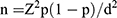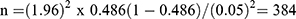Back to Journals » Risk Management and Healthcare Policy » Volume 13
Magnitude of Streptococcus pneumoniae Among Under-Five Children with Symptom of Acute Respiratory Infection at Hiwot Fana Specialized University Hospital, Harar, Ethiopia: Associated Risk Factors and Antibacterial Susceptibility Patterns
Authors Bayu D, Mekonnen A, Mohammed J, Bodena D
Received 25 September 2020
Accepted for publication 22 November 2020
Published 8 December 2020 Volume 2020:13 Pages 2919—2925
DOI https://doi.org/10.2147/RMHP.S283860
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Marco Carotenuto
Dejene Bayu,1 Abiyu Mekonnen,2 Jemal Mohammed,3 Dagne Bodena1
1Hiwot Fana Specialized University Hospital, College of Health and Medical Sciences, Haramaya University, Harar, Ethiopia; 2Department of Medical Laboratory Sciences, Menelik-II College of Health and Medical Sciences, Kotobe Metropolitan University, Addis Ababa, Ethiopia; 3Department of Medical Laboratory Sciences, College of Health and Medical Sciences, Haramaya University, Harar, Ethiopia
Correspondence: Dagne Bodena
Hiwot Fana Specialized University Hospital, College of Health and Medical Sciences, Haramaya University, PO Box 235, Harar, Ethiopia
Tel +251 910127241
Email [email protected]
Purpose: Streptococcus pneumoniae is the major cause of pneumoniae infection among under-five children that leads to high morbidity and mortality. Thus, the aim of this study was to determine the magnitude of Streptococcus pneumoniae in under-five children of an acute respiratory infection, assess its antimicrobial susceptibility patterns, and define the associated factors.
Methods: An institutional-based cross-sectional study was conducted on a total of 384 under-five children of acute respiratory infection attending outpatient department of Hiwot Fana Specialized University Hospital, Harar, Ethiopia, from March 1 to 30, 2020. Socio-demographic and clinical data were collected from the study participants using a structured questionnaire. Sputum samples were collected and processed to identify Streptococcus pneumoniae pathogen using the culture and biochemical tests as per the standard procedures. The Kirby–Bauer disk diffusion method was used for antimicrobial susceptibility testing. Data were entered into Epi-data version 3.1 and analyzed by using Statistical Product and Service Solutions version 22.
Results: The proportion of Streptococcus pneumoniae in under-five children with acute respiratory infection was 11.2%. About 50% of isolated Streptococcus pneumoniae was resistant to tetracycline and cotrimoxazole, whereas more than 90% of it was susceptible to Ceftriaxone and amoxicillin-clavulanate. Children who lived in rural areas were 3.6 times more likely to have S. pneumoniae compared to children who lived in urban areas (AOR: 3.6, 95% CI: 1.2– 11) and children with familysmokers in a house were 3 times at risk to be infected with S. pneumoniae (AOR: 3, 95% CI: 1.8– 8.0).
Conclusion: High antimicrobial resistance of S. pneumoniae against tetracycline and cotrimoxazole was observed and children who lived in rural areas and live with a family of cigarette smoker are factors associated with Streptococcus pneumoniae. Therefore, providing health educations to the family of children rural residents and isolating smokers from the house where children lived are recommended actions to reduce bacteria caused by Streptococcus pneumoniae.
