Back to Journals » Local and Regional Anesthesia » Volume 16
Magnesium Sulfate in Pediatric Abdominal Cancer Surgery: Safety and Efficacy in Ultrasound-Guided Transversus Abdominis Plane (US-TAP) Block in Conjugation with Levobupivacaine
Authors El Sherif F , Sayed DG, Fares KM, Mohamed SA, Osman AM , Sayed AK , Kamal SM
Received 8 July 2023
Accepted for publication 31 August 2023
Published 12 September 2023 Volume 2023:16 Pages 133—141
DOI https://doi.org/10.2147/LRA.S425649
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Stefan Wirz
Fatma El Sherif,1 Doaa Gomaa Sayed,1 Khaled Mohamed Fares,1 Sahar Abdel-Baky Mohamed,1 Amira Mahmoud Osman,2 Ahmed Kamal Sayed,1 Shereen Mamdouh Kamal1
1Department of Anesthesia, ICU, and Pain Management, South Egypt Cancer Institute, Assiut University, Assiut, Egypt; 2Department of Pediatric Oncology, South Egypt Cancer Institute, Assiut University, Assiut, Egypt
Correspondence: Ahmed Kamal Sayed, Department of anesthesia, ICU, and pain management, South Egypt Cancer Institute, Assiut University, Assiut, Egypt, Tel +20 100677881, Fax +20 88 2348609, Email [email protected]
Purpose: Magnesium sulfate (MgSO4) may enhance the effects of local anesthetics when used as an adjuvant in peripheral nerve blocks. Our objective was to evaluate efficiency and safety of utilizing MgSO4 alongside levobupivacaine in bilateral ultrasound-guided transversus abdominis plane (US-TAP) block for postoperative pain in pediatric cancer patients who underwent abdominal surgery.
Methodology: A randomized double-blinded controlled trial at South Egypt Cancer Institute, Assiut University, Assiut, Egypt, included that 40 pediatric patients with Wilms’ tumor or neuroblastoma were randomly allocated to get bilateral (US-TAP) block and divided into two groups; M group: received US-TAP with 0.6 mL/kg levobupivacaine 0.25% + 2 mg/kg MgSO4 and C group: received with 0.6 mL/kg levobupivacaine 0.25% only. FLACC scores (Face, Leg, Activity, Cry, Consolability) were used to evaluate post-operative pain, first analgesic request, total analgesic consumption, adverse effects, as well as hemodynamics were monitored for 24 h and recorded at time points (2, 4, 6, 8, 12, 18, and 24h). Parent’s satisfaction at discharge, also, was evaluated.
Results: FLACC score in M group was significantly lower than in C group from 4 h to 24 h with the first analgesic request being longer (15.95 ± 1.99 vs 7.70 ± 0.80 (h); p < 0.001) and lower total analgesic consumption (231.75 ± 36.57 vs 576.00 ± 170.71 (mg); p < 0.001) when comparing M group to C group, respectively. Both groups had insignificant differences regarding hemodynamics, parent satisfaction, postoperative agitation, and side effects except vomiting occurred in two patients in the C group and one patient in the M group.
Conclusion: We conclude that adding magnesium sulphate as an adjuvant to local anaesthetic in US-TAP block for pain management in pediatric abdominal cancer surgeries resulted in better and longer analgesia, with less consumption of rescue analgesics with no serious side effects.
