Back to Journals » Clinical Ophthalmology » Volume 14
Management of Adenoviral Keratoconjunctivitis: Challenges and Solutions
Authors Labib BA, Minhas BK , Chigbu DI
Received 26 November 2019
Accepted for publication 25 February 2020
Published 17 March 2020 Volume 2020:14 Pages 837—852
DOI https://doi.org/10.2147/OPTH.S207976
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Bisant A Labib, Bhawanjot K Minhas, DeGaulle I Chigbu
Pennsylvania College of Optometry, Salus University, Elkins Park, PA 19027, USA
Correspondence: DeGaulle I Chigbu Tel +1 215 991 4790
Email [email protected]
Abstract: Human adenovirus (HAdV) is the most common cause of infectious conjunctivitis, accounting for up to 75% of all conjunctivitis cases and affecting people of all ages and demographics. In addition to ocular complications, it can cause systemic infections in the form of gastroenteritis, respiratory disease, and dissemination in immunocompromised individuals. HAdV causes lytic infection of the mucoepithelial cells of the conjunctiva and cornea, as well as latent infection of lymphoid and adenoid cells. Epidemic keratoconjunctivitis (EKC) is the most severe ocular manifestation of HAdV infection, in which the presence of subepithelial infiltrates (SEIs) in the cornea is a hallmark feature of corneal involvement. SEIs have the tendency to recur and may lead to long-term visual disability. HAdV persistence and dissemination are linked to sporadic outbreaks of adenoviral keratoconjunctivitis. There is no FDA-approved antiviral for treating adenoviral keratoconjunctivitis, and as such, solutions should be proffered to handle the challenges associated with viral persistence and dissemination. Several treatment modalities have been investigated, both systemically and locally, to not only mitigate symptoms but reduce the course of the infection and prevent the risk of long-term complications. These options include systemic and topical antivirals, in-office povidone-iodine irrigation (PVI), immunoglobulin-based therapy, anti-inflammatory therapy, and immunotherapy. More recently, combination PVI/dexamethasone ophthalmic formulations have shown favorable outcomes and were well tolerated in clinical trials for the treatment of EKC. Possible, future treatment considerations include sialic acid analogs, cold atmospheric plasma, N-chlorotaurine, and benzalkonium chloride. Continued investigation and evaluation of treatment are warranted to reduce the economic burden and potential long-term visual debilitation in affected patients. This review will focus on how persistence and dissemination of HAdV pose a significant challenge to the management of adenoviral keratoconjunctivitis. Furthermore, current and future trends in prophylactic and therapeutic modalities for adenoviral keratoconjunctivitis will be discussed.
Keywords: human adenovirus, adenoviral keratoconjunctivitis, antivirals, immunotherapy, povidone-iodine, viral dissemination
Introduction
Human adenovirus (HAdV) is the most common cause of infection to the ocular surface, accounting for up to 75% of conjunctivitis cases.1 The most common presentation is pharyngoconjunctival fever (PCF), which often occurs in children and manifests clinically with fever, pharyngitis, rhinitis, follicular conjunctivitis, and regional lymphoid hyperplasia.2 Epidemic keratoconjunctivitis (EKC) is the most severe ocular form and is distinguished by its ability to invade the corneal epithelium, ranging in presentation from a keratitis to persistent and recurrent subepithelial infiltrates (SEIs). HAdV is highly contagious due to its unique structure and ability to evade the normal host’s immune system. It is distinguished from other types of conjunctivitis in that it often involves the cornea, with potentially devastating visual complications. These features contribute to a heavy economic burden and necessitate the establishment of a standard treatment protocol.1 In addition to the potential ocular manifestations of this virus, HAdV infections have the propensity to manifest systemically, in cases such as respiratory, urinary, and gastrointestinal tract (GIT) infections. This variety of presentations can infect a normal, healthy host, and also have an increased risk in immunocompromised individuals. Despite the detrimental effect that HAdV infections pose, there has yet to be an FDA-approved drug to treat these conditions, making management difficult. Even following the active phase of the disease, viral persistence and reactivation may occur. Oral and topical antivirals have been considered as off-label management solutions, but problems with efficacy, bioavailability, and therapeutic profiles have limited their use. With regards to EKC, topical disinfection during active cases as well as treatment of corneal sequelae using corticosteroids and immunosuppressive agents show promise. This review will focus on how persistence and dissemination of HAdV poses a significant challenge to the management of adenoviral keratoconjunctivitis. Furthermore, current and future trends in prophylactic and therapeutic modalities for adenoviral keratoconjunctivitis will be discussed.
Virology
HAdV belongs to the genus Mastadenovirus and family Adenoviridae. It is a nonenveloped virus with a linear dsDNA genome and icosahedral capsids. HAdV consists of 7 groups classified through genomic sequence analysis.3 Adenoviral-based ocular surface infections are attributed to several subtypes of Group B and D HAdV. Generally, these viruses bind CD46, a ubiquitously expressed transmembrane protein, to infect the host.4,5 Exposure of the host to HAdV is made possible through the interaction between adenoviral fiber protein and primary host cellular receptors such as CD46, sialic acid, and heparin-sulfate proteoglycan, all of which promote the attachment and internalization of HAdV.6,7 Interactions between the penton base of the virus and vitronectin-binding integrins of the host support internalization and acidification of the endosome, triggering conformational changes to the viral capsid. This process culminates in the release of viral DNA genome into the host nucleus, where viral replication occurs.2,7-9
Challenges
HAdV causes a lytic infection of the mucoepithelial cells of the conjunctiva and cornea as well as a latent infection of lymphoid and adenoid cells.10 Members of Groups B and D HAdV cause both GIT and ocular infections. See Table 1 for the subtypes of Group B and D HAdV.11,12 HAdV type 3, 7, and 21 of Group B can cause keratoconjunctivitis, urinary tract infection, respiratory infection, and GIT infection. Group D HAdV can also cause both ocular and GIT infection. Some group B HAdV subtypes infect the respiratory tract. Group B HAdV including type 3, 7, 14, and 21 have been associated with acute respiratory distress (ARD) outbreaks.2,12 HAdV types that cause ARD are transmitted through aerosolized droplets. It is important to note that both respiratory droplets and the fecal-oral transmission route from individuals with acute adenoviral infection, or even post-infection adenoviral shedding, play an important role in the transmission dynamics of HAdV infections.2,13
 |
Table 1 Overview of Subtypes of Group B and D HAdV with Sites of Infection |
Persistent HAdV secretions in the tears may also occur even years following the resolution of acute ocular infection. T cells in tonsillar and adenoid lymphoid tissue serve as reservoirs for harboring HAdV, making it possible to develop latent adenoviral infections.3,13,14 Reactivation of persistent latent adenovirus in the host is likely facilitated through the blockade of types I and II interferon (IFN) response that is required to inhibit expression of the HAdV E1A gene.3
Immunosuppressive steroid therapy can suppress the production of cytokines including TNF-alpha, type I IFN, and type II IFN, as well as depleted T cells and NK cells.3,15,16 This inadvertently reduces the secretion of antiviral cytokines that play a major role in inhibiting viral replication. Inhibition of the IFN response allows for expression of the HAdV E1A gene, which results in reactivation and replication of HAdV DNA in epithelial cell associated with latently infected lymphoid tissue and consequential dissemination of HAdV.3 Thus, immunosuppression could facilitate dissemination of adenovirus into the community since subclinical adenoviral infection of tonsillar and adenoid lymphoid tissue serve as a source of transmitting adenoviruses, particularly in immunocompromised children with no prior exposure and immunity to a particular strain of adenovirus.17 Additionally, asymptomatic passage of adenovirus in the stool of patients with previous adenoviral GIT infection can also occur.3,11
Kosulin et al suggested that latent HAdV infection of the gut lymphoid cell could serve as a source of release of HAdV particles in the community.18 Garnett et al demonstrated the asymptomatic persistence of group C adenovirus in human mucosal T lymphocytes or lymphoid tissue following primary adenoviral infection.17 The stimulation of these adenovirus containing mucosal T-lymphocytes results in reactivation of latent HAdV with consequential leakage of reactivated viruses into intestinal epithelial cells, where adenoviruses undergo replication and subsequent shedding in stool.11,17,19-21 This is indicative of persistent subclinical HAdV infection of the gut-associated lymphoid tissue.20
Immunocompetent individuals are likely to shed less HAdV into their stool, in contrast to those who are immunocompromised, where significant amounts of HAdV are released. Immunocompromised individuals are also more likely to have reactivation of HAdV with productive infection of intestinal epithelial cells and consequential extensive viral dissemination in the community. As such, in the immunocompromised state, reactivation of persistent latent HAdV is an essential cause of HAdV dissemination.18 These resultant, latent adenoviral infections are considered a significant challenge in managing and containing the virus within the community due to persistent adenoviral shedding and its high propensity of spread via hand-to-eye contact by those contaminated with fecal matter.18
Another significant challenge in managing adenoviral infections, particularly in Group D HAdV, is the propensity for this group to cause oculogenital infection. Several published cases have demonstrated that Group D HAdV can be associated with concurrent urethritis and conjunctivitis.22–26 HAdV 19 and 37 specifically have been sequestered from genital tracts of young adults with EKC, indicating the possibility of sexual transmission.25,26 Group D HAdV type 37, for example, has been isolated from sexually active men with adenoviral urethritis.24 Additionally, Liddle et al discussed eight cases of individuals presenting with concurrent conjunctivitis and adenoviral urethritis.23 Avolio et al also presented a case of two male patients with HAdV D37 associated urethritis and conjunctivitis, in which one of the spouses contracted adenoviral conjunctivitis via oculogenital contact. These cases highlight the importance of testing for the presence of adenovirus in clinical specimens collected from both urethral and conjunctival swab in men presenting with conjunctivitis, dysuria, and scant urethral discharge.22
Management
Due to the aforementioned HAdV characteristics and its interactions with host cells, HAdV has demonstrated a high likelihood of evading the immune response leading to infection. Additional research on its many modes of transmission contributes to the highly contagious nature of HAdV and dissemination into the community. Large-scale epidemics lead to high social and economic burdens, making health education in infected patients an invaluable tool to limit spread. Preventative methods require that patients exercise frequent hand washing with soaps and to keep hands dry, avoiding eye rubbing. Children are to be encouraged to stay home during the infectious phase to limit the spread of disease. Contact with infected towels, soap, bedding, door handles, etc. should also be avoided. Following resolution of the virus, bedding, and towels used by the infected individual should be washed thoroughly and exposed under sunshine (solar ultraviolet radiation) to further ensure elimination of the virus.27 EKC outbreaks are also commonly spread through ophthalmology clinics, warranting the need to evaluate disinfection procedures for ophthalmic instruments. Methods of spread include tonometry probes, tips of contaminated eye drop bottles, and foreign body removal instruments. A study comparing the efficacy of hydrogen peroxide disinfection versus alcohol swabs determined that there was a more significant reduction in log growth using hydrogen peroxide. The use of disposable prisms is also an option, though many times this method is of limited use due to cost.28,29 Because of these unique viral characteristics, ubiquity of HAdV, and its potential for epidemic, several treatment modalities have been investigated to establish an effective treatment protocol. However, at this stage, there is no FDA-approved antiviral treatment for HAdV infections.