Keywords: antibiotic susceptibility, children under-five, S. pneumoniae, Hiwot Fana Specialized University Hospital, Harar, Ethiopia
Background
Streptococcus pneumoniae is one of the most usual causes of childhood serious invasive infections, like pneumoniae, meningitis, and bacteremia. It is present in the commensal bacterial community in the human nasopharynx that is responsible for a public health problem and economic impact in both developed and developing countries.1,2
Host, socio-economic and environmental factors including daycare attendance, living in a household with other young children and symptoms of an upper respiratory tract infection are responsible for pneumococcal carriage.3
S. pneumoniae is the leading cause of bacterial pneumoniae and secondary pneumoniae following flu infection that kills more than under-five years of age each year than any other illness such as AIDS, malaria, and measles combined together world wide. Over 9 million cases of the pneumococcal disease were reported in children under five years, and more than 300,000 died due to these cases in 2015 and the majority of the cases occur in low- and middle-income countries.4,5
Moreover, the cost of antibiotic treatment for all children with pneumoniae in 42 of the world’s poorest countries is around US$ 600 million per year and around US$ 200 million incurred due to treating pneumoniae in South Asia and sub-Saharan Africa.6 Even though effective and inexpensive treatments are available, about 30% of children in Africa, particularly in sub-Saharan Africa of rural areas, receive antibiotics that cause bacterial pneumoniae remains a major cause of morbidity and mortality globally.7,8
Most of the developing countries with a high burden of morbidity and mortality associated with the acute respiratory infection including Ethiopia still lack the necessary data on the real burden, epidemiology, and etiology of ARI within their territories. Actually, in most of these countries, the estimation of the burden of ARI is based on specific surveys at the national or regional level.9
Lack of rapid diagnostic testing and limited data on antibiotic use for suspected childhood plays a major role in the continual existence of pneumoniae in the developing countries especially in vulnerable groups, like girls, rural-dwelling children, and poor communities.10,11
Despite a high burden of pneumoniae, there was no study done to determine pneumoniae among under-five children in Harar town, Ethiopia. Therefore, this study aimed to assess the magnitude, associated risk factors, and drug susceptibility patterns of Streptococcus pneumoniae among under-five children with a symptom of the acute respiratory infection in Hiwot Fana Specialized University Hospital, Harar, Ethiopia.
Materials and Methodology
Study Area, Design, and Period
Harar is the capital city of Harari Regional State, located 526 km away from East of Addis Ababa, the capital city of Ethiopia. Hiwot Fana Specialized University Hospital is one of the referrals and a teaching hospital, which was established during the occupation of Ethiopia by Italian soldiers. Currently, the hospital serves to about 5.2 million communities around Harari regional state and neighboring regions. The hospital has a total of 899 workers, 434 health professionals, and 465 administrative staff.12
Study Design and Period
An institutional cross-sectional study was conducted from March 1 to 30, 2020, in Hiwot Fana Specialized University Hospital, Harar, Ethiopia.
Study Population
All under-five children suspected of respiratory tract infection who visited Hiwot Fana Specialized university hospital pediatrics outpatient department were included. We have excluded children age of less than five years who start antibiotic treatment.
Sample Size Determination
The sample size was calculated by using a single population proportion formula
By considering the prevalence 48.6%, anticipated population proportion of S. pneumoniae from a study conducted in hospitals of Bahir Dar city, Ethiopia,13 at 95% confidence interval= 1.96 and 5% margin of error (d) =0.05
After adding 5% of non-response rate, the final sample size was 403.
Sampling Technique
A convenient sampling technique was used to select study participants until the required sample size was achieved.
Data Collection Methods
Data Collection by Face-to-Face Interview
The parents of the children were consented and interviewed for socio-demographic variables like age, gender, and factors of housing condition, vaccination status of the child, and a residence which was adopted from different literatures.14,15 Data were collected by trained data collectors after testing by pre-structured questionnaire. On-site supervision was carried out by a supervisor and principal investigator on a daily basis.
Sample Collection, Handling, and Transportation
A well-labeled, leak-proof, sterilized, wide mouth screw-cap universal container was given to the child’s parents to collect 5 mL of morning sputum, after that child’s parents were instructed to cleanse the area around the mouth opening with clean water. A deep breath was taken through the mouth and coughed up mucous with deep coughing. To avoid contamination, each individual was instructed on how to collect a cough specimen by laboratory personnel. All sputum specimens reached the laboratory as soon as collected.16 For those who cannot give sputum samples, lung aspiration was done by inserting a needle blindly over the top of a rib and applying a suction to the plunger of the syringe, and the needle was withdrawn while maintaining constant suction. The procedure was performed under sterile conditions by experienced nurses or doctors.
