Back to Journals » Infection and Drug Resistance » Volume 15
Magnitude of Drug-Resistant Gram-Positive Bacterial Pathogens, and Its Associated Factors from External Ocular Infected Patients Attending at Jinka General Hospital Ophthalmic Clinic, Southern Ethiopia
Authors Fenta F, Alemu D, Alelign D
Received 15 January 2022
Accepted for publication 4 March 2022
Published 9 March 2022 Volume 2022:15 Pages 947—959
DOI https://doi.org/10.2147/IDR.S356974
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Fikremariam Fenta,1 Derbie Alemu,1 Dagninet Alelign2
1Department of Medical Laboratory Science, Arba Minch College of Health Science, Arba Minch, Ethiopia; 2Department of Medical Laboratory Sciences, College of Medicine and Health Sciences, Arba Minch University, Arba Minch, Ethiopia
Correspondence: Dagninet Alelign, Tel: +251 964-428-118, Email [email protected]
Background: The emergence and the incidence of antimicrobial-resistant Gram-positive bacteria increase the risk of treatment failure of ocular infection. Thus, this study aimed to determine the magnitude of drug-resistant Gram-positive bacterial pathogens and associated factors among patients attending Jinka General Hospital Ophthalmic Clinic in Southern Ethiopia.
Methods: A facility-based cross-sectional study was conducted on 347 external ocular infected patients attending Jinka General Hospital Ophthalmic Clinic from 15th March to 20th June 2021. Study participants were selected by a systematic random sampling technique. Required data were collected by using structured questionnaires. Swabs of the external eye were obtained with a sterile cotton swab and processed in the Jinka branch Public Health Laboratory. Each sample was inoculated on blood agar, chocolate agar, and mannitol salt agar and incubated aerobically and micro-aerobically at 37°C for 48 hrs. Identification was done by standard microbiological protocols and antimicrobial resistance testing by Kirby Bauer’s disk diffusion technique. Logistic regression was used to identify the associated factors with Gram-positive bacterial external-ocular infection.
Results: The overall prevalence of Gram-positive bacterial pathogens among external ocular samples was 119/347 (34.3%). S. aureus 57/119 (47.39%), followed by coagulase-negative Staphylococcus spp. 38/119 (31.9%), and S. pneumoniae 13/119 (10.9%) were predominantly isolated. Overall, multi-drug resistance was observed in 72/119 (60.5%) of the bacteria isolates. The 78.9% of isolated S. aureus were MDR. Methicillin-resistant S. aureus (MRSA) and Methicillin-resistant CoNS (MR-CoNS) were accounted for 45.6% and 36.8%, respectively. Previous use of ocular antibiotics was statistically associated to external-ocular Gram-positive bacterial infection [AOR= 1.624, 95% CI: (1.037– 2.542)].
Conclusion: High levels of drug resistance were observed for commonly prescribed antibiotics, which attracted the attention of an ophthalmic clinic. Thus, for the effective treatment and management of bacterial eye infections, regular monitoring of drug resistance trends is essential.
Keywords: external-ocular infection, drug-resistant, gram-positive bacteria, Jinka, Ethiopia
Introduction
The eye is one of the body’s most sophisticated sensory organs, and it is remarkably resistant to most environmental contaminants owing to lysozymes, lactoferrin, defensins, and antibodies in tears, as well as the blinking mechanism, which reduces microbial colonization of the ocular surface.1–7 However, bacteria, followed by viruses, fungi, and parasites, are the most common causal agents that cause infections in the eyes. The most commonly infected regions of the eye are the conjunctiva, eye lid, and cornea. Infections associated with the surface of the eye like blepharitis, conjunctivitis, and keratitis are said to be external ocular infections and, if not treated effectively, can affect the normal structure of the eye, leading to vision impairment and blindness.3–6 The prevalence of blindness in Ethiopia is estimated to be 1.6%, with 87.4% of cases due to preventable causes.7,8
Bacterial infection of the eye in both adults and children accounts for up to 50% of all cases in adults and 70% to 80% of all cases in children.5–7 About 60% to 80% of eye infections are caused by Gram-positive bacterial pathogens.7 The most common bacteria involved in ocular infections are Staphylococcus aureus, coagulase-negative Staphylococci (CoNS), Streptococcus pneumoniae, Corynebacterium spp., Bacillus spp., and Streptococcus pyogenes.5–10 Blepharitis, conjunctivitis, dacryocystitis, keratitis, and endophthalmitis are commonly all caused by S. aureus. The reason for the high coverage of S. aureus may be due to virulence factors such as exo-enzymes and surface slime that may play a role in the pathogenesis.7 Despite being termed commensal skin flora, CoNS strains are a source of illness linked to previous surgical intervention or implanted foreign bodies.9 In Ethiopia, the prevalence of S. aureus and CoNS strains was 50.3% and 33.5%, respectively.2,9
