Back to Journals » Therapeutics and Clinical Risk Management » Volume 19
Magnitude and Impacts of Adverse Events of Injectable Containing Shorter Regimen in Programmatic Management of Multi-Drug Resistant Tuberculosis in Ethiopia: A Retrospective Cohort Study
Authors Achalu DL , Mohammed FG, Teferi M
Received 27 May 2023
Accepted for publication 23 October 2023
Published 10 November 2023 Volume 2023:19 Pages 889—901
DOI https://doi.org/10.2147/TCRM.S423163
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Garry Walsh
Daniel Legese Achalu,1 Foziya Getachew Mohammed,2 Mekonnen Teferi1
1Clinical Trial Directorate, Armauer Hansen Research Institute, Addis Ababa, Ethiopia; 2Knowledge Management Directorate, Armauer Hansen Research Institute, Addis Ababa, Ethiopia
Correspondence: Daniel Legese Achalu, Tel +251912750932, Email [email protected]
Background: Since its launch as a standardized treatment for multidrug-resistant tuberculosis (MDR-TB) in Ethiopia in April 2018, the safety profile of the shorter injectable regimen under a programmatic setting has not been well studied. Thus, this study aimed to assess the status of adverse events in patients treated with a shorter injectable regimen in Ethiopia.
Methods: This is a retrospective cohort study. Data were collected using a structured data abstraction form and analyzed using SPSS, version 25, both descriptively and analytically. Logistic regression was conducted to assess predictors, and Kaplan–Meier analysis was used to examine the time to AEs and survival experiences.
Results: Of 256 patients, 245 (95.7%) were eligible for the study. Of 245, 107 (43.7%) patients experienced at least one AE. In total, 276 AE cases were observed out of which the most common were nausea/vomiting (20.3%), dyspepsia (18.1%), and ototoxicity (11.6%). Of 276 AEs, approximately 49 (17.8%) were serious. AEs led to drug discontinuation, dose modification, and regimen change in 29 (27%), 15 (14%) and 10 (9.3%) patients, respectively. Only 19.2% of 276 the overall AEs and 22.6% of 62 AE of special interest (AESI) were reported to the National Pharmacovigilance Center.
Conclusion: Although the observed extent of AEs associated with the shorter regimen (SR) seemed to be moderate, it significantly influenced the treatment schemes and patient conditions. Reporting of AEs was low, irrespective of their severity and AESI. Therefore, strengthening the implementation of active drug safety monitoring and management is required.
Keywords: adverse events, adverse event of special interest, drug resistant tuberculosis, injectable, serious adverse event and shorter regimen
Introduction
Treatment of drug-resistant tuberculosis (DR-TB) is more difficult than that of drug-susceptible TB. Among the challenges in DR-TB therapy, regimen-related safety issues are a major concern.1 The management of DR-TB requires a longer duration and concurrent use of multiple second- and first-line drugs.2 As a result, DR-TB regimens are known to cause excessive adverse events (AEs).3,4 Worldwide meta-analysis revealed that 57.3% of multi-drug resistant TB (MDR-TB) patients experienced at least one kind of AE,5 and the figure was higher in HIV prevalence settings (83%).6 The AEs in the MDR-TB regimen were reported to be associated with discontinuation and loss to follow-up (LTFU), which further markedly reduces the treatment success rate.7
The most commonly reported MDR-TB regimens associated AEs were vomiting, ototoxicity, hyperglycemia, hepatotoxicity, arthralgia, gastritis, skin rash, neuropathy, optic neuritis and nephrotoxicity.8 Being HIV positive,6,9,10 old age,10,11 previous TB treatment,12 diabetes mellitus10 and anemia13 were identified as significant predictors of AEs.
The use of an injectable shorter regimen (SR) was recommended by the WHO in 2016 with some evidence from observational studies.2 Despite some studies having reported the effectiveness of SR in treating MDR-TB,2,14 there is uncertainty regarding its safety. Although a decreased burden of AEs was anticipated with a reduced duration of SR,15 later studies reported higher AEs with the regimen (89.2%).16 In fact, slightly higher rates of AEs were reported with SR compared to longer regimen (LR) in a randomized clinical trial.17 Moreover, evidence on the safety and outcomes of SR in young patients and HIV co-infection was inadequate. Recently, slightly more AE in children,8 more serious adverse events (SAEs), and deaths in HIV patients on SR than in those on LR17 were reported. Furthermore, the time to AEs and SAEs has not been well studied.18
Ethiopia introduced the use of a standardized injectable SR in April 2018 as standard regimen for the treatment of MDR-TB. However, the status of AEs associated with this regimen has not been formally studied. Thus, this study aimed to assess the type, magnitude, determinants, and consequences of AEs among MDR-TB patients during treatment with standardized injectable SR.