Conventional Identification of S. pneumoniae and Antimicrobial Susceptability Test
Conventional identification of S.pneumoniae and antimicrobial susceptibility testing (AST) should be done according to appropriate standards and Erythromycin (15 μg), Cotrimoxazole (25 μg), Ceftriaxone (30 μg), Tetracycline (30 μg), Chloramphenicol (30 μg), and Amoxicillin-clavulanate (5 μg) were used for AST.17
Operational Definition
Acute respiratory tract infection is an infectious cause disease of the upper or lower respiratory tract of Streptococcus pneumoniae.4
Data Quality Control
To ensure the quality of data, training of data collectors was undertaken and administration of pre-test among 5% of the total sample size to assess its clarity, length, completeness, and consistency. Also, the skip patterns were corrected and questions found to be difficult or misleading were rephrased. Supervisor and principal investigator were closely following the data collection process. Checking for completeness and accuracy of completed data collection forms was done at the end of each day of data collection and gaps identified were addressed with the respective research assistants. Sterility of culture media was checked by incubating 3–5% of the batch at 37°c and control each new batch of agar by testing it with a reference strain E. coli ATCC 25922.
Methods of Data Analyses
Data were entered and edited into EPI-data version 3.1, clean, and analyzed by using SPSS 22. Descriptive statistics of different variables were determined and the result was presented in texts and tables and summarized by using percentages. Logistic regression was carried out to identify the associated factors between variables and Streptococcus pneumoniae. Variable with P-value ≤0.25 was a candidate for the multivariate analysis. Finally, multivariate logistic regression analysis was performed for adjusted odds ratio with 95% confidence intervals and variables with a P-value <0.05 were declared as statistically significant.
Ethical Consideration
The study protocol was approved by the Health Research Ethics, and Review Committee (HRERC) of the College of Health and Medical Sciences, Haramaya University. Information on the study was explained to the participants, including the procedures, potential risks, and benefits of the study. Participants’ confidentiality of information was assured by excluding names and identifiers in the questionnaire. Informed written consent was obtained from all Childs’ parent prior to the study and there was a compliance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Results
Socio-Demographic Characteristics of the Respondents
Of 403 participants, 384 under-five children were included in the study with a response rate of 95%. 19 participants refused to give sputum samples and were excluded from the study. Among 384 under-five children patients, 206 (53.6%) of them were males. The majority of study participants 301 (78.4%) were Oromo and among all study participants, 247 (64.3%) were residents of rural areas (Table 1).
 |
Table 1 Socio-Demographic Characteristics of Under-Five Children Patients (n=384) at Hiwot Fana Specialized University Hospital, Harar, Ethiopia, March 1–30, 2020 |
A Behavioral Factors of the Study Participants
Among all study participants, 258 (67.2%) of the children’s families were cigarette smokers. Majority of them, 227 (59.1%), had diarrhea during the study period and frequency of diarrhea once, twice, three times, and more than three times per day were 7 (1.8%), 50 (13%), 108 (28%), and 62 (16.1%), respectively, and 3 (0.8%), 26 (6.8%), 53 (13.8%), 92 (24%) of their mothers have breastfeeding habit once per day, twice per day, three times per day, and more than three times per day, respectively (Table 2).
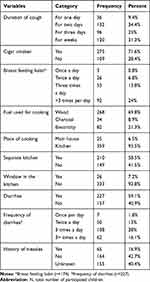 |
Table 2 Under-Five Children Duration of Diarrhea, Cough and Habit of Fuel Use of Their Family for a Cooking (N=384) at Hiwot Fana Specialized University Hospital, Harar, Ethiopia, March 1–30, 2020 |
Factors Associated with the Presence of S. pneumoniae Among Children Patients
The overall prevalence of S. pneumoniae among under-five children years of age with acute respiratory infection attending Hiwot Fana Specialized University Hospital was 11.2% (95% CI: 8.1–14.8).
Variables that showed association in the binary logistic regression of P-value of less than 0.25 were a family with Cigarette smoking habit, those children who were rural residents, those with a history of previous measles infection, and those who prepare their food within unsepareted kitchen. Cigarette smoking habit of family and children who were rural residents remained significantly associated after taken to the multiple logistic regressions (Table 3).
Antimicrobial Susceptibility Testing
About 23 (53.5%) of isolated Streptococcus Pneumoniae were resistant to Tetracycline and 21 (48.8%) to cotrimoxazole, whereas 95.4% and 93% of them were susceptible to Ceftriaxone and amoxicillin-clavulanate, respectively (Table 4).