A variety of clinical and behavioral factors, such as contact lens wear, trauma, previous surgery, ocular surface disease, age, dry eye state, ocular adnexal dysfunction (tear deficiencies), chronic nasolacrimal duct obstruction, systemic diseases, previous ocular infections, and other exogenous factors may determine the clinical outcome of microbial-caused eye infections and their epidemiological patterns.6,8,11–13
Apart from the prevalence of bacterial infection, antibiotic resistance is becoming a major problem in modern medicine, necessitating the development of coordinated and comprehensive national and international action plans.7,8 Multidrug-resistant (MDR) bacteria such as methicillin-resistant S. aureus (MRSA), vancomycin-resistant Enterococcus spp. (VRE), and β-lactamase-resistant S. pneumonia are a major worry and healthcare problem.8,14 Gram-positive bacteria can develop antibiotic resistance by generating beta-lactamases or decreasing the affinity and sensitivity of their target site, either by exogenous DNA acquisition or changes in the native penicillin-binding protein (PBP) genes.15
Bacterial resistance to topical antimicrobials could raise the chance of eye infection treatment failure, which could have serious consequences.4,8–15 The burden is particularly severe in developing countries like Ethiopia due to the inability of most patients to afford expensive alternative antibiotics, the unregulated and frequently misused antibiotics, and the rapid spread of antimicrobial-resistant organisms due to an increase in the number of infectious diseases.1–3,11–14 In various parts of Ethiopia, the prevalence of Gram-positive bacterial pathogens among patients with external ocular infections ranged from 30.1% to 55.6%, with more than half of the isolates being resistant to commonly prescribed antibiotics, implying that this should be evaluated on a regular basis.1,2,5–7
However, in the study area south of Omo Zone Jinka, in southern Ethiopia, there is a high trend of using traditional medicinal plants (not approved scientifically) and there is a wide use of human medicine on animals because of their pastoralist lifestyle, which results in antibiotic overuse or misuse. In this scenario, as in other clinical cases, developing antibiotic resistance by bacteria involved in ocular diseases necessitates observation to guide empirical therapy. Thus, this study aimed to determine Gram-positive bacterial pathogens involving external ocular infections, antimicrobial-resistant profiles, and associated factors among patients attending at Jinka General Hospital Ophthalmic Clinic, Southern Ethiopia.
Materials and Methods
Study Setting and Period
A facility-based cross-sectional study was conducted in the South Omo Zone, Jinka General Hospital Ophthalmic Clinic from 15th March to 20th June 2021. Jinka is the capital of the South Omo Zone, which had different pastoralist ethnic groups living together. Jinka General Hospital serves the population of the South Omo Zone and surrounding areas. The hospital provides preventive, curative, and rehabilitative care to over 500,000 individuals through outpatients, inpatients, pharmacy, and laboratory departments. The hospital also has an ophthalmology clinic that offers a variety of services for ocular-infected patients, including outpatient and inpatient.
Study Population
All patients attending in Jinka General Hospital Ophthalmic clinic with ocular infections were the source population, whereas those systematically selected patients with external ocular infections attending in Jinka General Hospital Ophthalmic clinic during the study period were the study population.
Eligibility Criteria
Patients clinically diagnosed with external ocular infections and those who were willing to give written informed consent or assent were included in the study. Patients with severe ocular trauma, recent ocular surgery (within one week), and patients on antibiotics for the past one week were excluded.
Sample Size Determination and Sampling Technique
The sample size was determined using a single population proportion formula by considering the proportion (p-value) of 31.3% from the previous finding at Hawassa, Southern Ethiopia,5 and d (0.05) was the margin of error, and then the final sample size was calculated as 347 after adding 5% of the non-response rate. Systematic random sampling was performed by calculating the K-value.
Data Collection Tool
A pre-tested, structured questionnaire was used as a data collection tool. A questionnaire translated into Amharic was administered through face-to-face interviews to collect socio-demographic characteristics and clinical features.