Methods
Study Area
The present study was conducted in Ethiopia. Ethiopia implemented a standardized injectable SR for MDR-TB treatment in April 2018. Out of 62 treatment initiation centers (TICs) in Ethiopia, eight potential TICs were selected based on the number of patients recruited. The total number of patients enrolled on SR in Ethiopia was estimated to 460 as obtained from unpublished Ministry of Health data. Thus, more than half, 256 (55.7%), of patients received SR were enrolled at the selected TICs. Therefore, the study was conducted at the Adama Hospital Medical College (Gada TIC), ALERT Hospital, Boru Meda Hospital, Gondar University Hospital, Hosaena Hospital, Shanen Gibe Hospital, Tulu Bollo Hospital, and Yirgalem Hospital.
Study Design
This was a multicenter, hospital-based, self-control retrospective cohort study. The records of all MDR-TB patients who had been enrolled in SR at selected sites from April 2018 to March 2020 were reviewed to evaluate AEs that occurred during treatment with a standardized SR of MDR-TB.
Study Participants and Selection Criteria
All the patients diagnosed with MDR-TB and treated with injectable SR (9–12 months) in selected TICs during the study period were included in the study population. MDR TB is defined as tuberculosis caused by organisms that are resistant to isoniazid and rifampicin, the two most powerful anti-TB drugs.19 The injectable SR refers to a course of treatment for MDR-TB lasting 9–12 months, which is largely standardized,20 and is composed of high-dose moxifloxacin/gatifloxacin, clofazimine, pyrazinamide and ethambutol throughout, supplemented by kanamycin, prothionamide, and high-dose isoniazid in the intensive phase for 4 months.21
In Ethiopia, to confirm the drug resistance status of TB, drug susceptibility testing (DST) is required. Gene expert (Xpert) is used for the detection of MTB and Rifampicin-resistant TB directly from the sputum, while Line Probe Assay (LPA) is a rapid DST technique that uses molecular technology to test resistance to other first-line TB drugs as well as resistance to fluoroquinolone and injectable core second-line TB drugs. In addition, clinical and laboratory safety monitoring parameters are conducted on a regular schedule during the follow-up period to ensure patient safety and know treatment progress.22
Therefore, diagnosis and monitoring of patients during the course of treatment was considered during patient selection. Accordingly, patients whose MDR-TB positive status was confirmed by Xpert or LPA were included. Clinical parameters, such as audiometry, visual acuity, ECG, liver function tests, electrolyte tests, and assessment/follow-up of AEs, were also used to select patients to differentiate underlying diseases from possible drug-related AEs. Patients with MDR-TB with complete medical records of relevant clinical information at baseline and during treatment were included in the study, while those with incomplete relevant data that could significantly influence the findings, thereby limiting the adequacy of the results for inferential analysis, were excluded.
Sample Size and Sampling Techniques
Overall, 256 MDR-TB patients enrolled in injectable SR treatment during the study period at the study TICs. All patients who fulfilled the inclusion criteria for the selected health facilities were included in the study. Accordingly, 245 of the 256 patients were selected for this study, while 11 patients who did not meet the inclusion criteria were excluded.
Study Variables
Adverse event status like magnitudes, types, impact and reporting of AEs were the dependent variables in this study. The independent variables included socio-demographic variables, behavioral characteristics, and clinical data at baseline and prior use of first- and second-line TB drugs.
As part of management, any treatment is report-able to the National Pharmacovigilance Center (NPC) by all healthcare givers.23 Serious adverse events (SAEs) are those leads to death or a life-threatening experience, to hospitalization or prolongation of hospitalization, to persistent or significant disability, or to a congenital anomaly; or AEs that require an intervention to prevent such an outcome from happening are included.24 Adverse Events of special interest (AESIs) is defined as an AE documented to have occurred during clinical trials and for which the monitoring program is specifically sensitized to report regardless of its seriousness, severity or causal relationship to the TB treatment.24 Those clinically important events need to be reported to NPC within defined timelines. Accordingly, SAEs are report-able within 24 hours while AESIs should be reported with 48 hours.23
All health care professionals including physicians, dentists, health officers, nurses, pharmacy professionals, and community health workers should report AEs to regional/national PV center.