Discussion
Streptococcus pneumoniae is a major pathogen of humans, causing diseases such as pneumoniae and meningitis that makes patients difficulty in breathing, wheezing, fever, irritability, chest pain, and chill.1 In this study, the proportion of S. pneumoniae was 11.2% (95% CI: 8.1–14.8), which was similar to a study conducted in 2018 in the northern part of Ethiopia (10.3%).18 But the present finding was lower than studies conducted in 2014 at northern part of Ethiopia by Fekadu et al, (16.1%),19 Jimma, Ethiopia (43%),20 and Southern Ethiopia (33.5%),15 (28.1%),21 however, higher than the studies reported from Spain (5.6%),22 Indian (3.7%),23 and Bangladesh (4.4%).24 This deference might be due to variation in the study of season, age, the difference in geography, site of study, and in implementation of vaccination status.
In this study, we have assessed various factors that could possibly cause the presence of S. pneumoniae. Children in the family of cigarette smokers were 3 times at risk to be infected with S. pneumoniae (AOR: 3, 95% CI: 1.8–8.0). This finding was in line with a study conducted by Vanker et al that antenatal environmental tobacco smoke exposure was associated with Streptococcus pneumoniae carriage in mothers (ARR: 1.73, 95% CI: 1.03–2.92) while postnatal environmental tobacco smoke exposure was associated with carriage in infants (AOR: 1.14, 95% CI: 1.00–1.30).24 However, the present study was not similar to the study reported from other part of Ethiopia (p=0.225).25
Children who lived in rural areas had 3.6 times more likely chance to be infected with S. pneumoniae compared to children who lived in urban areas (AOR: 3.6, 95% CI: 1.2–11). However, there was no significant association between the residence of rural and urban areas (AOR: 0.69, 95% CI: 0.33–1.4) in a study conducted in Gondar University Hospital, Ethiopia.25 This could be the fact that there was a difference in lifestyle between urban and rural areas in different places. In some urban dwellers, utilization of health-care services in time or better access to health-care facilities may be the reason; hence, childhood illnesses could receive a timely diagnosis that makes largest contributions to the rural–urban inequality in under-five children.26
In this study, the results of the antimicrobial susceptibility test revealed that 41 (95.4%) and 40 (93%) of S. pneumoniae were susceptible to Ceftriaxone and Amoxicillin-clavulanate, respectively, whereas 23 (53.5%) of Tetracycline and 21 (48.8%) of cotrimoxazole were resistant to it. This study was similar to the study reported from Hawassa, southern Ethiopia, in which 44 (64.6%) of cotrimoxazole and 29 (42.6%) of Tetracycline were resistant to S. pneumoniae.27 Another study revealed that 22.9% of Cotrimoxazole, in Ethiopia, 77.5% in Dakar, Senegal, and 100% in south Bangalore, India, were resistant to the isolated S. pneumoniae.26,28,29
On the other hand, a study conducted in Tanzania showed that the isolated 58.33% of S. pneumoniae were susceptible to erythromycin which was lower than this study.30 In contrary to this study, a study conducted in Kenya revealed that 100% of tetracycline was susceptible to isolated S. pneumoniae.31 In the present study, 40 (93%) of amoxicillin-clavulanate was the most active antibiotic against S. pneumoniae that was similar to a study conducted in Dakar, Senegal, in which 97.05% of amoxicillin-clavulanate was susceptible to S. pneumoniae.3 In this study, about 83.7% of Erythromycin was susceptible to the isolated bacteria that were similar to the study conducted at Hawassa, southern Ethiopia, that 85.3% of Erythromycin was susceptible to S. pneumoniae.25 The difference of antimicrobial susceptibility compared to other studies might be due to different bacterial strains, empirical treatment practice, use of antibacterial as a prophylactic, easy availability of some drugs without a prescription, a dose of the drug, and indiscriminate/prolonged use of common antibiotics.
Limitation of the Study
Since the study is cross-sectional, it was not able to sufficiently establish causality and the study may be subjected to response bias from the respondents.