Povidone-Iodine Irrigation
Though molecular iodine has long been established as an effective antiseptic agent, its formidable toxicity upon contact to mucosal surfaces deterred it for use in clinical the clinical setting.30 However, combining iodine with povidone allowed for this antiseptic to be safely and routinely used in the ophthalmic setting, and has even shown promise in the management of EKC affected individuals.
Povidone-iodine (PVI) is a broad-spectrum microbicide solution, which exists in multiple forms that are easily accessible, and a cost-effective disinfectant agent. Since its discovery, it has been routinely utilized in the medical field as an antiseptic agent for laboratory and surgical purposes. Furthermore, it has found much purpose in the ophthalmic setting as an effective disinfecting solution due to its proven toxicity against viruses, bacteria, parasites, fungi, yeasts, molds, and protozoans.31 Diluted forms of PVI are commercially sold as Betadine (Alcon Laboratories, Inc., Fort Worth, TX 76134) in 5% and 10% concentrations and can be further weakened as medical use requires. It is critical to note that, unlike other antiseptics, PVI does not lose antimicrobial activity with decreased available iodine concentration in solution when a diluent is added.30
The mode of action requires the oxidation of pathogen nucleotides, amino acids, and proteins, damaging vital bacterial cellular mechanisms.32 Additionally, in vitro investigation indicates that PVI impedes the host’s inflammatory response to a viral pathogen by affecting both host and pathogen parameters.33 This may give insight into how in-office PVI irrigation may alleviate inflammatory symptoms associated with EKC. Specific pathogen consequences include inhibition of production and release of exotoxins (such as α-hemolysin, phospholipase C, and lipase) and suppression of bacterial enzymes (such as elastase and ß-glucuronidase).32
Host factors involve modulation of antioxidant and free radical activity, inhibition of inflammatory effector cells and mediators (such as TNF-α and ß-galactosidase), inhibiting matrix metalloproteinase production, and enhancing healing signals via activation of T cells and macrophages.32 Globally, PVI characteristics that make it ideal for clinical use include broad antimicrobial spectrum, lack of resistance, ability to penetrate biofilms, low cytotoxicity, suitable tolerability, and overall favorable risk/benefit profile.32 Such versatility, ease of access, and ubiquitous use in antisepsis have been further promoted by biochemical characteristics of the compound. The combination of a synthetic carrier homopolymer (2-pyrrolidnone, 1-ethenyl-), which has no innate germicidal ability, and iodine forms PVI.34 In aqueous form, free iodine is released into solution from the PVI complex, which is what provides the microbicidal activity.32
Studies have shown that PVI exhibits antimicrobial activity proportional to the concentration of free iodine released in any given solution of specified dilution, regardless of PVI concentration.30 In addition to its well-documented antimicrobial activity, a study examining the virucidal efficacy of 0.01%, 0.1%, 1%, and 10% PVI demonstrated that 0.1% solution was actually the most effective against HAdV 3, as it maximized free iodine concentration.30 Specifically, PVI formulations have been proven effective against non-enveloped human viruses including HAdV, although, it has been postulated to be adenoviral type dependent.35,36
PVI has been shown to demonstrate virucidal reductions for ocular HAdV types 3, 4, 5, 7, and 8 at 1–5 mins and types 37, and 64 at 15–60 mins for various concentrations.36 This may indicate that time of exposure, not concentration of PVI, to disinfection is critical and that virucidal activity for PVI at different concentrations may require temporal consideration when evaluating specific virus types. Though PVI has been tested in vitro, in animal models, and clinically for its use in disinfection and wound healing for many decades, the use of a PVI irrigation in-office for EKC remains off-label.32 The theory behind in-office PVI irrigation is to reduce viral load on the ocular surface and to decrease viral shedding. A commonly implemented protocol in clinical practice involves anesthetizing the affected eye(s), then instilling a pre-irrigation NSAID drop, followed by four to five drops of 5% PVI solution. The patient then rolls his or her closed eye(s) for 60 s to maximize exposure (including swabbing of the eyelid margins), followed by lavage of the ocular surface with sterile saline irrigation solution (Figure 1). Finally, a post-irrigation NSAID drop is instilled. Anecdotally, patients may report exasperation of their conjunctivitis symptoms for 12–24 hrs after this procedure; however, the overall risk/benefit consideration regularly tips the decision in favor of preforming PVI irrigation.32
Interestingly, Cheung et al have indicated that multiple types of adenovirus can be involved in a single outbreak and as PVI has proven viricidal activity in multiple ocular types of HAdV, it would be sensible to consider PVI irrigation to decrease colonization of the ocular surface in this disease picture.37 The most powerful tool in limiting the severity of adenoviral conjunctivitis outbreaks includes reduction of viral shedding and limiting contamination of objects, workspaces, and surfaces in public places to avoid horizontal transmission, as mentioned previously in this manuscript.38 Gargling or flushing with PVI has been postulated as an effective measure in disrupting the transmission of respiratory viral spread.39 Hence, PVI irrigation can be a powerful tool to help in the reduction of transmission of adenoviral keratoconjunctivitis.