Data Collection and Management
Data were collected from patients’ medical records using a data abstraction form, which was adopted from the national registration logbook and patient treatment card. The form included socio-demographic information, medical history at baseline and follow-up, and adverse events. Data collection was conducted by trained and experienced nurses working in MDR-TB units and coordinated by the MDR-TB head. The principal investigator strictly supervised the data-collection process to ensure the reality, completeness, and consistency of the completed forms.
Data Analysis
SPSS version 25 was used for the data entry and analysis. Descriptive statistics were computed to obtain the summary results. Normality was checked for continuous variables using the Shapiro–Wilk test and plotting (histogram and line plot) to determine the type of statistics to be used for analysis. Accordingly, age and time to AEs were not normally distributed. Therefore, the median and interquartile range (IQR) were used to describe the central tendency. The severity of AEs during treatment with a shorter MDR-TB regimen was assessed using Hartwig’s Severity Assessment Scale. Severity describes the extent to which AEs influence patients’ daily lives. Hartwig et al categorized ADRs into seven levels of severity. Levels 1 and 2 are mild; Levels 3 and 4 are moderate; and Levels 5, 6, and 7 are classified as severe.25
Logistic regression analysis was used to identify the predictors of drug-related AEs. Outcome variables were compared with independent variables using bivariate and multivariable analyses to assess associated factors. A stepwise method was used to test the fitness of the model. The unadjusted (crude) and adjusted odds ratios (OR), together with their corresponding 95% confidence intervals, were computed, and the significance of the association was considered at p ≤ 0.05. Moreover, the Kaplan–Meier survival method was used to plot time to events (AEs) and examine survival experiences among different categories. Survival data were analyzed using the survival probability and hazard ratios.
Results
Baseline Characteristics
The median age of the patients was 27. More than half (129, 52.7%) of the patients were female. Of the total patients, 25 (10.2%) were khat chewers, 18 (7.3%) drank alcohol, and five (2%) were smokers. Most patients had no previous TB history (91, 37.1%). Approximately 15.5% of the patients had underlying diseases (Table 1).
 |
Table 1 Socio-Demographic and Clinical Data of MDR-TB Patients Treated with SR in Ethiopia |
Adverse Events During Treatment with Injectable Shorter Regimen
Of the 245 patients included in the study, 107 (43.7%) experienced at least one AE during the treatment course. As per the site of the study, a higher proportion of patients treated at ALERT Hospital 47 (87.0%) experienced AE, while there was no record of AE for patients who were treated at Boru Meda Hospital (Table 2).
 |
Table 2 Adverse Event Magnitude per Hospitals |
The most frequent AEs were nausea and vomiting. Of the 276 AEs, nausea and vomiting accounted for 20.3%, followed by dyspepsia (18.1%), ototoxicity (11.6%), tinnitus (8.7%), loss of appetite (8.3%), and others as shown in Figure 1. Eight (8) AEs (darkness/discoloration of the body, dysuria, hand pain, nasal congestion, other electronic imbalances, peripheral neuropathy, respiratory distress, and thyroid mass) with a frequency of one were categorized as others (Figure 1).
 |
Figure 1 Frequencies distribution of AE types during treatment with shorter MDR-TB regimen. |
Severity and Seriousness of Adverse Events
The severity of AEs during treatment with SR of MDR-TB was assessed using Hartwig’s severity assessment scale. Accordingly, the majority (238, 86.2%) of the AEs were identified as mild. The remaining patients were classified as moderate (26, 9.4%) or severe (12, 4.4%) (Table 3).
 |
Table 3 Hartwig’s Adverse Event Severity Assessment Scale |
Based on the definition of serious AEs, of 276 AEs, approximately 49 (17.8%) were evaluated as serious. Of the 49 serious AEs, 47 (89.8%) resulted in prolonged hospitalization, six (12.2%) led to permanent harm (hearing loss/deafness), and one (2.0%) was associated with death (Table 4).
 |
Table 4 Evaluation of Seriousness of Adverse Events |
Management Undertaken and Consequences of Adverse Events
The occurrence of drug-related AEs has compelled healthcare workers to implement various interventions. As obtained from the patients’ charts, to manage AEs, temporary suspected drug discontinuation, dose modification, and regimen change were performed in at least 29 (27%), 15 (14%), and 10 (9.3%) of the 107 patients with AEs, respectively. Of 10 regimen changes, majority 6 (60%) were linked to injectable drug-induced ototoxicity.
