Back to Journals » Clinical Ophthalmology » Volume 17
Magnification and Refocusing Comparison in Cataract Surgery Using a Heads-Up Three-Dimensional Visualization System versus Conventional Binocular Microscopy
Authors Ramírez Mejía M, Arroyo Muñoz L, Medina Perez AB, Mendoza Velasquez C, Ceja Martínez J, Camacho Ordonez A, Guerrero-Berger O
Received 16 June 2023
Accepted for publication 25 July 2023
Published 15 August 2023 Volume 2023:17 Pages 2333—2339
DOI https://doi.org/10.2147/OPTH.S423372
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Mariana Ramírez Mejía,1 Leticia Arroyo Muñoz,1 Ana Beatriz Medina Perez,1 Cristina Mendoza Velasquez,1 Jimena Ceja Martínez,1 Azyadeh Camacho Ordonez,1 Oscar Guerrero-Berger1,2
1Department of Anterior Segment Surgery, Fundación Hospital Nuestra Señora de la Luz, Mexico City, Mexico; 2Centro Oftalmológico Mira, Mexico City, México
Correspondence: Oscar Guerrero-Berger, Department of Anterior Segment Surgery, Fundación Hospital Nuestra Señora de la Luz, Ezequiel Montes 135, Tabacalera, Cuauhtémoc, Mexico City, 06030, Mexico, Tel +525551281140, Email [email protected]
Purpose: To compare magnification and refocusing during phacoemulsification with the NGENUITY® 3-D Visualization System (3-D) versus the conventional microscope (CM) OPMI LUMERA 700.
Setting: This study was performed in the Department of Anterior Segment of the Fundación Hospital Nuestra Señora de la Luz.
Design: Prospective, randomized, cross-sectional, multi-surgeon, and comparative study.
Methods: This study enrolled 100 patients (eyes) scheduled for phacoemulsification to measure the number of times changes in focusing and magnification were needed during cataract surgery.
Results: Our study included 100 patients. From the endpoints evaluated, “zoom-in” showed statistically significant differences for all of the four predefined cataract surgery steps (means: Step 1, 0.38 (CM) vs 0.08 (3-D); Step 2, 0.36 (CM) vs 0.06 (3-D); Step 3, 0.54 (CM) vs 0.22 (3-D); Step 4, 0.56 (CM) vs 0.24 (3-D); all comparisons, p < 0.05). In Step 4, there was a statistically significant increased use of “focus-out” for the 3-D system (mean 0.16 (CM) vs 0.58 (3-D); p < 0.05). “Focus-in” and “zoom-out” showed no group differences for all steps. The duration of surgery with the 3-D system was longer at each step and overall. The percentage of light intensity did not show a statistically significant difference between both systems, with a mean of 99.45 for CM vs 98.43% for the heads-up system.
Conclusion: The heads-up 3-D system is a safe option that offers excellent magnification for anterior segment visualization. The surgical time is longer, but adjusting settings like light intensity and brightness may facilitate some surgical steps early in the learning curve.
Keywords: three-dimensional visualization, cataract surgery, heads-up surgery, visualization systems
Introduction
Using conventional microscopes (CM) may predispose eye surgeons to musculoskeletal injuries, especially of the back and neck, leading to pain and disability due to poor ergonomics.1 To improve ergonomics and visualization, teaching, and training during ocular surgeries, three-dimensional (3-D) visualization systems were developed for the first time in 1999.2 These systems allow the surgeon a more comfortable “heads-up” position instead of looking down through a standard microscope with a flexed neck posture that induces muscle tension in the back and the neck. Another claimed advantage of 3-D systems in ophthalmology is the possibility of using lower light intensity due to the risk of phototoxicity.3 Finally, the smaller aperture of the High Dynamic Range (HDR) camera of the 3-D system provides a better depth of field that reduces the need for frequent focus adjustments.4
All these factors, in theory, would facilitate anterior segment surgery; however, the real-life performance of heads-up systems in cataract surgery has not been extensively studied. Some literature reports have not found differences between three-dimensional visualization and conventional microscopy.5 For example, a large retrospective study that included more than 2000 eyes that evaluated posterior capsular rupture and vitreous prolapse rates, as well as surgery duration, concluded that safety and efficiency are similar between the heads-up system and traditional binocular microscopy.5 On the contrary, other studies claim several advantages,6–9 such as enhanced operative fluency,6 surgeon´s perception of better ergonomics, visualization, safety, and improved training experience,7,8 as well as a reduction in the risk of phototoxicity.9
One of the main advantages claimed by the heads-up systems is the need for fewer magnification and refocusing adjustments, which is why in this study, we quantitatively report these factors considered differentiators of three-dimensional visualization as a superior technique for cataract surgery performed by experienced surgeons.