Keywords: abdominal cancer surgery, analgesia, levobupivacaine, magnesium sulfate, pediatrics, postoperative pain, US-TAP block
Introduction
Pediatric pain management is still misunderstood and not well managed especially in neonates and infants, due to the misconception that they are not able to sense pain as adults.1 This induces negative consequences, including a variety of autonomic, metabolic, hormonal, immunological or inflammatory, and neurobehavioral effects.2 Combining general anesthesia with regional or nerve blocks for this population enables a smooth intraoperative course, reduced need for general anesthetics, avoiding hazardous side effects from parenteral administration of narcotics during surgery, reduced stress response, pain-free awakening, and, most importantly, perfect postoperative pain control.3
Several regional block methods, including sciatic nerve block, fascia iliaca, ilioinguinal, and iliohypogastric blocks, as well as TAP block and topical analgesia, have been utilized on children,4 effectively and safely especially with the use the ultrasound to perform the transversus abdominis plane block (US-TAP), as a part of the multimodal anesthetic technique resulting in speedy recovery following lower abdominal surgeries.5
Because the widespread introduction of ultrasound guidance for peripheral nerve blocks, interest has developed in using the TAP block for perioperative analgesia in various types of surgery involving the abdomen. The anterior rami of the thoracolumbar nerves from T6 to L1 innervate the segmental cutaneous anterior abdominal wall; this includes the intercostal, subcostal, ilio inguinal and iliohypogastric nerves.6 The branches inter-communicate and form the upper and lower TAP plexuses and the rectus sheath plexus. These segmental nerve branches converge just above the transversus abdominis muscle or between the transversus and the internal oblique abdominal muscles.6,7 Therefore, local anesthetics applied in this plane will block these nerve plexus branches and provide analgesia to the anterolateral abdominal wall.6–8
Magnesium may influence the central nervous system’s (CNS) ability to transmit nociceptive signals and pain sensation in the central nervous system (CNS) by inhibiting N-methyl-D-aspartate (NMDA) receptor and calcium channels9 and was used recently as a powerful analgesic adjuvant in pediatric loco-regional anesthesia,10 resulting in reduced postoperative opioid requirements.11
Our objective was to study the efficiency and safety of utilizing MgSO4 alongside levobupivacaine in bilateral US-TAP block for the management of postoperative pain in pediatric cancer patients underwent abdominal cancer surgery.
Patients and Methods
Enrollment and Eligibility
Our current investigation was conducted as a randomized double-blinded controlled trial at South Egypt Cancer Institute, Assiut University, Assiut, Egypt, and approved by the local research ethical committee, South Egypt Cancer Institute, Assiut University, Assiut, Egypt (ID: IORG0006563/No. 456, March13th, 2019). It strictly adhered to the Helsinki Declaration’s guidelines and amendments. It was carried out on patients from the pediatric oncology departments.
Written informed consent was obtained from the guardians of all patients. Patients with Wilms tumor or neuroblastoma aged 1–7 years old, weighed 10–30 kg, and planned to undergo abdominal cancer surgery, with an American Society of Anesthesiology physical status of I or II (ASA), of both sex were included. We started to enroll the patients on April 15th, 2019, to determine how far they match the inclusion and exclusion criteria. However, we did not start the actual intervention until we obtained clinical trial approval of ClinicalTrials.gov on June 7th, 2019 (Identifier: NCT 03979599). The first patient was intervened on June 17th, 2019.
The exclusion criteria include patients with hemorrhagic diseases, local infection, and hypersensitivity to the medications under study, muscle disorders, substantial organic malfunction, and/or those who were unconscious or mentally retarded.
Randomization and Blindness
A random sequence was created using the randomization tool (http://www.randomizer.org), and each code was placed inside an opaque, sealed envelope. The envelope opening was done by someone else who was not a part of the study and did not possess authority over the evaluation of results.
Following anesthetic induction and 15 minutes before wound incision, 40 participants were randomly selected to receive a bilateral US-TAP block and divided into two groups as follows:
M Group (magnesium sulfate group): 20 patients received a bilateral US-TAP block with 0.6 mL/kg levobupivacaine 0.25% + 2 mg/kg MgSO4. Total dose was divided into 2 equal doses, with each dose was administered on either side.
C Group (control group): 20 patients received a bilateral US-TAP block with 0.6 mL/kg levobupivacaine 0.25% only, with half of the dose was administered on either side.
The anesthesiologist, doctors who prepared study medications, guardians of the patients, and data collectors were blinded to the study group assignment.
Anesthetic Procedure
General anesthesia was induced using inhalation anesthetic through a face mask in the operating room following the completion of the fasting hours and the use of routine monitors (pulse oximetry, electrocardiograph non-invasive blood pressure, capnography, and temperature). A secured intravenous cannula was inserted. After administering 1 mg/kg of fentanyl, 1 mg/kg of propofol, as well as 0.5 mg/kg of atracurium, the endotracheal tube was placed and secured.