From 276 AE cases, 166 (60.9%) necessitated additional medication (antidotes), 61 (22.1%) resulted in dose modification, temporal discontinuation, or a change in medication, 16.3% of AEs resulted in hospitalization and 6 (2.2%) led to permanent hearing loss. Of the 32 ototoxicity cases, 18.8% resulted in permanent hearing loss (Table 5).
 |
Table 5 Action Undertaken in Response to or Consequences of Adverse Events |
Complementary to AE management, only 53 (19.2%) patients were reported to the National Pharmacovigilance Center. AE reporting was also evaluated based on AEs of special interest. Of the 62 AEs of interest, only 14 (22.6%) were reported: ototoxicity (25%), blurred vision (optic) (33.3%), hepatotoxicity (20%), and QT prolongation (33.3%) (Table 5).
Factors Affecting Occurrence of Adverse Events
Factors associated with AE occurrence were assessed using bivariate and multivariable binary logistic regression analyses. Overall, comorbidity, resistance type, first exposure to TB treatment, and relapse were significantly associated with the occurrence of AEs during treatment with the shorter MDR-TB regimen. The probability of encountering AEs increased more than 2.5 folds in patients with other diseases (2.535 [1.156–5.559]). Compared with RR patients, those with MDR-type resistance were more likely (nine times) to experience AEs (9.673 [1.077–86.838]). Similarly, being new to TB treatment doubled the probability of experiencing AEs (2.397 [1.250–4.595]) (Table 6).
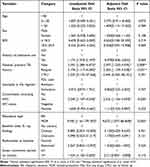 |
Table 6 Bivariate and Multivariable Analysis of Risk Factors for the Occurrence of AEs During Treatment with Injectable SR of MDR TB |
Survival Analysis
The Kaplan–Meier survival method was used to analyze and pilot the time-to-event survival function. Survival status is assessed over 9 months from the start of the treatment to assess whether any AE occurred. During this period, the median time to AE in patients was 0.970 months. Survival from AEs was analyzed over 9 months of treatment follow-up period. The cumulative survival rates at the end of 2nd, 4th, 6th and 7th month were 0.690, 0.591, 0.569, and 0.563, respectively. At the end of the study period, the cumulative survival from AEs was 0.563, as no AE occurred after 7 months (Figure 2). In general, the risk of experiencing AE increases with duration in the course of treatment (Figure 3).
 |
Figure 2 Kaplan–Meier curve showing cumulative survival MDR-TB patients from AEs. |
 |
Figure 3 Kaplan-Meier plot for cumulative hazard of AEs to treated MDR-TB patients. |
Patients with and without comorbidity were compared to determine whether their survival differed, and the median survival of patients with comorbidity was lower than that of patients without other diseases (3.680 vs 4.677) (Figure 4). Similarly, the cumulative hazard of AEs was higher in patients with comorbid (Figure 5).
 |
Figure 4 Plot of cumulative survival of MDR-TB patients with and without a comorbidity from experiencing AE. |
 |
Figure 5 Curve showing hazards of AEs to MDR-TB patients with and without a comorbidity status. |
Discussion
This study evaluated the adverse events associated with medication during SR treatment of MDR-TB under programmatic management. We identified that 43.7% of the patients developed at least one event during treatment with SR. Our finding was lower compared to results of previous studies in different countries; retrospective study in Niger (68%),8 prospective study in Africa (89.2%)16 and a multi-country randomized controlled trial (RCT) (48.2%).17 This difference can be due to the retrospective nature of our study because prospective and RCT provides stronger evidence than retrospective study to detect and record every AEs. The results of the RCT seem to be close to ours because the trial focused only on severe adverse events. Furthermore, the low degree of AE in our findings may be due to the gaps in detection and record keeping of AE at TICs. We observed inter-site variations in the AE detection. For example, at ALERT Hospital, 87% of patients faced AEs, while none of the patients’ charts showed AE case in course of treatment at Boru Meda. This higher difference in AE magnitude among sites may be indicative of gaps in the implementation of AEs monitoring and recording at some sites.