Methods
Study Design
Prospective, randomized, cross-sectional, and comparative study of the heads-up NGENUITY 3-D® Visualization System (Alcon Laboratories, USA) and a conventional microscope (OPMI LUMERA 700; Carl Zeiss Meditec, Germany).
Study Population
We included adult patients (50 to 70 years of age) diagnosed with grade 3 or 4 cataracts according to the LOCS III grading standard, with a formal indication for phacoemulsification, and who provided informed consent. After informed consent, patients underwent a complete slit-lamp examination to confirm cataract grade, exclude corneal disorders that would compromise visualization during surgery, and confirm the presence of red reflex. Patients with a history of ocular surgery were excluded.
Surgical Technique, Treatment, and Assessment
Cataract surgery was divided into four steps to register the number of refocusing and magnification adjustments for each step: I. Clear corneal incision, Capsulorrhexis, hydrodissection, and nucleus rotation; II. Phacoemulsification; III. Cortex aspiration and intraocular lens implantation. IV. Viscoelastic aspiration and incision suture. An engineer at our institution designed a counter connected to the microscope footswitch that recorded the number of times the “zoom-in/zoom-out/focus-in/focus-out” rocker was pressed (Figure 1).
 |
Figure 1 Counter connected to the microscope footswitch. |
All microscope parameters were standardized before surgery, using neutral magnification and focus and 70% light intensity. These were considered the baseline parameters. Phacoemulsification was performed through a 2.4 mm clear corneal incision using the Centurion® Vision System (Alcon Laboratories, USA). A foldable acrylic intraocular lens (single-piece AcrySof SA60AT, Alcon Laboratories, USA) was inserted in the capsular bag using the Monarch IIB implantation system. All phaco incisions were sutured with 10–0 nylon.
Endpoints
Primary Endpoint
The number of refocusing and magnification adjustments during cataract surgery.
Secondary Endpoints
- Percentage of light intensity registered on the microscope display during surgery.
- Total duration of surgery (in minutes and seconds).
- Duration of the different surgical steps (in minutes and seconds).
Exploratory Endpoint
The number of intraoperative complications.
Statistical Analysis
Since no studies report the number of magnification and refocusing adjustments needed during cataract surgery, we used convenience sampling. We selected a sample size of 50 subjects per group.
The analyses were carried out using the statistical packages RStudio and IBM SPSS version 28, utilizing descriptive statistics with tables and graphs representing the absolute and relative values of the qualitative variables. The assumption of normality was verified using the Shapiro–Wilk test for quantitative variables. We used the Mann–Whitney test to compare the means, and statistical significance was established with a p-value <0.05.
This study obtained written informed consent from all patients before enrollment. The study protocol was approved by the Ethics Committee of the Fundación Hospital Nuestra Señora de la Luz I.A.P. and conformed to the ethical guidelines of the Declaration of Helsinki.
Results
We included 100 patients (eyes) with a mean age of 70.91 years, 56 women and 44 men; 52% were right eyes, and 48% were left (Table 1).
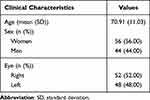 |
Table 1 Clinical Characteristics of the Patients |
When comparing both visualization systems, we observed statistically significant differences regarding the duration of each step, with shorter times in all steps for CM (means: Step 1, 2.92 minutes (CM) vs 4.64 minutes (3-D); Step 2, 4.3 minutes (CM) vs 5.9 minutes (3-D); Step 3, 3.44 minutes vs 5.42 minutes (3-D); Step 4, 3.28 minutes (CM) vs 5.08 minutes (3-D); all comparisons with a p-value <0.05; see Table 2). This was also reflected in the total duration of surgery (see Figure 2).
 |
Table 2 Comparison of Surgery Duration |
 |
Figure 2 Comparison of duration of surgery by system. |
Regarding magnification and focusing endpoints, “focus-in” and “zoom-out” showed no differences between groups for all steps. On the other hand, “zoom-in” showed statistically significant differences for all steps, with lower use of this parameter for the heads-up system (means: Step 1, 0.38 (CM) vs 0.08 (3-D); Step 2, 0.36 (CM) vs 0.06 (3-D); Step 3, 0.54 (CM) vs 0.22 (3-D); Step 4, 0.56 (CM) vs 0.24 (3-D); Total, 1.84 (CM) vs 0.6 (3-D); all comparisons with a p-value <0.05; see Table 3). Finally, Step 4 was the only one with a statistically significant increased use of “focus-out” for the 3-D system (CM 0.16 vs 3-D 0.58; p-value 0.008).
 |
Table 3 Comparison of Magnification and Focus Between Systems per Step |
The percentage of light intensity did not show statistically significant differences between both systems, with a mean of 99.45 for CM and 98.43 for 3-D; p-value 0.695; see Table 2). There were no intraoperative complications.