Ultrasound bilateral oblique subcostal TAP block was carried out by two anesthesiologist specialists in TAP blocks. Using a high-frequency linear ultrasound probe (Sonosite®, Inc., USA) and an in-plane 50 mm 22 G needle (Pajunk® SonoPlex Stim cannula U.S.A.), US-TAP block was carried out on a patient while they were lying on their backs. The needle and ultrasound entry site were sterile, and the ultrasound probe was positioned obliquely to the sagittal plane and parallel to the subcostal border near the xiphoid process.
The transversus abdominis muscle appeared to be the most hypoechoic layer of muscle located below the rectus abdominis muscle close to the xiphoid between the medial and lateral borders of the external and internal oblique muscles. The first layer below the subcutaneous tissue was the aponeurosis, which was located above the transversus abdominis. After that, 2 mL of 0.9% NaCl were injected to confirm the location of the needle tip and the correct injection plane by hydro-dissection.
Local anesthesia was placed with intermittent aspiration and observed as a hypoechoic layer transecting the TAP when the needle was moved in-plane with the probe at the xiphoid, passing slightly below the rectus to the TAP. Skin incision was permitted 15 minutes following the block. A mixture of oxygen-air (50–50%) and sevoflurane (2%) were utilized to sustain anesthesia. Until the surgery was finished, hemodynamics were monitored and evaluated at intervals of ten minutes. Hypotension, which was defined as a systolic arterial pressure below 70 and linked with decreased peripheral perfusion, as well as bradycardia, which was defined as a heart rate below 60 beats per minute, were both monitored and managed during the surgery. The patient was extubated and transferred to the PCU (pediatric care unit) after the surgical procedure finished and patient recovery. Following recovery, a 3-point emergency agitation scale evaluation was performed using the following scale (1 = calm; 2 = restless but calms to verbal instructions; and 3 = combative and disoriented), and the results were documented.12
Postoperative Follow-Up
Postoperatively, pain intensity was assessed by FLACC pain score1 (Face, Leg, Activity, Cry, Consolability) at 2, 4, 6, 8, 12, 18, and 24 h, as follows (0 = No pain; 1–2 = mild pain; 3–5 = moderate pain; 6–8 = severe pain; 9–10 = extreme–maximum pain) when FLACC score was ≥4, rescue analgesia in the form of intravenous acetaminophen (perfalgan®) 15mg/kg was given, if the patient was still in pain additionally diclofenac 25 mg suppository was given. The overall quantities of consumed analgesia and duration for the initial request of analgesia in the first 24 h postoperatively were measured and documented.
Patients were monitored for hemodynamics immediately after the surgical procedures and then at 2, 4, 6, 8, 12, 18, and 24 h and documented. At the same intervals, the sedation level was evaluated using the Ramsay sedation score,13 as follows: 1 = anxious, agitated, or restless, or both; 2 = cooperative, oriented, and calm; 3 = responsive to commands only; 4 = exhibiting brisk response to a light glabellar tap or loud auditory stimulus; 5 = exhibiting sluggish response to a light glabellar tap or loud auditory stimulus; 6 = unresponsive.
During the first 24 hours following surgery, undesirable symptoms (sedation, hypotension, respiratory depression, and vomiting), as well as block-negative outcomes (seizure, hematoma, transient femoral nerve palsy, and ventricular arrhythmia), were noted and documented.
At the time of the patient’s discharge from the PCU, the level of the patient’s parents’ satisfaction was evaluated on a 5-point scale (totally unsatisfied, unsatisfied, neither fully satisfied nor unsatisfied, satisfied, or totally satisfied).14
Statistical Analysis
Power of the Study
A pilot trial with 10 patients per group as there was no previously published data was conducted to detect an effect size of 0.5 difference of the mean of FLACC score between the two studied groups (2.9 ± 0.32 in C group and 2.40 ± 0.52 in M group), with a p-value <0.05 and 80% power, confidence level (CL) 0.95, the sample size should include 17 patients for each group. However, to avoid patient dropout, we enrolled 20 patients in each group. This was calculated using G Power 3.1.15
Statistical Tests
SPSS (Statistical Package for the Social Science, version 20, IBM, Armonk, New York) was used to gather and analyze the data. The Shapiro test was utilized to check if the data adhered to a normal distribution. The Student’s t-test is used to compare quantitative data that were reported as mean ± standard deviation (SD). The Mann–Whitney test was used to examine quantitative data with abnormal distributions that were reported as median (range). Nominal data were represented as a number (n) and a percentage (%). Comparing qualitative data was done using the chi-square and Fisher Exact tests. A P-value of 0.05 or less was interpreted as significant since the CL was maintained at 95%.