Conclusion and Recommendations
The proportion of S. pneumoniae among studied children was high with 11.2%. Cotrimoxazole and Tetracycline were not enough effective to treat S. pneumoniae. Children in the family of cigarette smokers and rural residents were at risk to be infected with S. pneumoniae.
Based on the findings of this study, the following recommendations are made:
Health education should be provided for families on the identified predisposing factors that needs screening and early treatment of children with S. pneumoniae. Ceftriaxone, amoxicillin-clavulanate, and Erythromycin can be used for the treatment of infected patients with isolated bacteria in this finding at Hiwot Fana Specialized University Hospital. Further studies should be conducted with different possible associated factors by using advanced laboratory tests.
Abbreviations
ARI, Acute Respiratory Infection; HRERC, Institutional Health Research Ethics, and Review Committee; HFSUH, Hiwot Fana Specialized University Hospital.
Data Sharing Statement
The raw data set used/analyzed during the current study is available from Mr. Dejene Bayu on a reasonable request.
Acknowledgments
The authors would express great gratitude to Hiwot Fana Specialized University Hospital for providing Sponsorship.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; and agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work.
Funding
Fund for data collection for this research was covered by Haramaya University College of Health and Medical Sciences.
Disclosure
The authors report that there is no potential conflicts of interest for this work.
References
1. Abolwafa NF. Effect of educational program on mothers knowledge about prevention of pneumonia for their children under five years. IOSR-JNHS. 2017;6(5):05–12.
2. Hans-Christian S, Tiny D, Steen H. The effect of pneumococcal conjugate vaccines on the incidence of invasive pneumococcal disease caused by ten non-vaccine serotypes in Denmark. Vaccine J Elsevier. 2016;34(6):769–774.
3. Timkete M. Factor’s affecting healthcare-seeking for children below five years with symptoms of acute respiratory tract infection in Ethiopia: a cross-sectional study based on the 2016. Demographic Health Survey. 2018.
4. World Health Organization. Measuring Impact of Streptococcus Pneumoniae and Haemophilus Influenzae Type b Conjugate Vaccination; 2012.
5. Wahl B, Sharan A, Knoll MD, et al. National, regional, and state-level burden of Streptococcus pneumoniae and Haemophilus influenzae type b disease in children in India: modelled estimates for 2000–15. Lancet Glob Health. 2019;7(6):735–747. doi:10.1016/S2214-109X(19)30081-6
6. World Health Organization. Fact sheet on pneumonia. Wkly Epidemiol Rec. 2013;88(11):126–127.
7. Buchner DL, Awor P. A protocol for engaging unlicensed private drug shops in rural eastern Uganda for Integrated Community Case Management (ICCM) of malaria, pneumonia and diarrhoea in children under 5 years of age. BMJ Open. 2015;5(10):e009133. doi:10.1136/bmjopen-2015-009133
8. Shrestha S, Foxman B, Weinberger DM, Steiner C, Viboud C, Rohani P. Identifying the interaction between influenza and pneumococcal pneumonia using incidence data. Sci Transl Med. 2013;5(191):191ra84–191ra84. doi:10.1126/scitranslmed.3005982
9. Zar HJ, Madhi SA, Aston SJ, Gordon SB. Pneumonia in low and middle income countries: progress and challenges. Thorax. 2013;68(11):1052–1056. doi:10.1136/thoraxjnl-2013-204247
10. Richai G, Rav A. Committing to child survival: a promise renewed. Aust Med J. 2012.
11. United Nations Children’s Fund (UNICEFP). Pneumonia and Diarrhea: Tackling the Deadliest Diseases for the World’s Poorest Children. New York; 2012.
12. Hiwot Fana Specialized University Hospital annual report. 2019.
13. Bantie G, Zemene M, Melkamu B, Abebayehu B. The prevalence and root causes of delay in seeking healthcare among mothers of under-five children with pneumonia in hospitals of Bahir Dar city, North West Ethiopia. BMC Pediatr. 2019;19(1):1–10. doi:10.1186/s12887-019-1869-9
14. Gordon SB, Bruce NG, Grigg J, et al. Respiratory risks from household air pollution in low and middle income countries. Lancet Respir Med. 2014;2(10):823–860. doi:10.1016/S2213-2600(14)70168-7
15. Abuka T. Prevalence of pneumonia and factors associated among children 2–59 months old in Wondo Genet district, Sidama Zone, SNNPR, Ethiopia. Cur Pediar Res. 2017;21(1):19–25.