PVI has also shown value in treatment formulations. A large clinical trial for the use of 1.25% PVI ophthalmic solution in the treatment of pediatric conjunctivitis displayed efficacy in treatment of bacterial, chlamydial, and viral conjunctivitis.40 Interestingly, a clinical trial looking at 0.5% PVI (in combination with artificial tears at pH 4.2 for enhanced tolerability) for the treatment of adenoviral keratoconjunctivitis found faster recovery from disease at two weeks, with three drops administered thrice daily.34
Antiviral Therapy
Antiviral activity against systemic HAdV infections has been thoroughly studied and paved the way for its more focused evaluation for the treatment of the ocular manifestations of this virus. Besides the aforementioned forms of keratoconjunctivitis, HAdV may also cause upper and lower respiratory tract disease, hemorrhagic cystitis, and gastroenteritis due to its propensity to infect mucosal epithelium.3,41,42 Respiratory infection is typically mild and self-limiting in immunocompetent individuals, but in rare cases, severe respiratory infection and pneumonia may result in acute respiratory distress syndrome (ARDS). Additionally, in immunocompromised patients undergoing hematopoietic stem cell or solid organ transplantation, disseminated infections can be life-threatening, particularly in the pediatric population.3 Despite thorough investigation on the role of antiviral treatment both systemically and topically, there remains no standard of care. Possible therapeutic benefits have been demonstrated through the use of antiviral drugs such as ganciclovir, ribavirin, and cidofovir.41–44
Ganciclovir (GCV), a synthetic nucleoside analog of 2ʹ-deoxyguanosine, has been proven effective in the inhibition of the herpes family of viruses, specifically herpes simplex types 1 and 2, varicella-zoster virus, cytomegalovirus, and Epstein-Barr. In a series of experiments utilizing Syrian, immunocompromised hamsters infected with HAdV 5, GCV was able to suppress viral replication in the liver, a method that may involve the direct inhibition of DNA polymerase. It was proposed that GCV inhibited the advancement of viral infection into the late phase. Systemically, GCV was able to mitigate the effects of HAdV infection in these hamsters, decreasing the rate of mortality. This led to the investigation of GCV in the treatment of ocular infections of HAdV.43,44 The ophthalmic gel form (ganciclovir 0.15%, Virgan®; Farmila-Thea, Milan, Italy) has been the standard of care for the treatment of acute ocular herpetic keratitis, yet it has not been standardized in the management of adenoviral keratoconjunctivitis, particularly since studies in human subjects are lacking.43,44 Current research suggests, however, that with off-label topical use, GCV inhibits the replication of HAdV that leads to ocular infection. Several clinical trials have reported its efficacy.43–46
In one study, Huang et al investigated the antiviral activity of GCV against HAdV types 3, 4, 8, 19, and 37 using polymerase chain reaction (PCR) and concluded that there was a significant, dose-dependent inhibitory effect on those serotypes responsible for EKC.46 Systemically, Bruno et al described the potential therapeutic effect of GCV in that the incidence of HAdV infections was significantly reduced in stem cell-transplant patients treated with GCV for human cytomegalovirus prophylaxis.45
Sun et al conducted a study utilizing a series of eye drops to treat active EKC infections, including GCV ophthalmic gel, interferon eye drops, a tobramycin-dexamethasone combo, and topical diclofenac sodium. In this study, interferon was administered with the purpose of inhibiting viral replication, while GCV was used for its antiviral properties. To improve efficacy and limit potential ocular side effects, such as inflammation, congestion, and edema, tobramycin-dexamethasone and diclofenac sodium treatments were also employed. This combination of drugs demonstrated a 91.76% cure rate in a 6-week treatment period, with low risk of side effects limited to transient corneal epithelial defects and elevated intraocular pressure. While EKC is often considered to be self-limiting, this study concluded that the proposed treatment plan, specifically during the early phase of the disease, could markedly reduce the patient’s symptoms, shorten the course of the disease, and lessen the risk of corneal complications.27
Valganciclovir is a pro-drug of GCV that inhibits replication of adenoviral genomic DNA via blockade of HAdV DNA polymerase. However, because HAdV lacks thymidine kinase, a known target for valganciclovir, it would likely become a challenge in the treatment of adenoviral keratoconjunctivitis.47
Ribavirin and cidofovir have also been shown to exhibit antiviral activity against HAdV in vitro. However, many of these systemic antiviral therapies lead to the risk of significant side effects. Cidofovir (CDV) is an acyclic nucleoside phosphonate and nucleotide analog of cytosine. It is converted by cells to its diphosphate form and binds to the HAdV DNA polymerase, causing viral DNA chain termination and viral inhibition.13,44 Intravenous cidofovir is often used in transplant clinics with only mild efficacy. This is due to poor cellular uptake because of its phosphate group, leading to accumulation of the drug in the renal tubules and, when used systemically, leads to nephrotoxicity.44,46 Locally, CDV may also cause ocular toxicity around the skin of eyelids and conjunctiva.46 Similarly, ribavirin also results in poor systemic side effects and safety profile, associated with extravascular hemolysis, anemia, and bone marrow suppression. Due to these discoveries, it is necessary to determine an effective antiviral with a high therapeutic index for the treatment of HAdV associated infections.46
In animal models, CDV was administered utilizing topical and intrastromal inoculation three times per day for 20 consecutive days; the results displayed significant effectivity against HAdV type 5 when compared to the placebo group, reducing both viral shedding and the severity of sub-epithelial infiltrates. This study showed great promise in the future of cidofovir for the treatment of ocular HAdV infections in the future.48 Additionally, early systemic administration of CDV in immunocompetent patients with HAdV pneumonia was an effective treatment strategy.42 Due to the positive results in the aforementioned studies, CDV was tested as a prophylactic measure due to the epidemic nature of HAdV. Romanowski et al determined that antiviral prophylaxis with 1% and 0.5% concentrations of CDV significantly reduced viral replication of HAdV type 5 in animal models, giving promise to the use of CDV in prophylaxis.49
Despite favorable outcomes of cidofovir’s antiviral activity in rabbit models, several studies discussed the significant side effects to the ocular surface, which is a potential limitation for therapeutic use. Gordon et al described the effects of topical cidofovir in uninfected animals, determining that there was consistent clinically significant ocular toxicity at a total dose exceeding 15mg over 10 days.50 Significant eyelid redness and conjunctival hyperemia were also described in additional studies as well as nasolacrimal blockage and lacrimation.50,51 Even at lower and presumably ineffective dosages, 3.5 mg of cidofovir continued exhibit significant ocular surface toxicity in vivo.52
Resistance to CDV is likely due to changes in amino acid sequence within the encoded DNA polymerase genes in resistant HAdV, which has been suggested to confer resistance of adenovirus to CDV. Drug resistance poses a significant challenge to the management of adenoviral keratoconjunctivitis in the clinical setting.53 Romanowski et al were able to demonstrate that HAdV type 5 that are resistant to topical CVD therapy are less likely to constitute a significant challenge in management of adenoviral keratoconjunctivitis in immunocompetent patients. Drug-resistant viruses usually pose a minimal threat in immunocompetent patients, but CDV-resistant HAdV can retain their ability to replicate in permissive ocular epithelial cells.54 Another major challenge to the use of CDV for treating adenoviral keratoconjunctivitis is its propensity to lead to toxicity, which manifests as persistent epiphora from lacrimal canalicular blockade.53 As such, despite its potential, its high toxicity profile and poor bioavailability make CDV a less than ideal treatment option for the treatment of HAdV associated infections, both systemically and locally.
The solution to these challenges led to the investigation of brincidofovir (BCV), a lipid-linked derivative of CDV, which allows for cells to uptake the drug more readily when administered orally.13,44,55 Following cellular uptake, the lipid component is cleaved by phospholipases, leaving CDV. This allows for similar antiviral efficacy as seen with CDV, but without the subsequent nephrotoxicity. Averbuch et al. reported successful use of BCV in the treatment of adenoviral infection in a patient with primary immunodeficiency whereas Florescu et al demonstrated that it was clinically beneficial in treating adenoviral infection in immunocompromised individuals who were at risk of disseminated HAdV infection.56 It was suggested that BCV could be a potential anti-adenoviral therapeutic agent.57 Though this drug shows promise, there is still a need for more conclusive research regarding the effectivity and safety profile of BCV systemically, as well as its potential for the treatment of adenoviral ocular infections. Clinical development of this drug is ongoing, as there is yet to be an ophthalmic form.13,44 When antiviral comparison is made, ganciclovir appears to be the most favorable in terms of antiviral activity and limited systemic or local side effects.46
Immunoglobulin (Ig)-Based Therapy
In 2001, Goosens et al discussed the antiviral activity of anti-Ad IgG on gene transfer to synovial fluid. This was attributed to the antibodies' ability to target adenoviral capsid proteins, subsequently inhibiting infection by the virus.58 Topically, immunoglobulin (Ig) may offer the same effect on ocular tissue and prevent transmission and replication of the virus, to some degree. In addition to its antiviral properties, Ig has the added benefit of anti-inflammatory effects, which can aid in sub-epithelial infiltrate management.59 Nwanegbo et al found that Ig was actually comparable to cidofovir in its antiviral activity. While cidofovir acts intracellularly to block DNA replication, Ig neutralizes the virus on the ocular surface. In comparison to cidofovir and saline titers, Ig was more effective during the acute phase of the infection.59 It worked to aid in the clearance of the virus, shortening the duration of the infection, and thereby limiting the transmission of the virus. Additionally, Ig may be beneficial in prophylaxis to prevent clinical infections. Though both cidofovir and Ig were effective in this study, cidofovir demonstrated a significantly shorter duration of viral shedding. This is likely due to the mechanism of intracellular-mediated adenoviral DNA polymerase-blocking activities and prolonged tissue half-life after rapid uptake into cells. This study yielded promising results in the future of topical Ig use on ocular HAdV infections. The limitation lies in product consistency as Ig is derived from serum pooled by various donors, though anti-adenoviral efficacy appears stable over different lots.59,60
Topical Anti-Inflammatory Therapy
Corneal involvement in EKC occurs in approximately 80% of cases, varying in presentation as a superficial punctate keratitis, focal epithelial punctate keratitis, subepithelial infiltrate (SEI) formation, and reduced corneal sensitivity.1,2,12,61 SEIs specifically will often manifest anywhere from one to three weeks following the acute phase of the infection. Histologically they are comprised of residual antigen and lymphocytic accumulations that adhere to stromal cells in the cornea.62 This clinical finding may persist for months to years following resolution of the conjunctivitis, leading to subjective visual disturbances, such as decreased vision, photophobia, halos, and the development of irregular astigmatism. Due to their chronicity and visual impact, treatments including anti-inflammatory and immunosuppressive agents have been investigated to treat as well as prevent SEIs, though they often end up resolving without scarring or corneal neovascularization formation.2,12,62
Research demonstrates that mild corticosteroid treatment administered topically approximately three times per day can significantly improve EKC symptoms and, if used in the short term, acute phase of the disease, does not lead to significant ocular side effects. There is also additional benefit in the use of topical corticosteroids once the acute phase of the infection resolves, for the remaining, persistent SEIs.2,63 While chronic corticosteroid treatment has been proven to reduce these findings, a significant challenge to this mode of treatment is the risk of complications of long-term use, including glaucoma and cataract formation.64–69 Additionally, corticosteroid treatment of SEIs can result in a 17.5% recurrence rate and consequential, unsuccessful drug tapering.70 If discontinued abruptly, these viral antigens continue to attract lymphocytes, causing persistence of SEIs.62,66 Excimer laser ablation can be helpful in such cases.63 In a study comparing topical loteprednol with dexamethasone, similar outcomes in SEI treatment were observed. This is significant because milder topical steroid forms, such as loteprednol, are known to have less risk of adverse effects.71 However, it is important to note that short-term treatment with topical steroids of limited potency may also delay viral clearance.72
Nonsteroidal anti-inflammatory drugs (NSAIDs) are another alternative to corticosteroids, in that they are approved for topical, ocular use and exhibit anti-inflammatory effects without the substantial risk of glaucoma and cataract effect seen with steroid use. These agents work on the arachidonic acid pathway by inhibiting cyclooxygenase and, subsequently, the formation of prostaglandins, thromboxane, and prostacyclin. With specificity to ocular uses, they have been shown to be effective in cases of allergic conjunctivitis, alkali burns, herpetic uveitis, ocular trauma, and pre/post-operative cataract and refractive surgeries.73–76
Gordon et al were the first to investigate NSAID effects on adenoviral replication, specifically ketorolac tromethamine and diclofenac sodium ophthalmic solutions. During both the early and late phases of infection, the effect of NSAIDs on viral titers did not differ from control groups, nor did it affect the duration of viral shedding. In contrast, prednisolone treatment was shown to prolong viral shedding.73 As it pertained to sub-epithelial infiltrates, treatment with diclofenac or ketorolac did not yield a statistically significant reduction when compared to control groups, whereas prednisolone did. This study suggests that NSAIDs are not likely to have any clinically significant antiviral effects.73
It is important to note that even though NSAIDs offer a higher safety profile with long-term use in comparison to topical corticosteroids, they do not seem to be useful in cases of HAdV-associated conjunctivitis. They are also not without complication and associated with risk of corneal melt.74
Pseudomembranes are marked by fibrin rich exudation lacking blood or lymphatic vessels, while true membranes are formed by coalescence of the exudation over the substantia propria of mucous membranes; both are common in adenoviral keratoconjunctivitis and may hemorrhage upon removal.1 Histopathological studies indicate the presence of fibrin, neutrophils, macrophages, effector lymphocytes, and activated dendritic cells in these films.1
Because pseudomembrane formation can present in severe cases of adenoviral keratoconjunctivitis in the setting of an intense inflammatory response (Figure 2), in-office removal of the pseudomembrane could be beneficial in preventing further complications of conjunctival fibrosis and long-term sequelae. Additionally, pseudomembrane manifestation along with SEIs necessitates the need for topical anti-inflammatory management.
