Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Maculopapular Drug Eruption Caused by Finasteride: A Case Report
Received 6 August 2023
Accepted for publication 1 November 2023
Published 17 November 2023 Volume 2023:16 Pages 3359—3361
DOI https://doi.org/10.2147/CCID.S426747
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Hongxia Jia, Liwei Ran
Department of Dermatology, Beijing Chao-Yang Hospital, Capital Medical University, Beijing, People’s Republic of China
Correspondence: Liwei Ran, Department of Dermatology, Beijing Chao-Yang Hospital, Capital Medical University, 8 Gongren Tiyuchang Nanlu, Chaoyang District, Beijing, 100020, People’s Republic of China, Tel +86 010 85231374, Email [email protected]
Abstract: A 76-year-old male developed a maculopapular rash on his trunk and extremities. The rash appeared 2 months after Finasteride administration for his prostatic hyperplasia. Clinical suspicion was of drug exanthema due to Finasteride. The clinical and histologic data were compatible with pharmacologic eruption by Finasteride.
Keywords: maculopapular, drug eruption, Finasteride
A 76-year-old male developed a maculopapular rash on his trunk and extremities. The rash appeared 2 months after Finasteride administration for his prostatic hyperplasia and was referred to our department for evaluation of his skin eruption. He had no past history of skin diseases, such as atopic dermatitis. The patient was treated with “eczema” by several dermatology clinics and with oral antihistamines and external administration of glucocorticoids for 2 months, but there was no obvious improvement.
Physical examination revealed multiple palpable erythematous papules and maculopapules occurring on his trunk and extremities without the involvement of mucous membrane (Figure 1A and B).
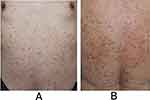 |
Figure 1 (A and B) palpable erythema and papules on his trunk. |
Blood tests showed that eosinophils 5.5% (0.5–5.0%), and IgE was within normal. Biochemical tests including liver and kidney function were normal.
The histopathological features of the back skin lesion showed mild hyperkeratosis and parakeratosis, acanthosis, spongiosis and spongiotic vesiculation, occasional apoptotic keratinocytes, and exocytosis of lymphocytes. There was a dense perivascular infiltrate of lymphocytes and a few eosinophils in the upper dermis, a few lymphocytes and eosinophils around follicles and sweat glands accompanied with follicular spongiosis (Figure 2A–C).
 |
Figure 2 (A–C) Histological features ((A): HE x40, (B and C): HE x400). |
The patient was diagnosed as maculopapular drug eruption caused by Finasteride. After stopping the use of Finasteride, the patient was given oral administration of ebastine tablets 20mg/day, topical application of halometasone cream and zinc oxide ointment twice daily. Discontinuation of Finasteride treatment showed a complete improvement. No recurrence of lesions was observed during 1-year follow-up time.
Discussion
Drug eruption, also known as drug dermatitis, belongs to cutaneous adverse drug reaction, refers to the inflammatory skin lesions of the skin and mucosa caused by drugs entering the human body through various ways including oral administration, injection, inhalation, etc. In severe cases, other organs may be involved. It is a dose-independent drug response, which is unpredictable and related to the individual’s constitution and the drug characteristics. Most patients can be cured by immediately stopping allergenic medication and giving anti-allergy treatment.1 The pathogenesis of drug eruption is often considered as drug toxicology and hypersensitivity reaction.2 The diagnosis of drug eruption is mainly based on the history of taking drugs, dermatological examination, skin histopathology, blood examination and immune examination. To determine the cause, specific immune tests for drug delay response can be performed one to six months after the complete resolution of clinical symptoms. These include patch testing, intradermal or skin prick tests, and in vitro tests such as the lymphocyte transformation tests.2
We all know that dihydrotestosterone is a key mediator of androgenic alopecia and benign prostatic hyperplasia, while Finasteride is a synthetic azosteroid that selectively inhibits type II and III 5α-reductase isoenzymes, thereby treating androgenic alopecia and benign prostatic hyperplasia by preventing the conversion of testosterone to dihydrotestosterone.3 So far, there are few reports about adverse drug reactions caused by Finasteride: T cell-mediated acute localized exanthematous pustulosis,4 urticarial rash,5 cutaneous vasculitis6, erythema annulare centrifugum,7 solitary fixed drug eruption,8 and photosensitivity reaction.9
Conclusion
In our case, based on the history of Finasteride use, blood tests, and histopathological changes, combined with the relief of symptoms after withdrawal and conventional anti-allergy therapy, we concluded that this was a macular papular eruption caused by Finasteride. Of course, the confirmatory test should be the oral Finasteride provocation test. However, in this case, the skin healed completely and the drug provocation test was unnecessary. We believe that the pathogenesis of this case may be associated with type IV delayed hypersensitivity, which is a drug-specific T-cell-induced inflammatory response to the skin.
In addition to our case, Finasteride has not been reported to cause maculopapular drug eruption. The case suggests that if apoptotic keratinocytes, eosinophilia and periappendicular inflammatory cell infiltration are visible in the histopathology, it is necessary to be alert to drug causation. In this case, conventional anti-allergy treatment is effective after cessation of the Finasteride use, which confirms the correct diagnosis, so it can also be distinguished from endogenous eczema. Meanwhile, for the patients with hyperplasia of the prostate who have drug eruption caused by Finasteride, we can choose Tamsulosin hydrochloride or Celodosinto as an alternative therapy.
Ethics Statements
Written informed consent for publication of their clinical details and clinical images was obtained from the patient. No institutional approval was required.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Hoetzenecker W, Nägeli M, Mehra ET, et al. Adverse cutaneous drug eruptions: current understanding. Semin Immunopathol. 2016;38(1):75–86. doi:10.1007/s00281-015-0540-2
2. Chu C-Y. Drug Eruptions: a great imitators. Clin Dermatol. 2020;38(2):193–207. doi:10.1016/j.clindermatol.2019.10.005
3. Motofei IG, Rowland DL, Tampa M, et al. Finasteride and androgenic alopecia; from therapeutic options to medical implications. J Dermatolog Treat. 2020;31(4):415–421. doi:10.1080/09546634.2019.1595507
4. Tresch S, Cozzio A, Kamarashev J, et al. T cell–mediated acute localized exanthematous pustulosis caused by finasteride. J Allergy Clin Immunol. 2012;129(2):589–594. doi:10.1016/j.jaci.2011.07.033
5. Moreno-Fernandez A, Mira Laguarda JM, Ruiz-Hornillos FJ, et al. Urticarial rush due to finasteride. Allergy. 2010;65(3):405–406. doi:10.1111/j.1398-9995.2009.02165.x
6. Lear JT, Byrne JP. Finasteride-related cutaneous vaculitis. Postgrad Med J. 1996;72(844):127. doi:10.1136/pgmj.72.844.127-a
7. Al Hammadi A, Asai Y, Patt ML, et al. Erythema annulare centrifugum secondary to treatment with finasteride. J Drugs Dermatol. 2007;6(4):460–463.
8. Oyama N, Kaneko F. Solitary fixed drug eruption caused by finasteride. J Am Acad Dermatol. 2009;60(1):168–169. doi:10.1016/j.jaad.2008.07.037
9. Yamada T. Photosensitivity reaction induced by finasteride. J Gen Fam Med. 2021;23(3):185–186. doi:10.1002/jgf2.510
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