Specimen Collection and Processing
External ocular samples were collected using a sterile cotton swab moistened with saline by gently swabbing the eye, the lower conjunctiva sac, and lid margins by asking the patient to look up, pull down the lower lid with the thumb on an absorbing tissue paper, and rub the swab over the lower conjunctival sac from medial to lateral side and back again.7 Swabs of ocular samples from patients would be aseptically obtained from ocular infection sites before the eye is cleaned with an antiseptic solution, topical anesthetic, and antibiotic. On the sample tubes, the patient’s code and time of swab collection were labeled. Then the swab would be immersed in 2 mL of brain heart infusion broth and transported to the Jinka Branch Public Health Laboratory for investigation. Ocular specimens were processed in the laboratory within 2 hours of collection, and specimens that were not processed within 2 hours were kept in a refrigerator at 4 °C until they were processed as per standard.16
Isolation and Identification
Ocular specimens were primarily inoculated onto Blood agar (Oxoid, LTD), Chocolate agar, and Mannitol salt agar (Oxoid, LTD) and incubated for 24 to 48 hours at 35°C–37°C. In each sample, one inoculated chocolate medium was incubated in a candle jar to maintain a 5–10% CO2 environment. Standard microbiological methods were used for the identification of isolates. Biochemical tests like catalase, coagulase, bacitracin, optochin test, and bile solubility test were used accordingly as per the isolated bacteria. A bacitracin sensitivity test was used for confirmation of S. pyogenes isolates, whereas the optochin test and bile solubility test were applied for confirmation of S. pneumoniae isolates.17
Antimicrobial Susceptibility Testing
Antimicrobial susceptibilities of the bacterial isolates were performed according to the criteria of the Clinical and Laboratory Standards Institute (CLSI), 2018 using the Kirby–Bauer disc diffusion method on Muller-Hinton Agar (Oxoid, Ltd., UK).18 A 3–4 colony of bacteria was taken from a pure culture colony and transferred to a tube containing 5mL of sterile normal saline (0.85% NaCl) and mixed gently until it formed a homogenous suspension. The turbidity of the suspension was adjusted to the turbidity of the McFarland 0.5 standard in a tube and swabbed on Muller Hinton. The used antibiotics were Penicillin 10 μg, Ampicillin 10 μg, Ceftriaxone 30 μg, Vancomycin 30 μg, Erythromycin 15μg, Azithromycin 15μg, Tetracycline 30μg, Chloramphenicol 30μg, Clindamycin 2μg, Cefoxitin 30 μg, Gentamicin 10 μg, Doxycycline 30μg, Cotrimoxazole 10 μg, and Ciprofloxacin 5μg. These antimicrobial agents were selected as per CLSI guidelines, and by considering the commonly prescribed antibiotics for the treatment of external ocular infections in the area. The results of the antimicrobial susceptibility test were interpreted as sensitive, intermediate, and resistant according to the CLSI, 2018 guideline. Multidrug resistance (MDR) was defined as isolated bacteria that resist at least one antimicrobial drug in three or more antimicrobial categories.18
Data Quality Control
To ensure data quality, 5% of the sample size was pre-tested before actual work began. Two ophthalmic nurses and two laboratory personnel were trained for three days for data and sample collection as well as sample processing. The implementation of quality control measures was applied throughout the data and specimen collection. The reliability of the research results was guaranteed. Staining reagents, culture media, and antibiotic discs were checked for their normal shelf life before use. After preparation, all culture plates and antibiotic plates were stored at the recommended refrigeration temperature. Reference strains of S. pyogenes (ATCC 19615) and S. aureus (ATCC 25923) were used as controls.18 All laboratory procedures were applied by strictly following the pre-analytical, analytical, and post-analytical stages of quality assurance.
Statistical Analysis
Data entry and analysis were done using SPSS statistical software version 21. Descriptive statistics like frequency, mean, and percentage were calculated to describe the socio-demographic characteristics. Bivariable logistic regressions analysis was used to assess the relationship between dependent and independent variables. Odds ratio (OR) and 95% confidence interval (CI) were estimated. Variables with P < 0.25 in the bivariable analysis were entered and analyzed by multivariable logistic regression analysis. Statistically, the significant variable was determined at a 95% confidence interval and P-value < 0.05. Finally, the compiled results were presented in words, percentages, graphs, and tables.