The prevalence of AE during treatment with SR observed in our investigation was lower than that of conventional LR, as reported by previous studies in Ethiopia (51%13 and 92%10), China (90.7%),26 Angola (82.9%),12 South Africa (83%),6 and a worldwide systematic review (57.3%).5 The observed difference is mainly due to regimen variation and excessive AE related to LR1 and reduced duration decreases the burden of AEs.15
Our study also assessed the magnitude and impacts of individual AE cases. Nausea and vomiting (20.3%), dyspepsia (18.1%), ototoxicity (11.6%), tinnitus (8.7%), anorexia (4.7%), blurred vision (4.3%), and headaches (4.0%) were identified as common AEs. The extent of these AEs was slightly comparable with the findings of a study in Niger;8 however, it was significantly lower with respect to the results of a multi-site African study.16 Studies on SR in other countries revealed that hyperglycemia (7%)8 and nephrotoxicity (15.7%10 and 2%8) were detected; however, none were reported in our study. We found one death (0.04%), which was significantly less than the result of the clinical trial in the same regimen (8.5%).17
The impact and consequences of AEs during treatment with injectable SRs were observed to be challenging. Our investigation identified that AE affected the patients’ treatment scheme; it caused at least dose modification, temporary discontinuation, or regimen change in 22.1% of the patients. Consequently, regimen change/treatment failure was observed in 9.3% of the patients. In addition, AEs caused hospitalization and hearing loss, and the causality was found to be higher with seriousness; 89.8% of serious AEs resulted in prolonged hospitalization. Approximately 18.8% ototoxicity led to permanent deafness, which is consistent with a previous study.27 So, this finding is alarming for ototoxicity/hearing loss, and other serious AEs should be carefully and actively monitored to avoid further complications.
Regarding determinants of AEs, our analysis showed that; comorbidity (22.535 [1.156–5.559]), being MDR resistance type (9.673 [1.077–86.838]), new patient to treatment (first exposure to TB drugs) (2.397 [1.250–4.595]) and having relapse history of past TB treatment (2.264 [1.129–4.538]) were independently associated with occurrence of AEs. Similar findings were reported in previous studies: HIV positive in Japan,9 previous TB treatment in Angola12 and in Ethiopia: HIV reactive and diabetes mellitus,10 co-morbid conditions, and baseline anemia13 were significantly correlated with poor outcomes. Furthermore, the baseline abnormal audiometer results did not show a statistically significant association with ototoxicity/hearing loss (3.190 [0.778–13.080]), indicating that ototoxicity was merely linked to the administered injectable SR of MDR-TB. However, in contrast to previous studies in Iran11 and Ethiopia10,13 which reported a significant association between older age and AE, we did not observe a significant relationship between age and the occurrence of AEs in our study. This may be due to age differences; unfortunately, the majority (96.7%) of our study patients were below extremely older ages; only eight and two patients were ≥55 and ≥65 years of age, respectively.
Time-to-event analysis showed that for cohorts with events, the median time-to-event was 0.970 months, showing that the occurrence of some AEs occurred immediately after the initiation of treatment, and the majority of them occurred during the early intensive phase of treatment. This may be due to a higher number of medications in the beginning phase and patients’ less adaptation to drugs at the commencement of treatment.28 This time to event warrants frequent patient monitoring and immediate intervention on AEs during the early months of treatment as an essential part of MDR-TB management.29 So, healthcare workers need to be vigilant for AE detection in the early phase of therapy.
Finally, it is possible to limit our study to certain constraints. As this was a retrospective study, some information could be incomplete and may lead to the underestimation of some results. However, we used all possible techniques to minimize its effects. Participants with unacceptable levels of missing data were excluded, and list-wise deletions were employed in the analysis. Being multicenter, our study can yield better results that can be used for policy-making at the national level.