Results
Participant Flow
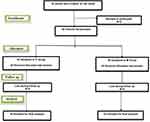
Forty-two pediatric cancer patients with Wilms tumor or neuroblastoma underwent abdominal cancer surgery were evaluated, 2 patients were refused to participate, and 40 patients were successfully recruited and randomized divided into two groups of 20 patients each. Figure 1 demonstrates the participants’ flowchart for the study.
Baseline and Clinical Data
Demographic data (age, sex, weight, and ASA) and clinical data (duration of surgery) of this study did not reveal significant differences between the two studied groups (p > 0.05) (Table 1).
 |
Table 1 Demographic and Clinical Data of Patients in the Studied Groups |
Primary Outcome
The median (range) FLACC scores in the M group were substantially lower than those in C group from 4 to 24 h when put in comparison following surgery (P < 0.001) as shown in Figure 2.
Secondary Outcomes
The mean (±SD) time of the initial analgesic request was significantly longer (15.95 ± 1.99 vs 7.70 ± 0.80 h; p < 0.001) and lower mean (±SD) total analgesic consumption (231.75 ± 36.57 vs 576.00 ± 170.71 mg acetaminophen; p < 0.001) when comparing M group to C group, respectively, during the first 24 h postoperatively with no patient required additional analgesics, with no significant differences in regard to parent satisfaction and postoperative agitation (Table 2).
 |
Table 2 Time of First Request for Analgesia, Total Amount of Analgesic Consumption, Agitation Scale, and Parent Satisfaction Among the Studied Groups During the First 24 h Postoperatively |
Intraoperative and postoperative hemodynamic variables like heart rate, mean arterial blood pressure, respiratory rate, and oxygen saturation did not differ significantly between the two groups at any time (P > 0.05). The postoperative sedation scores did not differ significantly between the two groups studied (P > 0.05).
Vomiting was experienced by two patients in the C group and one patient in the M group, but there was no substantial difference among them concerning the other adverse effects. There were no complications associated with the block (Table 3).
 |
Table 3 Side Effects and Complications Related to the Block Observed During the First 24 h Postoperatively of the Studied Groups |
Discussion
We found that combining MgSO4 as fascial plane block with levobupivacaine in a pediatric bilateral US-TAP block for postoperative analgesia in pediatric cancer surgery, patients exhibited satisfactory pain management by reducing pain intensity at the different time points during the first 24 hours following surgery as indicated by significantly lower FACC scores in M group put in comparison with C group with a longer period before the initial inquiry for analgesics and fewer overall consumption of analgesics compared to the C group with good parent satisfaction and patient agitation score. Additionally, there were no serious complications and no impact on intraoperative or postoperative hemodynamics.
There is a dearth of research on MgSO4’s usage in conjunction with levobupivacaine in US-TAP blocks for pediatric abdominal cancer surgery. Therefore, we conducted this trial to assess the safety and effectiveness of combining MgSO4 with levobupivacaine in reducing postoperative pain intensity, the length of time before the first request of analgesia, and the total amount of analgesic consumed in the first 24 h in 40 pediatric patients who underwent abdominal cancer surgery for the resection of Wilms tumor or neuroblastoma.
Due to the large subcostal flank cut wound that is typically used to provide a roomy surgical field, management of pain following open nephrectomy is a hard task. Because open nephrectomy necessitates extensive muscle cutting, this somatic pain constitutes 70–75% of the postoperative pain and lasts for 72 h.16,17 Because the widespread introduction of ultrasound guidance for peripheral nerve blocks, interest has developed in using the TAP block for perioperative analgesia in various types of surgery involving the abdomen. The anterior rami of the thoracolumbar nerves from T6 to L1 innervate the segmental cutaneous anterior abdominal wall.6 Therefore, LA applied in this plane will block these nerve plexus branches and provide analgesia to the anterolateral abdominal wall.6–8 There are several TAP block approaches, these include the subcostal TAP (T6 to T9), the oblique subcostal TAP (T6 to L1; which corresponds to the upper approach TAP).7
Numerous adjuvants, including morphine, tramadol, fentanyl, α2 agonists, epinephrine, neostigmine, ketamine, midazolam, sodium bicarbonate, and dexamethasone have been utilized in conjunction with local anesthetics since the length of postoperative analgesia is frequently a limiting issue.18 Nevertheless, there have been no definitive findings to date, and these medications are frequently linked to a variety of negative side effects.19
It has been demonstrated that MgSO4, an adjuvant for perioperative pain relief that acts as an NMDA-receptor blocker in the peripheral and central nervous systems, lowers the need for intraoperative and postoperative analgesics.20 Our investigation found that the M group took longer than the C group to make their initial request for analgesia. Additionally, the M group consumed less total paracetamol than the C group.