Ethical Considerations
Ethical clearance was obtained from the institutional review board of the Arba Minch College of Health Sciences (Ref. No. CMchs /01/20/7036). Besides, separate permission was obtained from Jinka General Hospital. To overcome the issue of COVID-19, all the standard precautions were followed, like wearing a mask and using personal protective equipment while collecting and processing the sample. Moreover, the objective of the study was clearly described to the study participants, including the benefits and risks, and confidentiality was maintained using an identification code. Informed written consent was obtained before the interview, and informed written assent was obtained from parents or guardians for assuring participants less than 18-year-old. A positive result was communicated to physicians for proper treatment with the most effective antibiotics. Overall, this study was conducted in accordance with the declaration of Helsinki.
Results
Socio-Demographic Characteristics
A total of 347 patients with external ocular infections participated in the study. About 186 (53.6%) patients were males, and the age range of the study participants was from 1 to 75 years old, with a mean age of 29.4 ±17.5 years old. Among the age distribution, 171 (49.3%) of the study participants were in the age group of 16–35-year-olds. The majority 206 (59.1%) of the study participants were living in rural areas, and 150 (43.2%) of the patients were unable to read and write [Table 1].
 |
Table 1 Socio-Demographic Characteristics of External Ocular Infected Patients at Jinka General Hospital Ophthalmic Clinic, Southern Ethiopia, 2021 |
Clinical and Behavioral Characteristics of Study Participants
Among a total of 347 study participants who had external ocular infection, 204, 120, and 23 patients had conjunctivitis, blepharitis, and keratitis, respectively. More than half of patients had a history of ocular surface disease, a history of ocular trauma, and ever-used ocular antibiotics accounted for 237 (68.3%), 119 (57.2%), and 202 (58.2%), respectively. Participants with an ocular infection for less than a week accounted for 167 (48.1%), while underlying systemic disease, cosmetic use, and cigarette smoking habits constituted for 40 (11.5%), 66 (19%), and 19 (5.5%), respectively [Table 2].
 |
Table 2 Clinical and Behavioral Characteristics of External Ocular Infected Patients at Jinka General Hospital Ophthalmic Clinic, Southern Ethiopia, 2021 |
Magnitude of Gram-Positive Bacterial Isolates from External Ocular Infections
A total of 347 external ocular specimens were subjected to culture. 119/347 (34.3%) were culture-positive. Among culture-positive ocular specimens, 72/119 (62.2%) were conjunctivitis, 41/119 (34.5%) were blepharitis, and 6/119 (5.0%) were keratitis. A mixed infection was not determined. The predominant Gram-positive isolates were S. aureus (47.9%), followed by coagulase-negative Staphylococcus spp. (31.9%) and S. pneumoniae (10.9%) [Figure 1].
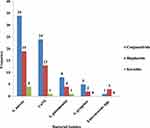 |
Figure 1 Gram-positive bacterial profile from external ocular infected patients at Jinka General Hospital Ophthalmic Clinic, Southern Ethiopia, 2021. |
Gram-positive bacterial isolates were high among males and study participants aged 16 to 35 years old, with 61/119 (52.1%) and 59/119 (49.6%), respectively. Likewise, the prevalence was higher among rural residents, at 71/119 (60.5%). Similarly, study participants who had previous use of ocular antimicrobials, a history of ocular trauma, and a history of ocular surface disease accounted for 60/119 (50.4%), 62/119 (52.1%), and 75/119 (63%), respectively [Table 3].
 |
Table 3 Bivariable and Multivariable Logistic Regressions of Factors Associated to External Ocular Gram-Positive Bacterial Infections |
Antimicrobial-Resistant Pattern of Gram-Positive Bacterial Isolates
Among isolated Gram-positive bacteria, resistance was observed against tetracycline (56.3%), doxycycline (53.8%), cefoxitin (53.8%), and penicillin (49.6%). However, isolated Gram-positive bacteria were sensitive to chloramphenicol (66.4%), clindamycin (60.5%), co-trimoxazole (56.3%), and azithromycin, gentamicin, and ciprofloxacin (52.9%). Isolates of S. aureus were 64.9% resistant to tetracycline and 61.4% sensitive to chloramphenicol and clindamycin, whereas 55.3% of isolated Coagulase Negative Staphylococcus were resistant to penicillin. Of the S. aureus isolates, 45.6% were MRSA. Likewise, 36.8% of isolated coagulase negative Staphylococcus spp. were methicillin-resistant (MR-CoNS). S. pneumoniae and S. pyogenes were found to be penicillin-resistant in 46.2% and 28.6% of isolates, respectively. Likewise, 25% of isolated Enterococcus spp. were found to be vancomycin-resistant (VRE) [Table 4].