Discussion
It has been claimed that heads-up surgery through a 3-D visualization display could offer advantages during cataract surgery compared to a conventional microscope, including improved visualization, depth of field, magnification, light intensity, and teaching, among others.3,4,6–9 In this study, we objectively assessed if there is a reduced need for adjustments of magnification and focus during cataract surgery and whether a presumed change in these adjustments influences the duration of surgery.
One of the parameters that showed a relevant difference between systems was the “zoom-in”, with a much lower statistically significant use with the 3-D system for all steps. This is not surprising since it is probably secondary to the magnification that the 3-D system offers, due to the screen size of 55 inches located 1.2 meters away from the surgeon, compared to the limited field of view of conventional microscopy.
We observed a large difference regarding “focus-out”, with a statistically significant greater use with the 3-D system. This was true only for Step 4, specifically during suture placement, when it was necessary to “focus-out” the image significantly to facilitate maneuvers on the ocular surface. In a surgical microscope, resolution and magnification must be sufficiently high, but if magnification is too high, there is an impairment of eye-to-hand coordination.10 While magnification is an advantage with heads-up 3-D systems, when working for the first time with this type of visualization device, there is an adjustment period for surgeons to get used to visualizing and performing specific steps, like in the case of suture placement.
Previous studies reported that with an aperture of 25%, the depth of field was significantly greater compared with a standard microscope; however, this aperture size was associated with a loss of brightness.11 Other authors that evaluated the 3-D heads‑up system versus CM issued recommendations on optimizing the surgical experience and suggested setting the aperture at 30% to get the best brightness and depth of field,12 as we did in our study. Theoretically, since the depth of field is greater than in CM, this would imply less need for Focus-in the 3-D system; however, we did not observe differences in this parameter. We believe that since only expert surgeons in the use of CM participated in the study, they did not need to use the “Focus-in” frequently, as is the case during the learning curve. However, we did not evaluate the endpoints of this study with less experienced surgeons, so further studies would be needed to confirm this.
Since some studies state that one of the advantages of three-dimensional systems is the reduction in surgical time, our team expected to see this reflected in the results. However, we observed a statistically significant increase in surgical time of 7.1 minutes with the 3-D system, as previously reported.8 When evaluating each step, we observed that the difference was about 1.5–2 additional minutes using the 3-D system for the four steps. We believe that this difference is secondary to the learning curve of the 3-D system, determined by the adaptation to the system’s magnification and the preset depth of field. The depth of field plays a determining role in 3-D surgery, and there is a difference in visualization between anterior and posterior segment surgery, where the work area is wider in the latter due to the depth of the vitreous cavity. On the contrary, in cataract surgery, the learning curve with CM is partly determined by the correct focus of the working areas. CM-trained cataract surgeons are used to working on the anterior capsule while the posterior capsule is out of focus, and vice versa. In 3-D surgery, since the depth of field is greater, all the structures are in focus; that is, the anterior and posterior capsules are in focus simultaneously, something that can generate in the surgeon the perception that one is “dangerously” near a particular structure. This can slow down the surgery during the learning curve with the 3-D system. To reduce this effect, the aperture can be enlarged to >30%, as this would reduce the depth of focus and increase brightness, as explained above. The correct setting of the parameters in each surgical step can facilitate the learning curve and decrease the surgical time.
Regarding light intensity, the baseline value was established at 70% before surgery, and surgeons were allowed to modify it at their convenience. Although the literature reports the use of lower light intensity with 3-D technology, in our study, all surgeons changed the light intensity level to almost 100% with both CM and 3-D. This result is inconsistent with previous reports13,14 in anterior and posterior segment surgery. Our results could be explained by the difference that operating in a dark cavity such as the posterior segment implies, which, when illuminated by an endoilluminator that transmits light directly to a specific structure, induces more notorious contrasts that make it easier to reduce light intensity, unlike anterior segment surgery in which light intensity is essential, especially during the learning curve, and is entirely dependent on external coaxial illumination. There are two other factors to consider. The first is that this work was not intended to evaluate the lowest light intensity that allows performing cataract surgery safely, as previously reported,15 but to record the light intensity used by surgeons in their learning curve and compare it with the light intensity of the CM. The second is that we did not adjust the brightness of the 3-D screen, a tool that can be implemented to compensate for reduced microscope illumination. Although we could have increased the brightness to decrease light intensity, per protocol, we did not want to modify this variable and used the system’s default brightness.
Finally, in addition to the fact that there were no intraoperative complications in any of the groups, confirming the previously reported safety of the 3-D visualization system,5 we observed other advantages. For example, live collective learning is essential for surgical understanding in teaching hospitals. Complications related to inadequate visualization by residents are significantly reduced with 3-D surgery. What the surgeon sees is seen by everyone in the room. In addition, ergonomic comfort for both the main surgeon and the assistant surgeon are essential, especially when performing substantial numbers of procedures per day, as in our teaching hospital. Lastly, although we did not assess ergonomics and comfort as endpoints in this study, we plan to conduct a study that evaluates such parameters since the surgical team agreed that the system feels more comfortable, as reported in other studies.7
What was Known
Heads-up 3-D systems have improved visualization and surgical efficiency in posterior segment surgery.