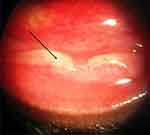 |
Figure 2 Presentation of an inflamed inferior palpebral conjunctiva with pseudomembrane (black arrow) in a patient with adenoviral keratoconjunctivitis.Note: Copyright ©2018. Dove Medical Press. Reproduced from Chigbu DI, Labib BA. Pathogenesis and management of adenoviral keratoconjunctivitis. Infect Drug Resist. 2018;11:981–993.2 |
Immunotherapy and Cardiotonic Steroids
Cyclosporine, a non-steroidal immunomodulatory, was first developed in the 1970s as a novel drug to inhibit T cells both specifically and reversibly. Its first use was in whole organ transplants in attempts to avoid transplant rejection by tempering the host’s own immune response.77 Ocular use in HAdV keratoconjunctivitis should be considered when there is a need to dampen the immune response as in corneal complications of the virus, especially when there are contraindications of using corticosteroids or to limit associated corticosteroid complications.
Topical cyclosporine (both 1% and 2% concentrations) is an alternative option which, if used in the acute phase of adenoviral keratoconjunctivitis, is successful in both reducing the risk of developing corneal findings as well as the chronic treatment of persistent SEIs, with most cases resolving over a course of 3–4 weeks.64,66,69,78 Okumus et al studied the efficacy of 0.05% cyclosporine (Restasis®, Allergan, Irvine, California, USA) once per day or once every other day, in treatment of SEIs secondary to adenoviral epidemic keratoconjunctivitis in cases persisting for more than three months. Patients in this study had also initially been treated with topical corticosteroids for several months with no regression, or who had to discontinue due to subsequent, elevated IOP. After one month of 0.05% cyclosporine treatment, 81.75% of eyes had cleared SEIs while the remaining 18.2% had decreased in number. No systemic or ocular side effects were observed. Few cases (11.12%) in this study resulted in recurrence of SEIs after discontinuing treatment.69
Cyclosporine is likely effective in these cases due to its action in the inhibition of T cell proliferation and activation, thereby reducing ocular surface inflammation.77 It has been shown to be efficacious in various ocular diseases, such as vernal keratoconjunctivitis, ulcerative keratitis, Thygeson superficial punctate keratitis, herpes stromal keratitis, dry eye disease, superior limbic keratoconjunctivitis, and many others.69,79-81
Asena et al also evaluated treatment options for acute adenoviral keratoconjunctivitis using either topical 1% prednisolone acetate in conjunction with non-preserved artificial tears, topical 2% Cyclosporine A with non-preserved artificial tears, or non-preserved artificial tears alone. In comparison to artificial tears alone, both the corticosteroid and cyclosporine groups exhibited improvement in symptoms as well as a shorter duration. Both the Cyclosporine A and prednisolone groups were also similarly effective in preventing the development of SEIs when used during the active phase of infection, suggesting possible prophylactic benefit.72
Tacrolimus is another immunosuppressive agent that also demonstrates anti-inflammatory activity. Like cyclosporine, tacrolimus’ initial use was to prevent rejection in organ transplants. However, despite similar effects, tacrolimus and cyclosporine differ in their chemical makeup. Cyclosporine is a cyclic endecapeptide, in contrast to tacrolimus, which is a macrocyclic lactone. Both are calcineurin inhibitors, an enzyme necessary for T cell replication.82,83 When cyclosporine and tacrolimus enter T cells, they bind to their respective immunophilins, which are important proteins that interact with calcineurin, and inhibit their action. Blocking calcineurin subsequently inhibits the transcription of several cytokines that are necessary to the immune pathway and response, particularly IL-2. This cytokine is integral in the maintenance, differentiation, and survival of CD4+ T cells and CD8+ T cells.70,84-86
Topical tacrolimus 0.03% has ophthalmic uses, mainly in the treatment of giant papillary conjunctivitis and vernal keratoconjunctivitis. With regards to its effect on HAdV keratoconjunctivitis, tacrolimus was more effective than dexamethasone in the reduction of SEIs, and subsequently in the improvement of vision and symptomology. This study is significant in that tacrolimus was not only superior to topical corticosteroids in the resolution of clinical signs and symptoms but also offered a lower recurrence rate and no significant rise in IOP as is sometimes seen with steroid use. Adverse effects were observed in 17.8% of patients using tacrolimus, manifesting as burning, redness, and foreign body sensation.70 Generally, for SEIs that are resistant to tapering of corticosteroid, tacrolimus 0.03% was proven to be an effective corticosteroid-sparing agent.87
Another promising treatment for HAdV is adoptive T cell therapy. This involves the transfer of virus-specific T cells into Tcell-depleted patients to fight infection. HAdV-specific T cells are present in peripheral blood of healthy individuals in low frequencies; as a result, donor leukocytes were reportedly useful in patients with severe HAdV infection. HAdV-specific T cells play a significant role in viral clearance, and as such, adoptive transfer of HAdV-specific T cells would be an immunotherapeutic agent of immense benefit for patients at risk of disseminated adenoviral infection. The method of adoptive T cell therapy involves harvested leukocytes that are stimulated and expanded in vitro using peptide-MHC I tetramers, instead of using lymphocytes derived from the same donor as the transplant which yielded results as early as two weeks.13,44 This therapy along with antiviral use appears to be synergistic and efficacious.44
Cardiotonic steroids digoxin and digitoxin have been suggested to offer a new strategy to target and suppress HAdV. Historically, these drugs have been used to treat heart failure for over 200 years. More recently, they have also shown efficacy in cancer treatments, in that treated patients demonstrated a lesser chance of relapse.88 Hartley et al first noted its potential antiviral benefit, noting its efficacy against HAdV and herpesviruses.89 This is due to the drugs inhibiting Na+/K+ ATPase on the cell surface, which leads to increased intracellular levels of NA+ and, subsequently, Ca++. Na+/K+ ATPase is an important cell signaling molecule, where binding with digoxin or digitoxin initiates multiple signaling cascades, influencing gene expression. These drugs also alter RNA splicing, which is necessary to HAdV replication. Preliminary data supports a concentration-dependent reduction in the number of infected cells and no apparent damage. Although there is known toxicity, particularly with long-term use of digoxin, the antiviral uses of cardiotonic steroids would be short term, negating the associated systemic complications. As such, these drugs seem to be efficacious against HAdV serotypes A-D and serve as potential treatment therapies for the treatment and prophylaxis of EKC.88 Thus, repurposing of these cardiotonic steroids as an antiviral agent for short-term treatment of adenoviral keratoconjunctivitis as well as a prophylactic for use in individuals in close contact with patients with epidemic keratoconjunctivitis.88 Furthermore, Marrugal-Lorenzo et al reported that mifepristone, a synthetic steroid drug, possesses anti-adenoviral properties via its interference with viral entry into the nucleus. As such, it could be repurposed for treating adenoviral infections.90
Combination Dexamethasone/PVI Treatment
The potential benefits of PVI as well as topical immunosuppressive therapies gave rise to the development of combined PVI/steroid ophthalmic solutions. The idea of combining these elements is to employ the antiseptic properties of PVI in conjunction with symptomatic relief of inflammation, with the added benefit of reducing the risk and/or treatment of SEI formation and scarring.2,52,72,91 Initial efficacy of combo PVI 0.4%/dexamethasone 0.1% dosed QID for five days was evaluated in a small study of nine eyes utilizing Rapid Pathogen Screening Adeno Detector-positive acute viral conjunctivitis.91 The pilot study included both clinical (conjunctival injection and discharge) and serological (reduction of quantitative polymerase chain reaction titers and eradication of infectious virus as determined by cell culture with confirmatory immunofluorescence) endpoints.91