 |
Table 4 Antimicrobial-Resistant Patterns of Gram-Positive Bacterial Isolates from External Ocular Infections |
Magnitude of Multi-Drug Resistance in Isolated Gram-Positive Bacteria
Among 119 culture-positive samples, 72/119 (60.5%) bacterial isolates were multi-drug resistant (MDR). MDR bacteria isolates from conjunctivitis, blepharitis, and keratitis were found in 43/119 (36.1%), 24/119 (20.2%), and 5/119 (4.2%) of these cases, respectively. S. aureus constituted 45/57 (78.9%) of MDR isolates, while isolated CoNS comprised 18/38 (47.4%) [Figure 2].
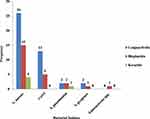 |
Figure 2 Multi-drug resistance pattern of isolated Gram-positive bacteria from external ocular infected patients at Jinka General Hospital Ophthalmic Clinic, Southern Ethiopia, 2021. |
Factors Associated for External Ocular Gram-Positive Bacterial Infections
Both bivariable and multivariable logistic regression analyses were done to determine factors associated with bacterial infection of external ocular. In binary logistic regression analysis, different socio-demographic, clinical, and behavioral factors were rolled up to show their crude association with bacterial infection of external ocular infection. Accordingly, those who met the cut-off criteria P < 0.25 were included in the multivariable analysis (previous history of ocular surface disease, history of ocular trauma, previous use of ocular antibiotics, and cosmetic use practice). Among these hypothesized associated factors, patients who had previous use of ocular antibiotics [AOR = 1.624, 95% CI: (1.037–2.542), P = 0.034] were statistically significant for bacterial infection of the external ocular. However, there was no statistically significant socio-demographic and behavioral factor for external ocular bacterial infections [Table 3].
Discussion
In developing countries, external ocular infections are the major cause of illness.7,12,19 Gram-positive bacterial pathogens are the most common cause of infection when they gain access to the eye through a variety of mechanisms, resulting in impaired vision and even blindness.3–7 In this study, the overall Gram-positive bacterial infection rates of external ocular infection were 34.3% (119/347) with a 95% CI (29.3, 39.3). The finding was in line with studies conducted in Jimma (30.1%),3 Hawassa (31.3%),5 Gondar (37.1%),19 and another study in Jimma (38.9%)12 of Ethiopia, Khartoum State, Sudan (36.8%)20 of Africa, and West Bengal (34.5%)21 and Sana’a, Yemen (38.6%).22 Our finding, however, was higher than that of research conducted in Bangalore, India (17.9%),23 Luigi Vanvitelli, Naples, Italy (26.7%),13 and New Delhi, India (27.5%).24 In contrast, our findings were lower than studies done in Tigray (41.8%),25 Shashemene (44.4%),1 Gonder (53.8%),2 Borumeda (55.6%),8 and Khartoum State, Sudan (57.0%).27 The variation in the occurrence might be due to differences in the geographical region, study period, season (data collection period), socio-demographic characteristics of the study population, and sample size variation.
The most common isolate observed in the current study was S. aureus (47.9%), CoNS (31.9%), S. pneumoniae (10.9%), S. pyogenes (5.9%), and Enterococcus spp. (3.4%). Similar studies were conducted in different parts of Ethiopia, like Hawassa,5 Jimma,12 Tigray,25 Shashemene,1 and Gonder.2 Likewise, Sudan,20 Khartoum,26 and Sana’a, Yemen22 also reported S. aureus as a predominant external ocular bacterial isolate. However, a study conducted in Bangalore, India,23 Naples, Italy,13 New Delhi, India,24 West Bengal,21 and various parts of Ethiopia1,3,7,8,19 identified CoNS as the most common external ocular bacterial isolate. The observed differences in isolate species could be due to variations in pathogenic bacteria distribution in the study areas.
Rapid use of antibiotics for severe ocular infections is routine in ophthalmic practice, resulting in increased drug resistance. Based on results from in-vitro susceptibility testing in this study, most Gram-positive cocci were susceptible to chloramphenicol (66.4%), clindamycin (60.5%), and gentamicin (56.2%). However, 56.2%, 53.8%, and 49.6% of all isolated strains were resistant to tetracycline, doxycycline, and penicillin, respectively, among the routinely used topical antibiotics.