Mg’s voltage-dependent antagonistic activity against NMDA receptors, which controls calcium ion influx and prevents central sensitization of peripheral nociceptive stimulation, may be the fundamental mechanism behind the analgesic effects of MgSO4. This would lessen immediate pain following tissue injury.21 An additional important mechanism is that Mg increases the release of excitatory neurotransmitters at the synaptic connection, which may intensify the effects of local anesthetics.22
Similar to our findings, Zeng et al discovered that adjuvant MgSO4 significantly decreased the total amount of analgesics consumed on the first postoperative day relative to the control group;23 a subgroup analysis showed that MgSO4 significantly decreased the total quantity of analgesics consumed in TAP blockade patients.21 Additionally, earlier research found that a single 50 mg bolus dose of MgSO4 given to children for caudal analgesia was effective in preventing intraoperative distress and delaying the beginning of postoperative pain.24,25 Furthermore, a previous study found that the mean length of analgesia in the experimental groups was much prolonged than in the control group.26
In agreement with us, Sayed et al study discovered a substantial decrease in FLACC pain score in the magnesium group.26 Our findings confirmed Kim et al findings, who used ropivacaine alone and in conjunction with MgSO4 in 80 children performing inguinal herniorrhaphy and found that those who received MgSO4 experienced much less postoperative pain levels.21 Our findings support a study carried out by Mukherjee et al who found that the MgSO4 group took longer to seek analgesia than the control group did and that the rescue analgesia was used less frequently in MgSO4 group compared to the control group.27 Umalkar et al, however, found no substantial difference amongst groups in the postoperative VAS score or intake of analgesia.28
In the current study, we found that there was no substantial difference among the intraoperative and postoperative hemodynamics of the studied groups at different periods of measurement. In agreement with this finding, Shahadah et al stated that both groups of children either received magnesium sulfate or not had comparable hemodynamics at different times of assessment.29 Furthermore, according to Refaee et al, the examined groups’ hemodynamic parameters (MAP) at baseline and after the block were comparable.30
Regarding postoperative adverse effects, there were no substantial differences among the two groups. According to several studies, MgSO4 can have several negative consequences when administered intravenously, including hypotension, bradycardia, irregular rhythms, transient facial and neck flushing, drowsiness poor reflexes, excessive perspiration, and skeletal muscular paralysis. However, it was discovered that employing MgSO4 alongside local anesthetics in a variety of nerve blocks at different doses does not produce any significant deleterious effects.31,32
The satisfaction of patient’s parents in the current study was not substantially different among the two groups, and this aligns with both groups’ optimal pain management, suggesting that both groups were similarly satisfied.
The main strengths of the current study include being a randomized trial and the first study that discussed the usage of MgSO4 as an adjuvant in pediatrics that underwent abdominal cancer surgery up to our knowledge. Also, we assessed the degree of satisfaction of parents after the usage of magnesium sulfate. However, the current study had some limitations. Although the results mentioned here suggest that MgSO4 could be used as an adjuvant to boost the effects of local anesthetics, the ideal dose for this purpose is yet unknown. Lastly, it is relatively small in size and is conducted in a single center. Further studies are required to confirm such results.
Conclusion
We conclude that adding magnesium sulphate as an adjuvant to local anaesthetic in US-TAP block for pain management in pediatric abdominal cancer surgeries resulted in better and longer analgesia, with less consumption of rescue analgesics with no serious side effects.
Data Sharing Information
Raw data (de-identified) used in this clinical trial are available from the corresponding author Dr. Ahmed Kamal Sayed; it will be available following publication and for a period of one year.