Combination treatment dosed QID for seven days has also been shown to improve clinical scores for scleral inflammation, ocular neovascularization, eyelid inflammation, friability of vasculature, inflammatory discharge, and epiphora as compared to treatment with 0.5% cidofovir, tobramycin/dexamethasone ophthalmic suspension, and balanced salt solution in rabbit models.52 Moreover, PVI/dexamethasone combination has proven effective in reducing viral titers and delaying viral shedding.52
When comparing PVI/dexamethasone combination treatment with that of dexamethasone alone, combination drops not only increase recovery but also, reduce the risk of development of SEIs more effectively than steroids alone.92 It is well documented that sole topical corticosteroid therapy has the risk of increased viral replication and prolonged shedding, further delaying the resolution of active infection. This significant treatment challenge is alleviated with the addition of PVI.92
In addition to the mentioned treatment benefits, combination PVI/dexamethasone ophthalmic formulation displayed a favorable safety profile and was well tolerated when administered for up to 14 days.93 There was also no statistically significant effect on intraocular pressure, as is often associated with topical corticosteroid use. Reported side effects were limited to increased stinging upon instillation when compared to treatment with palliative artificial tears.94
Surgical Management
Surgical management is usually not required in adenoviral keratoconjunctivitis; however, it may be necessary in cases of significant fibrotic remodeling of the conjunctiva or sustained corneal scarring with visual consequence. Membranes that develop on the mucosal surface can be differentiated into pseudomembranes or true membranes, with the latter being clinically distinguished by induced bleeding upon denuding.1
Persistence of pseudomembrane can lead to subepithelial fibrosis of the conjunctival mucosa, symblepharon formation, and punctal occlusion.1 Thus, rare cases may require repair of the fornix and associated lid anatomy defect (entropion or ectropion). Furthermore, streptococcal co-infection may be present in severe cases, with membranes that can precipitate corneal perforation and necessitate treatment.95
For patients with chronic adenoviral corneal opacification following resolution of acute infection, phototherapeutic keratectomy (PTK) can be an alternative option to other corneal surgeries or transplants. Corneal transparency can be compromised, or corneal irregularity may result with sequelae from the immune response to pathogen. Fortuitously, these scars are often superficial in nature by virtue of affecting the subepithelial layer. Transepithelial PTK low dose mitomycin C has shown benefit in such cases with reported improvements in photophobia, best corrected vision, and contrast sensitivity.96 Furthermore, a decrease in coma, secondary astigmatism, and total higher-order aberrations have been noted after PTK for SEIs in adenoviral keratoconjunctivitis.97
Conclusion and Future Perspectives
Though many consider adenoviral conjunctivitis a disease of self-limitation, the economic burden on affected individuals, high contagion risk, and potential long-term visual complications require a treatment protocol. Studies have indicated that the inflammatory process affecting the cornea begins in the prodromal period of adenoviral infections, thus questioning the theory of self-limitation and adding a potential factor immediate therapeutic management.98
Dissemination of HAdV is also a significant health burden particularly in individuals with factors that put them at great risk of morbidity and mortality from adenoviral infection. These individuals include children, elderly, immunocompromised individual, patients with acute Graft versus Host disease, those on immunosuppressants, and so on. Because there are no FDA-approved drugs to treat HAdV infection, eye care providers use multiple pharmaceuticals off-label to manage HAdV ocular infection. Finally, the propensity for latent HAdV to become reactivated and easily transmissible warrants the need for additional research in developing an effective, prophylactic antiviral drug. New treatments under consideration include sialic acid analogs, cold atmospheric plasma (CAP), N-chlorotaurine, and even benzalkonium chloride (BAK). Sialic acid plays a role in initial attachment of fiber knobs in adenoviral virions while facilitating accumulation.95 Sialic acid analogs have been theorized to block sialic acid-containing glycans which act as cellular receptors as a topical treatment.95,99
CAP is of use in dermatological disease and chronic wounds and has demonstrated adenoviral type-dependent antiviral effect that may be of benefit.100 N-chlorotaurine, an endogenous antimicrobial agent, has been shown to shorten duration of illness and is well tolerated in in vitro and in vivo experiments; however, Phase II clinical trials were prematurely ended due to inability to meet primary and secondary endpoints.101 These endpoints included clearing of bulbar conjunctiva, eradication of virus from tear film, fellow eye involvement, reduction of SEIs, and clearing of vision.102 BAK is a commonly found preservative in ophthalmic formulations typically present as 0.01% concentration. Studies prove the effectivity of BAK as an antiviral agent against adenovirus in concentrations higher than 0.1%; however, note the disadvantage of ocular toxicity from the known disinfectant.103
Future considerations in the management of adenoviral keratoconjunctivitis should require consideration of antisepsis and mitigation of sequelae from the host inflammatory response. Furthermore, special consideration for persistent latent HAdV subtypes and immunocompromised individuals is required. Identification of exact mechanisms that underlie the adaptive immune response in adenoviral infections is prudent to the development of novel therapeutic sources. However, the lack of animal models that accurately mimic complexity of human ocular anatomy while also illustrating proper replication and infection of human adenoviruses can prove decelerating for new discovery.95 Table 2 highlights the efficacy and adverse effects of antiviral and anti-inflammatory agents discussed in this review.2,46,55,63,64,66,69,87,104-124 In summary, there are several challenges regarding the treatment of adenoviral keratoconjunctivitis. Though there are solutions to some of these issues, more research is required to determine a standard protocol (Table 3).
 |
Table 2 Efficacy and Adverse Effects of Antiviral and Anti-Inflammatory Agents |
 |
Table 3 Challenges and Solutions in the Management of Adenoviral Keratoconjunctivitis |
Disclosure
The authors report no financial affiliations or conflicts of interest in this work.
References
1. Chintakuntlawar AV, Chodosh J. Cellular and tissue architecture of conjunctival membranes in epidemic keratoconjunctivitis. Ocul Immunol Inflamm. 2010;18(5):341–345. doi:10.3109/09273948.2010.498658
2. Chigbu DI, Labib BA. Pathogenesis and management of adenoviral keratoconjunctivitis. Infect Drug Resist. 2018;11:981–993. doi:10.2147/IDR.S162669
3. Radke JR, Cook JL. Human adenovirus infections: update and consideration of mechanisms of viral persistence. Curr Opin Infect Dis. 2018;31(3):251–256. doi:10.1097/QCO.0000000000000451
4. Iacobelli-Martinez M, Nemerow GR. Preferential activation of toll-like receptor nine by CD46-utilizing adenoviruses. J Virol. 2007;81(3):1305–1312. doi:10.1128/JVI.01926-06
5. Iacobelli-martinez M, Nepomuceno RR, Connolly J, Nemerow GR. CD46-utilizing adenoviruses inhibit C/EBPbeta-dependent expression of proinflammatory cytokines. J Virol. 2005;79(17):11259–11268. doi:10.1128/JVI.79.17.11259-11268.2005
6. Chen RF, Lee CY. Adenoviruses types, cell receptors and local innate cytokines in adenovirus infection. Int Rev Immunol. 2014;33(1):45–53. doi:10.3109/08830185.2013.823420
7. Hendrickx R, Stichling N, Koelen J, Kuryk L, Lipiec A, Greber UF. Innate immunity to adenovirus. Hum Gene Ther. 2014;25(4):265–284. doi:10.1089/hum.2014.001
8. Nemerow GR, Pache L, Reddy V, Stewart PL. Insights into adenovirus host cell interactions from structural studies. Virology. 2009;384(2):380–388. doi:10.1016/j.virol.2008.10.016
9. Smith JG, Wiethoff CM, Stewart PL, Nemerow GR. Adenovirus. Curr Top Microbiol Immunol. 2010;343:195–224. doi:10.1007/82_2010_16
10. Gordon YJ, Araullo-Cruz TP, Johnson YF, Romanowski EG, Kinchington PR. Isolation of human adenovirus type 5 variants resistant to the antiviral cidofovir. Invest Ophthalmol Vis Sci. 1996;37(13):2774–2778.