More than 49% of isolated S. aureus were resistant to tetracycline, doxycycline, and penicillin. Similarly, different findings were reported from other parts of Ethiopia; penicillin and tetracycline were the most common antimicrobials resistant to S. aureus. In Gonder, higher proportions of resistance were reported to penicillin (96.9%),2 while in Borumeda, a slightly lower proportion of resistance was reported to penicillin and doxycycline (33.3%).8 Whereas in Jimma, resistance to penicillin, erythromycin, ampicillin, tetracycline, co-trimoxazole, and chloramphenicol was reported at (86.2%), (82.8%), (75.9%), (69%), and 57.1%, respectively,3,12 and in Hawassa, isolated S. aureus was 83.3%, 70%, and 60% resistant to penicillin, ampicillin, and tetracycline, respectively.5
Likewise, CoNS were 55.3% and 47.7% resistant to penicillin and tetracycline, respectively. Similarly, high resistance to penicillin and tetracycline has been reported from different parts of Ethiopia and other parts of the world.11,18–20,22,25 However, the majority of the isolated CoNS (65.2%) were susceptible to ciprofloxacin and gentamicin. In this study, about 46.2% and 28.6% of isolated S. pneumoniae and S. pyogenes were penicillin-resistant, respectively, and 25% of isolates of Enterococcus spp. were vancomycin-resistant (VRE), which needs special attention both in community as well as clinical settings. However, isolated S. pneumoniae were 84.6%, 69.2%, and 69.2% susceptible to vancomycin, co-trimoxazole, and chloramphenicol, respectively.
The overall MDR pattern among Gram-positive bacterial isolates was 60.5%. The finding was higher than the previous study in Hawassa (54.5%).5 However, a high prevalence of multidrug resistance was previously reported in Gondar at 95.6%2 and Jimma at 78.1%.12 An increased proportion of MDR bacterial isolates indicates a rising problem with MDR and, as a result, emphasizes the necessity for ongoing antimicrobial resistance surveillance in the research region to affect infection control and prevention.
MRSA and MR-CoNS infections were found in 45.6% and 36.8% of ophthalmology patients, respectively. The resistance pattern of cefoxitin was used to determine the prevalence of MR Staphylococcus infection. Inducible macrolide Lincosamide-streptogramin B (MLSB) resistance phenotypes were found in 31.6% and 15.8% of S. aureus and CoNS strains, respectively. The fact that we employed different types and generations of antibiotics for susceptibility testing could explain the higher occurrence of drug resistance in this study. The prevalence of MR Staphylococcus ocular infections varied significantly across geographical areas and historical periods.1–5 Antibiotic therapy is also a significant risk factor for MR Staphylococcus, as it provides a selective advantage for the bacteria’s survival and dissemination.3,5 In general, the geographical distribution of strains, trends, and frequency of antibiotic prescriptions, community drug usage practices (low cost and purchasing drugs without a prescription), MDR interpretation differences, and antimicrobial sensitivity testing techniques could all contribute to the discrepancy in this result’s microbial susceptibility pattern.
Different socio-demographic, clinical, and behavioral features of individuals with external ocular infection were analyzed by considering only common variables that potentially contribute to the pathophysiology of ocular bacterial infection. Among these, patients who had previous use of ocular antibiotics had a 1.624 times higher risk of developing an external ocular bacterial infection than patients who had not previously used ocular antibiotics. This was similar to research done at Gondar, Ethiopia,2 and Kampala, Uganda.27 The causes could be related to bacterial strain selection pressure, incorrect antibiotic use (dosage and duration), and poor infection prevention control (IPC) practices.
Limitation of the Study
Our study is limited by the fact that the tests to determine the MIC of the antimicrobials were not carried out. Moreover, Gram-negative bacteria, Chlamydia trachomatis, and Corynebacterium species were not isolated due to resource constraints.