Conclusion
Our study demonstrated that less than half of the patients developed AEs under programmatic management of MDR-TB with an injectable shorter regimen, probably with underestimated magnitude owed to poor detection/recording of events. Even though the magnitude of AEs associated with the use of SR seems intermediate, it caused significant impacts on the treatment scheme. Underreporting of AEs was observed, regardless of their seriousness and AE of special interest (AESI), which is indicative of poor implementation of PV program. Thus, these findings suggest the necessity for strict implementation of pharmacovigilance initiatives: continuous patient follow-up, active safety monitoring and documentation, and reporting of AEs to manage them in a timely manner.
Data Sharing Statement
All data generated or analyzed during this study are included in this published article.
Ethical Approval
Our study complies with the Declaration of Helsinki. Ethical clearance was obtained from the Armauer Hansen Research Institute (AHRI)/ALERT Ethics committee (Ref. No. PO-34-20). Permission for waiver of consent was obtained from the ethics committee and/or the management of each facility. All potential identifiers (names and mobile numbers) were removed from the data collection forms and the analysis reports.
Acknowledgment
We would like to express our deepest gratitude to all hospitals for their cooperation in granting approval for our study. We would also like to acknowledge all data collectors for their honest efforts in providing us with accurate and relevant patient data. Lastly, we would like to show our appreciation and thanks to the PAVIA project and AHRI for their financial support and overall facilitation in conducting our study successfully.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
Data collection expenses for this study were supported by the Pharmacovigilance in Africa (PAVIA) project in collaboration with AHRI. The funder had no involvement in study design, data collection, analysis, or manuscript preparation.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Falzon D, Jaramillo E, Schünemann H. The 2011 Update of the World Health Organization Guidelines for the Programmatic Management of Drug-Resistant Tuberculosis. Eur Respiratory Soc; 2011.
2. World Health Organization. WHO Treatment Guidelines for Drug- Resistant Tuberculosis 2016. World Health Organization; 2016.
3. Bloss E, Kukša L, Holtz TH, et al. Adverse events related to multidrug-resistant tuberculosis treatment, Latvia, 2000–2004. Int J Tuberc Lung Dis. 2010;14(3):275–281.
4. Carroll MW, Lee M, Cai Y, et al. Frequency of adverse reactions to first-and second-line anti-tuberculosis chemotherapy in a Korean cohort. Inter J Tubercul Lung Dis. 2019;16(7):961–966. doi:10.5588/ijtld.11.0574.Frequency
5. Wu S, Zhang Y, Sun F, et al. Adverse events associated with the treatment of multidrug-resistant tuberculosis: a systematic review and meta-analysis. Am J Ther. 2016;23(2):e521–e530. doi:10.1097/01.mjt.0000433951.09030.5a
6. Schnippel K, Firnhaber C, Berhanu R, Page-Shipp L, Sinanovic E. Adverse drug reactions during drug-resistant TB treatment in high HIV prevalence settings: a systematic review and meta-analysis. J Antimicrob Chemother. 2017;72(7):1871–1879. doi:10.1093/jac/dkx107
7. World Health Organization. Global tuberculosis report 2018. Geneva: World Health Organization. 2018; Available from: https://apps.who.int/iris/handle/10665/274453.
8. Harouna SH, Ortuno-Gutierrez N, Souleymane MB, et al. Short-course treatment outcomes and adverse events in adults and children-adolescents with MDR-TB in Niger. Int J Tuberc Lung Dis. 2019;23(5):625–630. doi:10.5588/ijtld.17.0871
9. Matono T, Nishijima T, Teruya K, et al. Substantially higher and earlier occurrence of anti-tuberculosis drug-related adverse reactions in HIV coinfected tuberculosis patients: a matched-cohort study. AIDS Patient Care STDS. 2017;31(11):455–462. doi:10.1089/apc.2017.0116
10. Seid Y, Ketema B, Yilma M, Desalegn Z. Severe adverse effects associated with multidrug resistant tuberculosis medications among patients attending alert hospital, Ethiopia. Ethiop Med J. 2019;57:1.