11. Kosulin K. Intestinal HAdV infection: tissue specificity, persistence, and implications for antiviral therapy. Viruses. 2019;11:9. doi:10.3390/v11090804
12. Ghebremedhin B. Human adenovirus: viral pathogen with increasing importance. Eur J Microbiol Immunol (Bp). 2014;4(1):26–33. doi:10.1556/EuJMI.4.2014.1.2
13. Lion T. Adenovirus infections in immunocompetent and immunocompromised patients. Clin Microbiol Rev. 2014;27(3):441–462. doi:10.1128/CMR.00116-13
14. Kalu SU, Loeffelholz M, Beck E, et al. Persistence of adenovirus nucleic acids in nasopharyngeal secretions: a diagnostic conundrum. Pediatr Infect Dis J. 2010;29(8):746–750. doi:10.1097/INF.0b013e3181d743c8
15. Flammer JR, Dobrovolna J, Kennedy MA, et al. The type I interferon signaling pathway is a target for glucocorticoid inhibition. Mol Cell Biol. 2010;30(19):4564–4574. doi:10.1128/MCB.00146-10
16. Hu X, Li WP, Meng C, Ivashkiv LB. Inhibition of IFN-gamma signaling by glucocorticoids. J Immunol. 2003;170(9):4833–4839. doi:10.4049/jimmunol.170.9.4833
17. Garnett CT, Talekar G, Mahr JA, et al. Latent species C adenoviruses in human tonsil tissues. J Virol. 2009;83(6):2417–2428. doi:10.1128/JVI.02392-08
18. Kosulin K, Geiger E, Vecsei A, et al. Persistence and reactivation of human adenoviruses in the gastrointestinal tract. Clin Microbiol Infect. 2016;22(4):381 e381–381 e388. doi:10.1016/j.cmi.2015.12.013
19. Lion T. Adenovirus persistence, reactivation, and clinical management. FEBS Lett. 2019;593:3571–3582. doi:10.1002/1873-3468.13576
20. Garnett CT, Erdman D, Xu W, Gooding LR. Prevalence and quantitation of species C adenovirus DNA in human mucosal lymphocytes. J Virol. 2002;76(21):10608–10616. doi:10.1128/JVI.76.21.10608-10616.2002
21. Kosulin K, Pichler H, Lawitschka A, Geyeregger R, Lion T. Diagnostic parameters of adenoviremia in pediatric stem cell transplant recipients. Front Microbiol. 2019;10:414. doi:10.3389/fmicb.2019.00414
22. Avolio M, De Rosa R, Modolo ML, Stano P, Camporese A. When should adenoviral non-gonococcal urethritis be suspected? Two case reports. New Microbiol. 2014;37(1):109–112.
23. Liddle OL, Samuel MI, Sudhanva M, Ellis J, Taylor C. Adenovirus urethritis and concurrent conjunctivitis: a case series and review of the literature. Sex Transm Infect. 2015;91(2):87–90. doi:10.1136/sextrans-2014-051868
24. Samaraweera GR, Garcia K, Druce J, et al. Characteristics of adenovirus urethritis among heterosexual men and men who have sex with men: a review of clinical cases. Sex Transm Infect. 2016;92(3):172–174. doi:10.1136/sextrans-2015-052243
25. Tabrizi SN, Ling AE, Bradshaw CS, Fairley CK, Garland SM. Human adenoviruses types associated with non-gonococcal urethritis. Sex Health. 2007;4(1):41–44. doi:10.1071/SH06054
26. de Jong JC, Wigand R, Wadell G, et al. Adenovirus 37: identification and characterization of a medically important new adenovirus type of subgroup D. J Med Virol. 1981;7(2):105–118. doi:10.1002/jmv.1890070204
27. Sun G, Su G, Bai Y, Yang H. Analysis on the treatment and prevention of epidemic conjunctivitis in 108 cases. Pak J Pharm Sci. 2017;30(4(Suppl.)):1501–1503.
28. Buehler JW, Finton RJ, Goodman RA, et al. Epidemic keratoconjunctivitis: report of an outbreak in an ophthalmology practice and recommendations for prevention. Infect Control. 1984;5(8):390–394. doi:10.1017/S0195941700062238
29. Omar Akhtar A, Singh H, Si F, Hodge WG. A systematic review and cost-effectiveness analysis of tonometer disinfection methods. Can J Ophthalmol. 2014;49(4):345–350. doi:10.1016/j.jcjo.2014.04.003
30. Vermeulen H, Westerbos SJ, Ubbink DT. Benefit and harm of iodine in wound care: a systematic review. J Hosp Infect. 2010;76(3):191–199. doi:10.1016/j.jhin.2010.04.026
31. Reimer K, Wichelhaus TA, Schafer V, et al. Antimicrobial effectiveness of povidone-iodine and consequences for new application areas. Dermatology. 2002;204(Suppl 1):114–120. doi:10.1159/000057738
32. Bigliardi PL, Alsagoff SAL, El-kafrawi HY, Pyon JK, Wa CTC, Villa MA. Povidone iodine in wound healing: a review of current concepts and practices. Int J Surg. 2017;44:260–268. doi:10.1016/j.ijsu.2017.06.073
33. Beukelman CJ, van den Berg AJ, Hoekstra MJ, Uhl R, Reimer K, Mueller S. Anti-inflammatory properties of a liposomal hydrogel with povidone-iodine (Repithel) for wound healing in vitro. Burns. 2008;34(6):845–855. doi:10.1016/j.burns.2007.11.014
34. Yazar H, Yarbag A, Balci M, Teker B, Tanyeri P. The effects of povidone iodine (pH 4.2) on patients with adenoviral conjunctivitis. J Pak Med Assoc. 2016;66(8):968–970.
35. Sauerbrei A, Wutzler P. Virucidal efficacy of povidone-iodine-containing disinfectants. Lett Appl Microbiol. 2010;51(2):158–163. doi:10.1111/j.1472-765X.2010.02871.x
36. Yates KA, Shanks RMQ, Kowalski RP, Romanowski EG. The in vitro evaluation of povidone-iodine against multiple ocular adenoviral types. J Ocul Pharmacol Ther. 2019;35(2):132–136. doi:10.1089/jop.2018.0122
37. Cheung D, Bremner J, Chan JT. Epidemic kerato-conjunctivitis–do outbreaks have to be epidemic? Eye (Lond). 2003;17(3):356–363. doi:10.1038/sj.eye.6700330
38. Dart JK, El-amir AN, Maddison T, et al. Identification and control of nosocomial adenovirus keratoconjunctivitis in an ophthalmic department. Br J Ophthalmol. 2009;93(1):18–20. doi:10.1136/bjo.2007.130112
39. Eggers M, Eickmann M, Zorn J. Rapid and effective virucidal activity of povidone-iodine products against Middle East Respiratory Syndrome Coronavirus (MERS-CoV) and Modified Vaccinia Virus Ankara (MVA). Infect Dis Ther. 2015;4(4):491–501. doi:10.1007/s40121-015-0091-9
40. Isenberg SJ, Apt L, Valenton M, et al. A controlled trial of povidone-iodine to treat infectious conjunctivitis in children. Am J Ophthalmol. 2002;134(5):681–688. doi:10.1016/S0002-9394(02)01701-4
41. Pham TT, Burchette JL
42. Kim SJ, Kim K, Park SB, Hong DJ, Jhun BW. Outcomes of early administration of cidofovir in non-immunocompromised patients with severe adenovirus pneumonia. PLoS One. 2015;10(4):e0122642. doi:10.1371/journal.pone.0122642
43. Ying B, Tollefson AE, Spencer JF, et al. Ganciclovir inhibits human adenovirus replication and pathogenicity in permissive immunosuppressed Syrian hamsters. Antimicrob Agents Chemother. 2014;58(12):7171–7181. doi:10.1128/AAC.03860-14
44. Wold WS, Toth K. New drug on the horizon for treating adenovirus. Expert Opin Pharmacother. 2015;16(14):2095–2099. doi:10.1517/14656566.2015.1083975
45. Bruno B, Gooley T, Hackman RC, Davis C, Corey L, Boeckh M. Adenovirus infection in hematopoietic stem cell transplantation: effect of ganciclovir and impact on survival. Biol Blood Marrow Transplant. 2003;9(5):341–352. doi:10.1016/S1083-8791(03)00102-2
46. Huang J, Kadonosono K, Uchio E. Antiadenoviral effects of ganciclovir in types inducing keratoconjunctivitis by quantitative polymerase chain reaction methods. Clin Ophthalmol. 2014;8:315–320. doi:10.2147/OPTH.S55284
47. Toth K, Ying B, Tollefson AE, et al. Valganciclovir inhibits human adenovirus replication and pathology in permissive immunosuppressed female and male Syrian hamsters. Viruses. 2015;7(3):1409–1428. doi:10.3390/v7031409
48. de Oliveira CB, Stevenson D, LaBree L, McDonnell PJ, Trousdale MD. Evaluation of Cidofovir (HPMPC, GS-504) against adenovirus type 5 infection in vitro and in a New Zealand rabbit ocular model. Antiviral Res. 1996;31(3):165–172. doi:10.1016/0166-3542(95)00962-0
49. Romanowski EG, Yates KA, Gordon YJ. Antiviral prophylaxis with twice daily topical cidofovir protects against challenge in the adenovirus type 5/New Zealand rabbit ocular model. Antiviral Res. 2001;52(3):275–280. doi:10.1016/S0166-3542(01)00166-8
50. Gordon YJ, Romanowski EG, Araullo-Cruz T. Topical HPMPC inhibits adenovirus type 5 in the New Zealand rabbit ocular replication model. Invest Ophthalmol Vis Sci. 1994;35(12):4135–4143.
51. Inoue H, Sonoda KH, Ishikawa M, Kadonosono K, Uchio E. Clinical evaluation of local ocular toxicity in candidate anti-adenoviral agents in vivo. Ophthalmologica. 2009;223(4):233–238. doi:10.1159/000205585
52. Clement C, Capriotti JA, Kumar M, et al. Clinical and antiviral efficacy of an ophthalmic formulation of dexamethasone povidone-iodine in a rabbit model of adenoviral keratoconjunctivitis. Invest Ophthalmol Vis Sci. 2011;52(1):339–344. doi:10.1167/iovs.10-5944
53. Kinchington PR, Araullo-Cruz T, Vergnes JP, Yates K, Gordon YJ. Sequence changes in the human adenovirus type 5 DNA polymerase associated with resistance to the broad spectrum antiviral cidofovir. Antiviral Res. 2002;56(1):73–84. doi:10.1016/S0166-3542(02)00098-0
54. Romanowski EG, Gordon YJ, Araullo-Cruz T, Yates KA, Kinchington PR. The antiviral resistance and replication of cidofovir-resistant adenovirus variants in the New Zealand White rabbit ocular model. Invest Ophthalmol Vis Sci. 2001;42(8):1812–1815.