Conclusion
The magnitude of external ocular Gram-positive bacterial infection in this study was comparable to other similar studies conducted in different parts of Ethiopia. External ocular Gram-positive bacterial infections were statistically associated with patients who had previously used ocular antibiotics. S.aureus, followed by CoNS, was the most predominantly isolated from the ocular sample. More than half of the bacterial isolates were multidrug-resistant, and nearly half of the isolated S. aureus and more than one-third of the coagulase-negative Staphylococcus spp. were methicillin-resistant. Moreover, about 46.2% and 28.6% of isolated S. pneumoniae and S. pyogenes were penicillin-resistant, respectively, and one-fourth of isolated Enterococcus spp. were vancomycin-resistant. Thus, precise identification of causative agents and periodic evaluation of drug susceptibility patterns of bacterial pathogens associated with external ocular infection, as well as effective infection prevention and control strategies, should be used to help reduce the level of bacterial infections and the emergence of drug-resistant bacterial pathogens.
Abbreviations
ATCC, American Type Culture Collection; AOR, adjusted odds ratio; CLSI, Clinical Laboratory Standard Institute; OR, odds ratio; MRSA, methicillin resistance Staphylococcus aureus; MRCNS, methicillin resistance coagulase-negative Staphylococcus; MSA, mannitol salt agar; SPSS, Statistical Package Social Sciences.
Data Sharing Statement
Due to ethical and confidentiality concerns, the data sets evaluated in this work are available from the principal author upon reasonable request.
Acknowledgments
We appreciate Arba Minch College of Health Sciences and the research directorate for the technical and financial support. The Jinka General Hospital human resource office, eye clinic, and Jinka branch public health regional laboratory employees deserve special thanks. Finally, we want to extend our gratitude to all of the study participants for their time and willingness to participate.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Mohammed AA, Ali MM, Zenebe MH. Bacterial etiology of ocular and particular infections, antimicrobial susceptibility profile and associated factors among patients attending eye unit of Shashemene comprehensive specialized hospital, Shashemene, Ethiopia. BMC Ophthalmol. 2020;20:1–8. doi:10.1186/s12886-020-01398-w
2. Getahun E, Gelaw B, Assefa A, Assefa Y, Amsalu A. Bacterial pathogens associated with external ocular infections alongside the eminent proportion of multidrug-resistant isolates at the University of Gondar Hospital, Northwest Ethiopia. BMC Ophthalmol. 2017;17(1):151. doi:10.1186/s12886-017-0548-6
3. Diriba K, Kassa T, Alemu Y, Bekele S. In Vitro Biofilm Formation and Antibiotic Susceptibility Patterns of Bacteria from Suspected External Eye Infected Patients Attending Ophthalmology Clinic, Southwest Ethiopia. Int J Microbiol. 2020;2020:1–12. doi:10.1155/2020/8472395
4. World Health Organization. World report on disability; 2011. Available from: https://www.who.int/disabilities/world_report/2011/report.pdf.
5. Amsalu A, Abebe T, Mihret A, Delelegne D, Tadesse E. Potential bacterial pathogens of external ocular infections and their antibiotic susceptibility pattern at Hawassa University Teaching and Referral Hospital, Southern Ethiopia. African J Microbiol Res. 2015;9(14):1012–1019. doi:10.5897/AJMR2014.7282
6. Teweldemedhin M, Gebreyesus H, Atsbaha AH, Asgedom SW, Saravanan M. Bacterial profile of ocular infections: a systematic review. BMC Ophthalmol. 2017;17(1):212. doi:10.1186/s12886-017-0612-2
7. Aklilu A, Bitew A, Dessie W, Hailu E, Asamene N, Mamuye Y. Prevalence and drug susceptibility pattern of bacterial pathogens from ocular infection in St. Paul’s Hospital Millennium Medical College, Ethiopia. J Bacteriol Mycol. 2018;5(8):1085.
