Lee S, Hwang SH. Usefulness of nasal endoscopy for diagnosing patients with chronic rhinosinusitis: a meta-analysis. Am J Rhinol Allergy. 2020;34(2):306–314. doi:10.1177/1945892419892157
20. Gevaert P, Calus L, Van Zele T, et al. Omalizumab is effective in allergic and nonallergic patients with nasal polyps and asthma. J Allergy Clin Immunol. 2013;131(1):110–6 e1. doi:10.1016/j.jaci.2012.07.047
21. Hopkins C, Browne JP, Slack R, Lund V, Brown P. The Lund-Mackay staging system for chronic rhinosinusitis: how is it used and what does it predict? Otolaryngol Head Neck Surg. 2007;137(4):555–561. doi:10.1016/j.otohns.2007.02.004
22. Cho SH, Hamilos DL, Han DH, Laidlaw TM. Phenotypes of chronic rhinosinusitis. J Allergy Clin Immunol Pract. 2020;8(5):1505–1511. doi:10.1016/j.jaip.2019.12.021
23. Jarvis D, Newson R, Lotvall J, et al. Asthma in adults and its association with chronic rhinosinusitis: the GA2LEN survey in Europe. Allergy. 2012;67(1):91–98. doi:10.1111/j.1398-9995.2011.02709.x
24. Castagnoli R, Licari A, Brambilla I, Tosca M, Ciprandi G, Marseglia GL. An update on the role of chronic rhinosinusitis with nasal polyps as a co-morbidity in severe asthma. Expert Rev Respir Med. 2020;14(12):1197–1205. doi:10.1080/17476348.2020.1812388
25. Philpott CM, Erskine S, Hopkins C, et al. Prevalence of asthma, aspirin sensitivity and allergy in chronic rhinosinusitis: data from the UK national chronic rhinosinusitis epidemiology study. Respir Res. 2018;19(1):129. doi:10.1186/s12931-018-0823-y
26. Crimi C, Campisi R, Nolasco S, et al. Type 2-high severe asthma with and without bronchiectasis: a prospective observational multicentre study. J Asthma Allergy. 2021;14:1441–1452. doi:10.2147/JAA.S332245
27. Agache I, Song Y, Alonso-Coello P, et al. Efficacy and safety of treatment with biologicals for severe chronic rhinosinusitis with nasal polyps: a systematic review for the EAACI guidelines. Allergy. 2021;76(8):2337–2353. doi:10.1111/all.14809
28. Fokkens W, Van Der Lans R, Reitsma S. Dupilumab for the treatment of chronic rhinosinusitis with nasal polyposis. Expert Opin Biol Ther. 2021;21(5):575–585. doi:10.1080/14712598.2021.1901881
29. Bachert C, Zhang N, Cavaliere C, Weiping W, Gevaert E, Krysko O. Biologics for chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2020;145(3):725–739. doi:10.1016/j.jaci.2020.01.020
30. Laury AM, Wise SK. Chapter 7: allergic fungal rhinosinusitis. Am J Rhinol Allergy. 2013;27(1):S26–7. doi:10.2500/ajra.2013.27.3891
31. Bulkhi AA, Mirza AA, Aburiziza AJ, Marglani OA. Dupilumab: an emerging therapy in allergic fungal rhinosinusitis. World Allergy Organ J. 2022;15(3):100638. doi:10.1016/j.waojou.2022.100638
32. Steehler AJ, Vuncannon JR, Wise SK, DelGaudio JM. Central compartment atopic disease: outcomes compared with other subtypes of chronic rhinosinusitis with nasal polyps. Int Forum Allergy Rhinol. 2021;11(11):1549–1556. doi:10.1002/alr.22819
33. Lal D, Borish L, Detwiller KY, et al. The rationale for multidisciplinary management of chronic rhinosinusitis with nasal polyposis. J Allergy Clin Immunol Pract. 2020;8(5):1565–1566. doi:10.1016/j.jaip.2020.03.001
34. Han JK, Bosso JV, Cho SH, et al. Multidisciplinary consensus on a stepwise treatment algorithm for management of chronic rhinosinusitis with nasal polyps. Int Forum Allergy Rhinol. 2021;11(10):1407–1416. doi:10.1002/alr.22851
35. Alfallaj R, Obaid SB, Almousa H, et al. Demographic and clinical profile of patients with chronic rhinosinusitis in Saudi Arabia. Saudi Med J. 2023;44(4):401–405. doi:10.15537/smj.2023.44.4.20220947
36. Fokkens WJ, Lund V, Luong AU, Orlandi RR. A Comparison of International Guidelines for Rhinosinusitis. J Allergy Clin Immunol Pract. 2022;10(6):1418–1422. doi:10.1016/j.jaip.2022.01.013