55. Grimley MS, Chemaly RF, Englund JA, et al. Brincidofovir for asymptomatic adenovirus viremia in pediatric and adult allogeneic hematopoietic cell transplant recipients: a randomized placebo-controlled phase II trial. Biol Blood Marrow Transplant. 2017;23(3):512–521. doi:10.1016/j.bbmt.2016.12.621
56. Averbuch D, Safadi R, Dar D, et al. Successful brincidofovir treatment of metagenomics-detected adenovirus infection in a severely ill signal transducer and activator of transcription-1-deficient patient. Pediatr Infect Dis J. 2019;38(3):297–299. doi:10.1097/INF.0000000000002090
57. 2017 American transplant congress abstracts. Am J Transplant. 2017;17(Suppl 3):5–815.
58. Goossens PH, Vogels R, Pieterman E, et al. The influence of synovial fluid on adenovirus-mediated gene transfer to the synovial tissue. Arthritis Rheum. 2001;44(1):48–52. doi:10.1002/1529-0131(200101)44:1<48::AID-ANR7>3.0.CO;2-D
59. Nwanegbo EC, Romanowski EG, Gordon YJ, Gambotto A. Efficacy of topical immunoglobulins against experimental adenoviral ocular infection. Invest Ophthalmol Vis Sci. 2007;48(9):4171–4176. doi:10.1167/iovs.07-0491
60. Martin T, Duffy SF, Carson DA, Kipps TJ. Evidence for somatic selection of natural autoantibodies. J Exp Med. 1992;175(4):983–991. doi:10.1084/jem.175.4.983
61. Ozturk HE, Sonmez B, Beden U. Corneal sensitivity may decrease in adenoviral epidemic keratoconjunctivitis–a confocal microscopic study. Eye Contact Lens. 2013;39(4):264–268. doi:10.1097/ICL.0b013e31828ef19b
62. Meyer-rusenberg B, Loderstadt U, Richard G, Kaulfers PM, Gesser C. Epidemic keratoconjunctivitis: the current situation and recommendations for prevention and treatment. Dtsch Arztebl Int. 2011;108(27):475–480. doi:10.3238/arztebl.2011.0475
63. Kaufman HE. Treatment of viral diseases of the cornea and external eye. Prog Retin Eye Res. 2000;19(1):69–85. doi:10.1016/S1350-9462(99)00004-X
64. Hillenkamp J, Reinhard T, Ross RS, et al. The effects of cidofovir 1% with and without cyclosporin a 1% as a topical treatment of acute adenoviral keratoconjunctivitis: a controlled clinical pilot study. Ophthalmology. 2002;109(5):845–850. doi:10.1016/S0161-6420(02)00992-2
65. Hillenkamp J, Reinhard T, Ross RS, et al. Topical treatment of acute adenoviral keratoconjunctivitis with 0.2% cidofovir and 1% cyclosporine: a controlled clinical pilot study. Arch Ophthalmol. 2001;119(10):1487–1491. doi:10.1001/archopht.119.10.1487
66. Levinger E, Slomovic A, Sansanayudh W, Bahar I, Slomovic AR. Topical treatment with 1% cyclosporine for subepithelial infiltrates secondary to adenoviral keratoconjunctivitis. Cornea. 2010;29(6):638–640. doi:10.1097/ICO.0b013e3181c33034
67. Reinhard T, Godehardt E, Pfahl HG, Sundmacher R. [Local cyclosporin A in nummuli after keratoconjunctivitis epidemica. A pilot study]. Ophthalmologe. 2000;97(11):764–768. German. doi:10.1007/s003470070025.
68. Kilic A, Gurler B. Topical 2% cyclosporine A in preservative-free artificial tears for the treatment of vernal keratoconjunctivitis. Can J Ophthalmol. 2006;41(6):693–698. doi:10.3129/i06-061
69. Okumus S, Coskun E, Tatar MG, et al. Cyclosporine a 0.05% eye drops for the treatment of subepithelial infiltrates after epidemic keratoconjunctivitis. BMC Ophthalmol. 2012;12:42. doi:10.1186/1471-2415-12-42
70. Bhargava R, Kumar P. Comparison of the safety and efficacy of topical Tacrolimus (0.03%) versus dexamethasone (0.05%) for subepithelial infiltrates after adenoviral conjunctivitis. Indian J Ophthalmol. 2019;67(5):594–598. doi:10.4103/ijo.IJO_1352_18
71. Kocluk Y, Sukgen EA, Cevher S, Mat E. Symptomatic treatment of subepithelial infiltrates after viral conjunctivitis: loteprednol or dexamethasone? Ocul Immunol Inflamm. 2017;25(5):649–653. doi:10.3109/09273948.2016.1149593
72. Asena L, Singar Ozdemir E, Burcu A, Ercan E, Colak M, Altinors DD. Comparison of clinical outcome with different treatment regimens in acute adenoviral keratoconjunctivitis. Eye (Lond). 2017;31(5):781–787. doi:10.1038/eye.2017.4
73. Gordon YJ, Araullo-cruz T, Romanowski EG. The effects of topical nonsteroidal anti-inflammatory drugs on adenoviral replication. Arch Ophthalmol. 1998;116(7):900–905. doi:10.1001/archopht.116.7.900
74. Kessel L, Tendal B, Jorgensen KJ, et al. Post-cataract prevention of inflammation and macular edema by steroid and nonsteroidal anti-inflammatory eye drops: a systematic review. Ophthalmology. 2014;121(10):1915–1924. doi:10.1016/j.ophtha.2014.04.035
75. Malik A, Sadafale A, Gupta YK, Gupta A. A comparative study of various topical nonsteroidal anti-inflammatory drugs to steroid drops for control of post cataract surgery inflammation. Oman J Ophthalmol. 2016;9(3):150–156. doi:10.4103/0974-620X.192268
76. Dunn JP. Uveitis. Prim Care. 2015;42(3):305–323. doi:10.1016/j.pop.2015.05.003
77. Nussenblatt RB, Palestine AG. Cyclosporine: immunology, pharmacology and therapeutic uses. Surv Ophthalmol. 1986;31(3):159–169. doi:10.1016/0039-6257(86)90035-4
78. Romanowski EG, Pless P, Yates KA, Gordon YJ. Topical cyclosporine A inhibits subepithelial immune infiltrates but also promotes viral shedding in experimental adenovirus models. Cornea. 2005;24(1):86–91. doi:10.1097/01.ico.0000127481.23714.b6
79. Liegner JT, Yee RW, Wild JH. Topical cyclosporine therapy for ulcerative keratitis associated with rheumatoid arthritis. Am J Ophthalmol. 1990;109(5):610–612. doi:10.1016/S0002-9394(14)70704-4
80. Holland EJ, Olsen TW, Ketcham JM, et al. Topical cyclosporin A in the treatment of anterior segment inflammatory disease. Cornea. 1993;12(5):413–419. doi:10.1097/00003226-199309000-00008
81. Perry HD, Doshi-carnevale S, Donnenfeld ED, Kornstein HS. Topical cyclosporine A 0.5% as a possible new treatment for superior limbic keratoconjunctivitis. Ophthalmology. 2003;110(8):1578–1581. doi:10.1016/S0161-6420(03)00538-4
82. Fahr A. Cyclosporin clinical pharmacokinetics. Clin Pharmacokinet. 1993;24(6):472–495. doi:10.2165/00003088-199324060-00004
83. Thomson AW, Bonham CA, Zeevi A. Mode of action of tacrolimus (FK506): molecular and cellular mechanisms. Ther Drug Monit. 1995;17(6):584–591. doi:10.1097/00007691-199512000-00007
84. Barbarino JM, Staatz CE, Venkataramanan R, Klein TE, Altman RB. PharmGKB summary: cyclosporine and tacrolimus pathways. Pharmacogenet Genomics. 2013;23(10):563–585. doi:10.1097/FPC.0b013e328364db84
85. Boyman O, Sprent J. The role of interleukin-2 during homeostasis and activation of the immune system. Nat Rev Immunol. 2012;12(3):180–190. doi:10.1038/nri3156
86. Almawi WY, Melemedjian OK. Clinical and mechanistic differences between FK506 (tacrolimus) and cyclosporin A. Nephrol Dial Transplant. 2000;15(12):1916–1918. doi:10.1093/ndt/15.12.1916
87. Ghanem RC, Vargas JF, Ghanem VC. Tacrolimus for the treatment of subepithelial infiltrates resistant to topical steroids after adenoviral keratoconjunctivitis. Cornea. 2014;33(11):1210–1213. doi:10.1097/ICO.0000000000000247
88. Grosso F, Stoilov P, Lingwood C, Brown M, Cochrane A. Suppression of adenovirus replication by cardiotonic steroids. J Virol. 2017;91:3. doi:10.1128/JVI.01623-16
89. Hartley C, Hartley M, Pardoe I, Knight A. Ionic Contra-Viral Therapy (ICVT); a new approach to the treatment of DNA virus infections. Arch Virol. 2006;151(12):2495–2501. doi:10.1007/s00705-006-0824-x
90. Marrugal-lorenzo JA, Serna-gallego A, Gonzalez-gonzalez L, et al. Inhibition of adenovirus infection by mifepristone. Antiviral Res. 2018;159:77–83. doi:10.1016/j.antiviral.2018.09.011
91. Pelletier JS, Stewart K, Trattler W, et al. A combination povidone-iodine 0.4%/dexamethasone 0.1% ophthalmic suspension in the treatment of adenoviral conjunctivitis. Adv Ther. 2009;26(8):776–783. doi:10.1007/s12325-009-0062-1