Acknowledgments
We are grateful to the operating theatre and PICU staff for their co-operation in data collection.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Mencía S, Alonso C, Pallás-Alonso C, López-Herce J. Maternal and child health and development network Ii Samid Ii. evaluation and treatment of pain in fetuses, neonates and children. Children. 2022;9(11):1688. doi:10.3390/children9111688
2. Bosenberg AT. Regional anesthesia in children: an update. South African J Anaesth Analg. 2013;19(6):282–288.
3. Rai E, Naik V, Singariya G, Bathla S, Sharma R, Pani N. Recent advances in paediatric anaesthesia. Indian J Anaesth. 2023;67(1):27–31. doi:10.4103/ija.ija_973_22
4. Roberts S. Regional anesthesia in children. Princ Pract Region Anesth. 2012;2012:230.
5. Johns N, O’Neill S, Ventham NT, Barron F, Brady RR, Daniel T. Clinical effectiveness of transversus abdominis plane (TAP) block in abdominal surgery: a systematic review and meta-analysis. Colorectal Dis. 2012;14(10):e635–e642. doi:10.1111/j.1463-1318.2012.03104.x
6. McDonnell JG, O’Donnell BD, Farrell T, et al. Transversus abdominis plane block: a cadaveric and radiological evaluation. Reg Anesth Pain Med. 2007;32(5):399–404. doi:10.1016/j.rapm.2007.03.011
7. Tsai HC, Yoshida T, Chuang TY, et al. Transversus abdominis plane block: an updated review of anatomy and techniques. Biomed Res Int. 2017;2017:8284363. doi:10.1155/2017/8284363
8. Finnerty O, Carney J, McDonnell JG. Trunk blocks for abdominal surgery. Anesthesia. 2010;65(Suppl 1):76–83. doi:10.1111/j.1365-2044.2009.06203.x
9. De Baaij JH, Hoenderop JG, Bindels RJ. Magnesium in man: implications for health and disease. Physiol Rev. 2015;95(1):1–46. doi:10.1152/physrev.00012.2014
10. Cho HK, Park IJ, Yoon HY, Hwang SH. Efficacy of adjuvant magnesium for posttonsillectomy morbidity in children: a meta-analysis. Otolaryngol Head Neck Surg. 2018;158(1):27–35. doi:10.1177/0194599817730354
11. Monaa G, Nabil H, Elmetwally M, Elzahaby Islam AC. Ultrasound-guided transversus abdominis plane block for total abdominal hysterectomy: comparison between magnesium sulfate and dexamethasone as adjuvants. Res Opin Anesth Intensive Care. 2019;6(2):243–248. doi:10.4103/roaic.roaic_24_19
12. Zanaty OM, El Metainy SA. A comparative evaluation of nebulized dexmedetomidine, nebulized ketamine, and their combination as premedication for outpatient pediatric dental surgery. Anesth Analg. 2015;121(1):167–171. doi:10.1213/ANE.0000000000000728
13. Fares KM, Othman AH, Alieldin NH. Efficacy and safety of dexmedetomidine added to caudal bupivacaine in pediatric major abdominal cancer surgery. Pain Phys. 2014;17(5):393–400.