37. Hopkins C, Gillett S, Slack R, Lund VJ, Browne JP. Psychometric validity of the 22-item sinonasal outcome test. Clin Otolaryngol. 2009;34(5):447–454. doi:10.1111/j.1749-4486.2009.01995.x
38. Toma S, Hopkins C. Stratification of SNOT-22 scores into mild, moderate or severe and relationship with other subjective instruments. Rhinology. 2016;54(2):129–133. doi:10.4193/Rhino15.072
39. Alanazy F, Dousary SA, Albosaily A, Aldriweesh T, Alsaleh S, Aldrees T. Psychometric Arabic sino-nasal outcome test-22: validation and translation in chronic rhinosinusitis patients. Ann Saudi Med. 2018;38(1):22–27. doi:10.5144/0256-4947.2018.22
40. Hopkins C, Rudmik L, Lund VJ. The predictive value of the preoperative Sinonasal Outcome Test-22 score in patients undergoing endoscopic sinus surgery for chronic rhinosinusitis. Laryngoscope. 2015;125(8):1779–1784. doi:10.1002/lary.25318
41. Chowdhury NI, Mace JC, Bodner TE, et al. Does medical therapy improve sinonasal outcomes test-22 domain scores? An analysis of clinically important differences. Laryngoscope. 2019;129(1):31–36. doi:10.1002/lary.27470
42. Soler ZM, Kohli P, Storck KA, Schlosser RJ. Olfactory impairment in chronic rhinosinusitis using threshold, discrimination, and identification scores. Chem Senses. 2016;41(9):713–719. doi:10.1093/chemse/bjw080
43. Bachert C, Zhang L, Gevaert P. Current and future treatment options for adult chronic rhinosinusitis: focus on nasal polyposis. J Allergy Clin Immunol. 2015;136(6):1431–1440. doi:10.1016/j.jaci.2015.10.010
44. Neubauer PD, Schwam ZG, Manes RP. Comparison of intranasal fluticasone spray, budesonide atomizer, and budesonide respules in patients with chronic rhinosinusitis with polyposis after endoscopic sinus surgery. Int Forum Allergy Rhinol. 2016;6(3):233–237. doi:10.1002/alr.21688
45. Grayson JW, Harvey RJ. Topical corticosteroid irrigations in chronic rhinosinusitis. Int Forum Allergy Rhinol. 2019;9(S1):S9–S15. doi:10.1002/alr.22331
46. Head K, Chong LY, Hopkins C, Philpott C, Schilder AG, Burton MJ. Short-course oral steroids as an adjunct therapy for chronic rhinosinusitis. Cochrane Database Syst Rev. 2016;4:CD011992. doi:10.1002/14651858.CD011992.pub2
47. Hox V, Lourijsen E, Jordens A, et al. Benefits and harm of systemic steroids for short- and long-term use in rhinitis and rhinosinusitis: an EAACI position paper. Clin Transl Allergy. 2020;10:1. doi:10.1186/s13601-019-0303-6
48. Weber RK, Hosemann W. Comprehensive review on endonasal endoscopic sinus surgery. GMS Curr Top Otorhinolaryngol Head Neck Surg. 2015;14:Doc08. doi:10.3205/cto000123
49. Gill AS, Smith KA, Meeks H, et al. Asthma increases long-term revision rates of endoscopic sinus surgery in chronic rhinosinusitis with and without nasal polyposis. Int Forum Allergy Rhinol. 2021;11(8):1197–1206. doi:10.1002/alr.22779
50. Laidlaw TM, Buchheit KM. Biologics in chronic rhinosinusitis with nasal polyposis. Ann Allergy Asthma Immunol. 2020;124(4):326–332. doi:10.1016/j.anai.2019.12.001
51. Mullol J, Azar A, Buchheit KM, Hopkins C, Bernstein JA. Chronic rhinosinusitis with nasal polyps: quality of life in the biologics era. J Allergy Clin Immunol Pract. 2022;10(6):1434–1453.
52. Anselmo-Lima WT, Tamashiro E, Romano FR, et al. Guideline for the use of immunobiologicals in chronic rhinosinusitis with nasal polyps (CRSwNP) in Brazil. Braz J Otorhinolaryngol. 2021;88(3):471–480. doi:10.1016/j.bjorl.2021.03.003
53. Fokkens WJ, Viskens AS, Backer V, et al. EPOS/EUFOREA update on indication and evaluation of biologics in chronic rhinosinusitis with nasal polyps 2023. Rhinology. 2023;61(3):194–202. doi:10.4193/Rhin22.489
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.