8. Shiferaw B, Gelaw B, Assefa A, Assefa Y, Addis Z. Bacterial isolates and their antimicrobial susceptibility pattern among patients with external ocular infections at Borumeda hospital, Northeast Ethiopia. BMC Ophthalmol. 2015;15(1):103. doi:10.1186/s12886-015-0078-z
9. Petrillo F, Pignataro D, Lella FM, et al. Antimicrobial Susceptibility Patterns and Resistance Trends of Staphylococcus aureus and Coagulase-Negative Staphylococci Strains Isolated from Ocular Infections. Antibiotics. 2021;10(5):527. doi:10.3390/antibiotics10050527
10. Mohager MO, Kaddam LA, Mohager SO. External ocular bacterial infections among Sudanese children at Khartoum State, Sudan. African J Microbiol Res. 2016;10(40):1694–1702. doi:10.5897/AJMR2016.8092
11. Assefa Y, Moges F, Endris M, et al. Bacteriological profile and drug susceptibility patterns in dacryocystitis patients attending Gondar University Teaching Hospital, Northwest Ethiopia. BMC Ophthalmol. 2015;15(1):34. doi:10.1186/s12886-015-0016-0
12. Tesfaye T, Beyene G, Gelaw Y, Bekele S, Saravanan M. Bacterial profile and antimicrobial susceptibility pattern of external ocular infections in Jimma University specialized hospital, Southwest Ethiopia. Am J Infect Dis Microbiol. 2013;1(1):13–20. doi:10.12691/ajidm-1-1-3
13. Petrillo F, Folliero V, Santella B, et al. Prevalence and Antibiotic Resistance Patterns of Ocular Bacterial Strains Isolated from Pediatric Patients in University Hospital of Campania “Luigi Vanvitelli,” Naples, Italy. Int J Microbiol. 2020:2020;987.
14. Sharma S. Antibiotic resistance in ocular bacterial pathogens. Indian J Med Microbiol. 2011;29(3):218–222. doi:10.4103/0255-0857.83903
15. Jubeh B, Breijyeh Z, Karaman R. Resistance of gram-positive bacteria to current antibacterial agents and overcoming approaches. Molecules. 2020;25(12):2888. doi:10.3390/molecules25122888
16. Cheesbrough M District laboratory practice in tropical countries, part 2: Cambridge University Press; 2006. Available from: https://www.medbox.org/preview/5255d6e1-05d4-41a9-beb2-02b60e695ecc/doc.pdf.
17. Musa AA, Nazeerullah R, Sarite SR. Bacterial profile and antimicrobial susceptibility pattern of anterior blepharitis in Misurata region, Libya. Dentistry Med Res. 2014;2(1):8. doi:10.4103/2348-1471.131557
18. Wayne PA; CLSI. Performance Standards for Antimicrobial Susceptibility Testing.
19. Belyhun Y, Moges F, Endris M, et al. Ocular bacterial infections and antibiotic resistance patterns in patients attending Gondar Teaching Hospital, Northwest Ethiopia. BMC Res Notes. 2018;11(1):597. doi:10.1186/s13104-018-3705-y
20. Mohager MO, Kaddam LA, Mohager SO. External ocular bacterial infections among Sudanese children at Khartoum State, Sudan. African J Microbiol Res. 2016;10(40):1694–1702.
21. Mondal S, Gupta I, Nandi A, Mitra G. A study on the bacteriological profile of conjunctivitis patients attending in a peripheral tertiary medical college of West Bengal. J Adv Med Dental Sci Res. 2016;4(5):5.
22. Alshamahi EYA, Al-Shami HZ, Al-Shamahy HA, Musawa YA. Bacterial Causes And Antimicrobial Sensitivity Pattern Of External Ocular Infections In Selected Ophthalmology Clinics In SANA’A CITY. Pharm Res. 2020;5(3):12–16.
23. HeMavatHi PS, SHenoy P. Profile of microbial isolates in ophthalmic infections and antibiotic susceptibility of the bacterial isolates: a study in an eye care hospital, Bangalore. J Clin Diagnostic Res. 2014;8(1):23. doi:10.7860/JCDR/2014/6852.3910
24. Singh D. Spectrum of Ocular Infections and the Emerging Epidemic of Resistance to Fourth Generation Fluoroquinolones. EC Ophthalmol. 2018;9:430–436.
25. Teweldemedhin M, Saravanan M, Gebreyesus A, Gebreegziabiher D. Ocular bacterial infections at Quiha Ophthalmic Hospital, Northern Ethiopia: an evaluation according to the risk factors and the antimicrobial susceptibility of bacterial isolates. BMC Infect Dis. 2017;17(1):207. doi:10.1186/s12879-017-2304-1
26. Ahmed OB, Hamdan EM. Profile of bacterial conjunctivitis in Sudan. Sch J App Med Sci. 2016;4(4B):1217–1221.
27. Mshangila B, Paddy M, Kajumbula H, Ateenyi-Agaba C, Kahwa B, Seni J. External ocular surface bacterial isolates and their antimicrobial susceptibility patterns among pre-operative cataract patients at Mulago National Hospital in Kampala, Uganda. BMC Ophthalmol. 2013;13(1):71. doi:10.1186/1471-2415-13-71
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