14. Al-Sadek WM, Rizk SN, Selim MA. Ultrasound guided transversus abdominis plane block in pediatric patients undergoing laparoscopic surgery. Egypt J Anaesth. 2014;30(3):273–278. doi:10.1016/j.egja.2014.01.011
15. Hsieh FY, Bloch DA, Larsen MD. A simple method of sample size calculation for linear and logistic regression. Stat Med. 1998;17(14):1623–1634. doi:10.1002/(SICI)1097-0258(19980730)17:14<1623::AID-SIM871>3.0.CO;2-S
16. Niraj G, Tariq Z, G N. Continuous erector spinae plane (ESP) analgesia in different open abdominal surgical procedures: a case series. J Anesth Surg. 2018;5(1):57–60. doi:10.15436/2377-1364.18.1853
17. Chapman E, Pichel A. Anesthesia for nephrectomy. BJA Educ. 2015;16(3):98–101. doi:10.1093/bjaceaccp/mkv022
18. Kirksey MA, Haskins SC, Cheng J, Liu SS. Local anesthetic peripheral nerve block adjuvants for prolongation of analgesia: a systematic qualitative review. PLoS One. 2015;10(9):e0137312. doi:10.1371/journal.pone.0137312
19. Swain A, Nag DS, Sahu S, Samaddar DP. Adjuvants to local anesthetics: current understanding and future trends. World J Clin Cases. 2017;5(8):307–323. doi:10.12998/wjcc.v5.i8.307
20. Do SH. Magnesium: a versatile drug for anesthesiologists. Korean J Anesthesiol. 2013;65(1):4–8. doi:10.4097/kjae.2013.65.1.4
21. Kim EM, Kim MS, Han SJ, et al. Magnesium as an adjuvant for caudal analgesia in children. Paediatr Anaesth. 2014;24(12):1231–1238. doi:10.1111/pan.12559
22. Shin HJ, Na HS, Do SH. Magnesium and Pain. Nutrients. 2020;12(8):2184. doi:10.3390/nu12082184
23. Zeng J, Chen Q, Yu C, Zhou J, Yang B. The use of magnesium sulfate and peripheral nerve blocks: an updated meta-analysis and systematic review. Clin J Pain. 2021;37(8):629–637. doi:10.1097/AJP.0000000000000944
24. Albrecht E, Kirkham KR, Liu SS, Brull R. The analgesic efficacy and safety of neuraxial magnesium sulphate: a quantitative review. Anaesthesia. 2013;68(2):190–202. doi:10.1111/j.1365-2044.2012.07337.x
25. Yousef GT, Ibrahim TH, Khder A, Ibrahim M. Enhancement of ropivacaine caudal analgesia using dexamethasone or magnesium in children undergoing inguinal hernia repair. Anesth Essays Res. 2014;8(1):13–19. doi:10.4103/0259-1162.128895
26. Sayed JA, Kamel EZ, Riad MAF, Abd-Elshafy SK, Hanna RS. Dexmedetomidine with magnesium sulphate as adjuvants in caudal block to augment anaesthesia and analgesia in paediatric lower abdominal surgeries. Egypt J Anaesth. 2018;34(4):115–122.
27. Mukherjee K, Das A, Basunia SR, Dutta S, Mandal P, Mukherjee A. Evaluation of Magnesium as an adjuvant in Ropivacaine-induced supraclavicular brachial plexus block: a prospective, double-blinded randomized controlled study. J Res Pharm Pract. 2014;3(4):123–129. doi:10.4103/2279-042X.145387
28. Umalkar M, Londhe N. Evaluation of magnesium sulfate as an adjuvant to bupivacaine for postoperative analgesia in ultrasound-guided transversus abdominis plane block in patients scheduled for lower segment caesarean section under subarachnoid block–A prospective, randomized, double-blind study. Indian J Pain. 2020;34(3):189.
29. Refaee HH, Elela AHA, Hanna MG, Ali MA, Khateeb AME. Dexmedetomidine versus magnesium as adjuvants to bupivacaine-induced caudal block in children: a randomized, double-blinded, placebo-controlled, and trial. Open Access Maced J Med Sci. 2019;7(1):73–76. doi:10.3889/oamjms.2019.024
30. Singh J, Verma V, Pathania A, Thakur A, Chaudhary C, Sood P. Comparative evaluation of magnesium as an adjuvant to 0.5% bupivacaine and 0.5% ropivacaine in ultrasound guided interscalene brachial plexus block. Ann Int Med Den Res. 2017;3:22–27. doi:10.21276/aimdr.2017.3.4.AN6
31. Muthiah T, Arora MK, Trikha A, Sunder RA, Prasad G, Singh PM. Efficacy of magnesium as an adjuvant to bupivacaine in 3-in-1 nerve block for arthroscopic anterior cruciate ligament repair. Indian J Anaesth. 2016;60(7):491–495. doi:10.4103/0019-5049.186018
32. Shahadah HH, Goda AM, Abdelrazek GMI, Elhossary ZEA. Intraoperative hemodynamic changes in dexamethasone and magnesium sulphate as an adjunct to bupivacaine for caudal blockade anesthesia and analgesia in children undergoing lower abdominal surgeries. Egypt J Hosp Med. 2021;85(2):3514–3518. doi:10.21608/ejhm.2021.200579
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.