11. Farazi A, Sofian M, Jabbariasl M, Keshavarz S. Adverse reactions to antituberculosis drugs in Iranian tuberculosis patients. Tuberc Res Treat. 2014;2014:1–6. doi:10.1155/2014/412893
12. Aznar ML, Segura AR, Moreno MM, et al. Treatment outcomes and adverse events from a standardized multidrug-resistant tuberculosis regimen in a rural setting in Angola. Am J Trop Med Hyg. 2019;101(3):502–509. doi:10.4269/ajtmh.19-0175
13. Merid MW, Gezie LD, Kassa GM, Muluneh AG. Incidence and predictors of major adverse drug events among drug-resistant tuberculosis patients on second-line anti- tuberculosis treatment in Amhara regional state public hospitals; Ethiopia: a retrospective cohort study. BMC Infect Dis. 2019;2019:1–12.
14. Khan FA, Salim MAH, Cros P, et al. Effectiveness and safety of standardised shorter regimens for multidrug-resistant tuberculosis: individual patient data and aggregate data meta-analyses. Europ Respirat J. 2017;304:1–13. doi:10.1183/13993003.00061-2017
15. World Health Organization. Global tuberculosis report 2016. Geneva: World Health Organization; 2016. Available from: http://AppsWhoInt/Iris/Bitstream/10665/91355/1/9789241564656_EngPdf.
16. Trebucq A, Schwoebel V, Kashongwe Z, et al. Treatment outcome with a short multidrug-resistant tuberculosis regimen in nine African countries. Int J Tuberc Lung Dis. 2018;22(1):17–25. doi:10.5588/ijtld.17.0498
17. Nunn AJ, Phillips PPJ, Meredith SK, et al. A trial of a shorter regimen for rifampin-resistant tuberculosis. N Engl J Med. 2019;380(13):1201–1213. doi:10.1056/NEJMoa1811867
18. World Health Oorganization. Consolidated guidelines on drug-resistant tuberculosis treatment. Geneva: World Health Organization; 2019. Available from: https://apps.who.int/iris/bitstream/handle/10665/311389/9789241550529-eng.pdf?.
19. World Health Organization. A practical handbook on the pharmacovigilance of medicines used in the treatment of tuberculosis: enhancing the safety of the TB patient. World Health Organization; 2012. Available from: https://apps.who.int/iris/handle/10665/336226.
20. World Health Organization. WHO consolidated guidelines on drug-resistant tuberculosis treatment. World Health Organization; 2019. Available from: https://apps.who.int/iris/bitstream/handle/311389/9789241550529-eng.pdf.
21. World Health Organization. Global tuberculosis report 2018. Geneva: World Health Organization; 2018. Available from: http://apps.who.int/iris/bitstream/handle/10665/274453/9789241565646-eng.pdf.
22. Federal Democratic Republic of Ethiopia Ministry of Health. Guidelines for Clinical and Programmatic Management of TB, TB/HIV and Leprosy in Ethiopia. Addis Ababa; 2018:217.
23. Ethiopian Food and drug Authority. Guideline for Adverse Drug Events Monitoring (Pharmacovigilance) of Medicines. Addis Ababa; 2023:74.
24. World Health Organization. Active tuberculosis drug-safety monitoring and management (aDSM): framework for implementation. World Health Organization; 2015. Available from: https://apps.who.int/iris/bitstream/handle/10665/204465/WHO_HTM_TB_2015.28_eng.pdf?sequence=1.
25. Hartwig SC, Siegel J, Schneider PJ. Preventability and severity assessment in reporting adverse drug reactions. Am J Hosp Pharm. 1992;49(9):2229–2232. doi:10.1093/ajhp/49.9.2229
26. Zhang Y, Wu S, Xia Y, et al. Adverse events associated with treatment of multidrug-resistant tuberculosis in China: an ambispective cohort study. Med Sci Monit. 2017;23:2348–2356. doi:10.12659/MSM.904682
27. Ausi Y, Santoso P, Sunjaya DK, Barliana MI. Between curing and torturing: burden of adverse reaction in drug-resistant tuberculosis therapy. Patient Prefer Adherence. 2021;15(November):2597–2607. doi:10.2147/PPA.S333111
28. Sahile Z, Yared A, Kaba M. Patients’ experiences and perceptions on associates of TB treatment adherence: a qualitative study on DOTS service in public health centers in Addis Ababa, Ethiopia. BMC Public Health. 2018;18(1). doi:10.1186/s12889-018-5404-y
29. World Health Organization. Companion handbook to the WHO guidelines for the programmatic management of drug-resistant tuberculosis. World Health Organization; 2014. Available from: https://www.who.int/publications-detail-redirect/9789241548809.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