16. Cheesbrough M. District Laboratory Practice in Tropical Countries. Cambridge University Press; 2006.
17. CLSI. Performance standards for antimicrobial susceptibility testing.
18. Mulu W, Yizengaw E, Alemu M, et al. Pharyngeal colonization and drug resistance profiles of Morraxella catarrrhalis, Streptococcus pneumoniae, Staphylococcus aureus, and Haemophilus influenzae among HIV infected children attending ART Clinic of Felegehiwot Referral Hospital, Ethiopia. PLoS One. 2018;13(5):e0196722. doi:10.1371/journal.pone.0196722
19. Fekadu GA, Terefe MW, Alemie GA. Prevalence of pneumonia among under-five children in Este Town and the surrounding rural Kebeles, Northwest Ethiopia: a community based cross sectional study. Sci J Public Health. 2014.
20. Gebre T, Tadesse M, Aragaw D, et al. Nasopharyngeal carriage and antimicrobial susceptibility patterns of Streptococcus pneumoniae among children under five in Southwest Ethiopia. Children. 2017;4(4):27. doi:10.3390/children4040027
21. Lema K, Murugan R, Tachbele E. Prevalence and associated factors of pneumonia among under-five children at public hospitals in Jimma zone, South West of Ethiopia. J Pulmonol Clin Res. 2018;2(1):25–31.
22. Oporto CR, Tania -C-C, María LG, et al. Prevalence and clinical impact of Streptococcus pneumoniae nasopharyngeal carriage in solid organ transplant recipients. BMC Infect Dis. 2019;19(697):1–9.
23. Brooks AW, Goswami D, Rahman M, et al. Influenza is a major contributor to childhood in a tropical developing country. Pediatr Infect Dis J. 2010;29(3):216–221. doi:10.1097/INF.0b013e3181bc23fd
24. Vanker A, Nduru PM, Barnett W, et al. Indoor air pollution and tobacco smoke exposure: impact on nasopharyngeal bacterial carriage in mothers and infants in an African birth cohort study. ERJ Open Res. 2019;5(1):00052–2018. doi:10.1183/23120541.00052-2018
25. Haile AA, Gidebo DD, Ali MM. Colonization rate of Streptococcus pneumoniae, its associated factors and antimicrobial susceptibility pattern among children attending kindergarten school in Hawassa, southern Ethiopia. BMC Res Notes. 2019;344(12).
26. Abate A, Gelaw S, Tigabu Y, Zemene T. Nasopharyngeal carriage and antimicrobial susceptibility pattern of Streptococcus pneumoniae among pediatric outpatients at Gondar University Hospital, North West Ethiopia. Pediatr Neonatol. 2013;54(5):315–321. doi:10.1016/j.pedneo.2013.03.017
27. Sanni Y, Olalekan AU, Friday O, Ghose B. Decomposing the rural-urban gap in the factors of under-five mortality in sub-Saharan Africa? Evidence from 35 countries. BMC Public Health. 2019;19(616):1–10.
28. Kumar VS, Sagar P, Laxman G, et al. Emerging roles of inflammasomes in acute pneumonia. Am J Respir Crit Care Med. 2018;197(2):160–171. doi:10.1164/rccm.201707-1391PP
29. Abdoulaye D, Makhtar C, Abdoulaye S, et al. Antibiotic susceptibility profile of Streptococcus pneumoniae isolated from acute respiratory infection in Dakar: a cross sectional study. Microbiol Med. 2018;33(7862):13–17.
30. Ndosa A, Kidenya BR, Mushi MF, Mirambo MM, Hokororo A, Mshana SE. Factors associated with colonization of Streptococcus pneumoniae among under-fives attending clinic in Mwanza City, Tanzania. Tanzania J Health Res. 2015;17(1).
31. Githii S, Revathi G, Muigai A, Kariuki S. Carriage rate and serotype distribution of Streptococcus pneumoniae amongst children in Thika Hospital, Kenya. Afr J Lab Med. 2013;2(1). doi:10.4102/ajlm.v2i1.45
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.