What this Paper Adds
Heads-up 3-D systems are safe and valuable in anterior segment surgery; it is essential to consider the different features and specifics when using the system compared to posterior segment surgery.
Acknowledgments
A special acknowledgment to Engineer Dorian Rodrigo Gómez Núñez for designing and manufacturing the counter used in this study.
Funding
An Investigator-Initiated Trial (IIT) grant from Alcon Laboratories partially funded this work (IIT number 65552033).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Aaron KA, Vaughan J, Gupta R, et al. The risk of ergonomic injury across surgical specialties. PLos One. 2021;16(2):e0244868. doi:10.1371/journal.pone.0244868
2. Miyake K, Ota I, Miyake S, Tanioka K, Kubota M, Mochizuki R. Application of a newly developed, highly sensitive camera and a 3-dimensional high-definition television system in experimental ophthalmic surgeries. Arch Ophthalmol. 1999;117(12):1623–1629. doi:10.1001/archopht.117.12.1623
3. Postel EA, Pulido JS, Byrnes GA, et al. Long-term follow-up of iatrogenic phototoxicity. Arch Ophthalmol. 1998;116(6):753–757. doi:10.1001/archopht.116.6.753
4. Ohno H. Utility of three-dimensional heads-up surgery in cataract and minimally invasive glaucoma surgeries. Clin Ophthalmol. 2019;13:2071–2073. doi:10.2147/OPTH.S227318
5. Weinstock RJ, Diakonis VF, Schwartz AJ, Weinstock AJ. Heads-up cataract surgery: complication rates, surgical duration, and comparison with traditional microscopes. J Refract Surg. 2019;35(5):318–322. doi:10.3928/1081597X-20190410-02
6. Berquet F, Henry A, Barbe C, et al. Comparing heads-up versus binocular microscope visualization systems in anterior and posterior segment surgeries: a retrospective study. Ophthalmologica. 2020;243(5):347–354. doi:10.1159/000507088
7. Bin Helayel H, Al-Mazidi S, AlAkeely A. Can the three-dimensional heads-up display improve ergonomics, surgical performance, and ophthalmology training compared to conventional microscopy? Clin Ophthalmol. 2021;15:679–686. doi:10.2147/OPTH.S290396
8. Kelkar JA, Kelkar AS, Bolisetty M. Initial experience with three-dimensional heads-up display system for cataract surgery - A comparative study. Indian J Ophthalmol. 2021;69(9):2304–2309. doi:10.4103/ijo.IJO_231_21
9. Velasque L, Arbousoff N, Rigaudier F, et al. Lux study: contribution of a three-dimensional, high dynamic range, ultra-high-definition heads-up visualization system to a significant delivered light intensity decrease during different types of ocular surgeries. J Fr Ophtalmol. 2021;44(8):1129–1141. doi:10.1016/j.jfo.2021.01.006
10. Kaschke M, Donnerhacke K, Rill M. Optical visualization, imaging, and structural analysis. In: Optical Devices in Ophthalmology and Optometry Technology: Design Principles and Clinical Applications. Weinheim, Germany: Wiley-VCH; 2014:152.
11. Eckardt C, Paulo EB. Heads-up Surgery for Vitreoretinal Procedures: an experimental and clinical study. Retina. 2016;36(1):137–147. doi:10.1097/IAE.0000000000000689
12. Zhao XY, Zhao Q, Li NN, et al. Surgery-related characteristics, efficacy, safety and surgical team satisfaction of three-dimensional heads-up system versus traditional microscopic equipment for various vitreoretinal diseases. Graefes Arch Clin Exp Ophthalmol. 2022;261(3):669–679.
13. Nariai Y, Horiguchi M, Mizuguchi T, Sakurai R, Tanikawa A. Comparison of microscopic illumination between a three-dimensional heads-up system and eyepiece in cataract surgery. Eur J Ophthalmol. 2021;31(4):1817–1821. doi:10.1177/1120672120929962
14. Kim YJ, Kim YJ, Nam DH, et al. Contrast, visibility, and color balance between the microscope versus intracameral illumination in cataract surgery using a 3D visualization system. Indian J Ophthalmol. 2021;69(4):927–931. doi:10.4103/ijo.IJO_1825_20
15. Matsumoto CS, Shibuya M, Makita J, et al. Heads-up 3D surgery under low light intensity conditions: new high-sensitivity HD camera for ophthalmological microscopes. J Ophthalmol. 2019;2019:5013463. doi:10.1155/2019/5013463
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