92. Kovalyuk N, Kaiserman I, Mimouni M, et al. Treatment of adenoviral keratoconjunctivitis with a combination of povidone-iodine 1.0% and dexamethasone 0.1% drops: a clinical prospective controlled randomized study. Acta Ophthalmol. 2017;95(8):e686–e692. doi:10.1111/aos.13416
93. Pepose JS, Narvekar A, Liu W, Haque R. A randomized controlled trial of povidone-iodine/dexamethasone ophthalmic suspension for acute viral conjunctivitis. Clin Ophthalmol. 2019;13:535–544. doi:10.2147/OPTH.S191275
94. Pinto RD, Lira RP, Abe RY, et al. Dexamethasone/povidone eye drops versus artificial tears for treatment of presumed viral conjunctivitis: a randomized clinical trial. Curr Eye Res. 2015;40(9):870–877. doi:10.3109/02713683.2014.964419
95. Gonzalez G, Yawata N, Aoki K, Kitaichi N. Challenges in management of epidemic keratoconjunctivitis with emerging recombinant human adenoviruses. J Clin Virol. 2019;112:1–9. doi:10.1016/j.jcv.2019.01.004
96. Yamazaki ES, Ferraz CA, Hazarbassanov RM, Allemann N, Campos M. Phototherapeutic keratectomy for the treatment of corneal opacities after epidemic keratoconjunctivitis. Am J Ophthalmol. 2011;151(1):35–43 e31. doi:10.1016/j.ajo.2010.07.028
97. Kepez Yildiz B, Urvasizoglu S, Yildirim Y, et al. Changes in higher-order aberrations after phototherapeutic keratectomy for subepithelial corneal infiltrates after epidemic keratoconjunctivitis. Cornea. 2017;36(10):1233–1236. doi:10.1097/ICO.0000000000001296
98. Subasi S, Yuksel N, Toprak M, Yilmaz Tugan B. In vivo confocal microscopy analysis of the corneal layers in adenoviral epidemic keratoconjunctivitis. Turk J Ophthalmol. 2018;48(6):276–280. doi:10.4274/tjo.59251
99. Nilsson EC, Storm RJ, Bauer J, et al. The GD1a glycan is a cellular receptor for adenoviruses causing epidemic keratoconjunctivitis. Nat Med. 2011;17(1):105–109. doi:10.1038/nm.2267
100. Bunz O, Mese K, Zhang W, Piwowarczyk A, Ehrhardt A, Hamblin MR. Effect of cold atmospheric plasma (CAP) on human adenoviruses is adenovirus type-dependent. PLoS One. 2018;13(10):e0202352. doi:10.1371/journal.pone.0202352
101. de Mercoyrol L, Corda Y, Job C, Job D. Accuracy of wheat-germ RNA polymerase II. General enzymatic properties and effect of template conformational transition from right-handed B-DNA to left-handed Z-DNA. Eur J Biochem. 1992;206(1):49–58. doi:10.1111/ejb.1992.206.issue-1
102. Garcia-zalisnak D, Rapuano C, Sheppard JD, Davis AR. Adenovirus ocular infections: prevalence, pathology, pitfalls, and practical pointers. Eye Contact Lens. 2018;44(Suppl 1):S1–S7. doi:10.1097/ICL.0000000000000226
103. Romanowski EG, Yates KA, Shanks RMQ, Kowalski RP. Benzalkonium chloride demonstrates concentration-dependent antiviral activity against adenovirus in vitro. J Ocul Pharmacol Ther. 2019;35(5):311–314. doi:10.1089/jop.2018.0145
104. Saedon H, Nosek J, Phillips J, Narendran N, Yang YC. Ocular surface effects of repeated application of povidone iodine in patients receiving frequent intravitreal injections. Cutan Ocul Toxicol. 2017;36(4):343–346. doi:10.1080/15569527.2017.1291665
105. Tollefson AE, Spencer JF, Ying B, Buller RM, Wold WS, Toth K. Cidofovir and brincidofovir reduce the pathology caused by systemic infection with human type 5 adenovirus in immunosuppressed Syrian hamsters, while ribavirin is largely ineffective in this model. Antiviral Res. 2014;112:38–46. doi:10.1016/j.antiviral.2014.10.005
106. Gordon YJ, Naesens L, DeClercq E, Maudgal PC, Veckeneer M. Treatment of adenoviral conjunctivitis with topical cidofovir. Cornea. 1996;15(5):546. doi:10.1097/00003226-199609000-00018
107. Kinchington PR, Romanowski EG, Jerold Gordon Y. Prospects for adenovirus antivirals. J Antimicrob Chemother. 2005;55(4):424–429. doi:10.1093/jac/dki057
108. Yabiku ST, Yabiku MM, Bottos KM, Araujo AL, Freitas D, Belfort R
109. Ozen S, Ozer MA. Ganciclovir ophthalmic gel treatment shortens the recovery time and prevents complications in the adenoviral eye infection. Int Ophthalmol. 2017;37(1):245–249. doi:10.1007/s10792-016-0260-1
110. Hartline CB, Gustin KM, Wan WB, et al. Ether lipid-ester prodrugs of acyclic nucleoside phosphonates: activity against adenovirus replication in vitro. J Infect Dis. 2005;191(3):396–399. doi:10.1086/jid.2005.191.issue-3
111. Van Genechten T, van Heerden J, Bauters T, Dhooge C. Successful treatment of adenovirus infection with brincidofovir in an immunocompromised patient after hematological stem cell transplantation. Case Rep Infect Dis. 2020;2020:5981289.
112. Meena JP, Phillips RS, Kinsey S. Brincidofovir as a salvage therapy in controlling adenoviremia in pediatric recipients of hematopoietic stem cell transplant. J Pediatr Hematol Oncol. 2019;41(7):e467–e472. doi:10.1097/MPH.0000000000001480
113. Sulejmani N, Nagai S, Safwan M, et al. Brincidofovir as salvage therapy for adenovirus disease in intestinal transplant recipients. Pharmacotherapy. 2018;38(4):470–475. doi:10.1002/phar.2018.38.issue-4
114. Chittick G, Morrison M, Brundage T, Nichols WG. Short-term clinical safety profile of brincidofovir: a favorable benefit-risk proposition in the treatment of smallpox. Antiviral Res. 2017;143:269–277. doi:10.1016/j.antiviral.2017.01.009
115. Marty FM, Winston DJ, Chemaly RF, et al. A randomized, double-blind, placebo-controlled phase 3 trial of oral brincidofovir for cytomegalovirus prophylaxis in allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2019;25(2):369–381. doi:10.1016/j.bbmt.2018.09.038
116. Daniel BS, Orchard D. Ocular side-effects of topical corticosteroids: what a dermatologist needs to know. Australas J Dermatol. 2015;56(3):164–169. doi:10.1111/ajd.2015.56.issue-3
117. Jeng BH, Holsclaw DS. Cyclosporine A 1% eye drops for the treatment of subepithelial infiltrates after adenoviral keratoconjunctivitis. Cornea. 2011;30(9):958–961. doi:10.1097/ICO.0b013e31820cd607
118. Muftuoglu IK, Akova YA, Gungor SG. Effect of 0.05% topical cyclosporine for the treatment of symptomatic subepithelial infiltrates due to adenoviral keratoconjunctivitis. Int J Ophthalmol. 2016;9(4):634–635. doi:10.18240/ijo.2016.04.26
119. Bayraktutar BN, Ucakhan OO. Comparison of efficacy of two different topical 0.05% cyclosporine a formulations in the treatment of adenoviral keratoconjunctivitis-related subepithelial infiltrates. Case Rep Ophthalmol. 2016;7(1):135–140. doi:10.1159/000444784
120. Pucci N, Caputo R, Mori F, et al. Long-term safety and efficacy of topical cyclosporine in 156 children with vernal keratoconjunctivitis. Int J Immunopathol Pharmacol. 2010;23(3):865–871. doi:10.1177/039463201002300322
121. Schultz C. Safety and efficacy of cyclosporine in the treatment of chronic dry eye. Ophthalmol Eye Dis. 2014;6:37–42. doi:10.4137/OED.S16067
122. Levinger E, Trivizki O, Shachar Y, Levinger S, Topical VD. 0.03% tacrolimus for subepithelial infiltrates secondary to adenoviral keratoconjunctivitis. Graefes Arch Clin Exp Ophthalmol. 2014;252(5):811–816. doi:10.1007/s00417-014-2611-9
123. Berisa Prado S, Riestra Ayora AC, Lisa Fernandez C, Chacon Rodriguez M, Merayo-lloves J, Alfonso Sanchez JF. Topical tacrolimus for corneal subepithelial infiltrates secondary to adenoviral keratoconjunctivitis. Cornea. 2017;36(9):1102–1105. doi:10.1097/ICO.0000000000001279
124. Barot RK, Shitole SC, Bhagat N, Patil D, Sawant P, Patil K. Therapeutic effect of 0.1% tacrolimus eye ointment in allergic ocular diseases. J Clin Diagn Res. 2016;10(6):NC05–NC09. doi:10.7860/JCDR/2016/17847.7978
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

