Back to Journals » International Medical Case Reports Journal » Volume 15
Lymphangioma of Colon Presenting as an Intramural Tumor
Authors Pham HD, Nguyen TA , Doan TG, Bui VG, Phan-Nguyen TV
Received 30 March 2022
Accepted for publication 31 May 2022
Published 11 July 2022 Volume 2022:15 Pages 361—366
DOI https://doi.org/10.2147/IMCRJ.S368610
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ronald Prineas
Hong Duc Pham,1,2 The Anh Nguyen,3 Thi Giang Doan,1,2 Van Giang Bui,2,4 Thanh Van Phan-Nguyen5
1Radiology Department, Saint Paul Hospital of Ha Noi, Ha Noi, Vietnam; 2Radiology Department, Hanoi Medical University, Ha Noi, Vietnam; 3Department of Respiratory Medicine, Huu Nghi Hospital, Hanoi, Vietnam; 4Radiology Centre, National Cancer Hospital, Ha Noi, Vietnam; 5Department of Biochemistry, Pham Ngoc Thach University of Medicine, Ho Chi Minh City, Vietnam
Correspondence: Thanh Van Phan-Nguyen, Department of biochemistry, Pham Ngoc Thach University of Medicine, 2 Duong Quang Trung street, Ho Chi Minh city, 700000, Vietnam, Tel +84919691770, Email [email protected]
Abstract: Lymphangiomas are rare and benign vascular malformations of the lymphatic system. They may arise in any location and at all ages and have variable presentation. These lesions in the intestinal wall are reported very rarely. In the case of colonic lymphangiomas, it is more common in late adulthood and old age, which, in this age group is thought to be associated with local disturbances of lymphatic circulation secondary to inflammation, degeneration, surgical procedure, trauma or radiation. The clinical presentation of colonic lymphangiomas varies from incidental findings on imaging to presenting with acute abdomen. The imaging features are usually multilocular cyst in intramural colon and submucosal mass on endoscopy. However, in the case of symptomatic lesions with atypical image findings, and the fact that the disease is rare, preoperative diagnosis is often difficult. On the other hand, although these cystic tumors do not transform into malignancy, they can be locally invasive or complicated, and often require resection. We report a 53-year-old male who had a cystic lymphangioma of the transverse colon illustrated by imaging modalities and recognized via postoperative histopathological examination.
Keywords: cystic lymphangioma, colonic lymphangioma, submucosal tumor
Introduction
Lymphangiomas are rare benign malformations of lymphatic vessels, therefore, they can occur in any organ of the body. The most common (95%) arise in the neck and axilla region; the rest (5%) occurs mainly in the abdomen and mediastinum.1–3 It is worth noting that they are common in children and related to the congenital origin where lymphangiectasis arises from the failure to establish a patent communication with the lymphatic system; its discovery in adults is rare and the acquired origin has been suggested to be a lymphatic obstruction as a result of inflammation, trauma, or degeneration.3
Among the abdominal lymphangiomas, the majority are located in the mesentery and retroperitoneum.4–6 These lesions in the colonic wall have been reported even more rarely, accounting for about 0.7% of all abdominal lymphangiomas.6 Various imaging modalities including ultrasound (US), computed tomography (CT), magnetic resonance imaging (MRI), and endoscopy/endoscopic ultrasound (EUS) are increasingly important in the diagnosis and assessment of these lesions before therapeutic intervention. Herein, we present a case of a 53-year-old male presenting with acute abdominal pain, who was found to have an intramural cystic mass in the transverse colon on imaging, which was confirmed as a lymphangioma on postoperative histopathology.
Case Presentation
A 53-year-old male presented to the emergency room with acute pain in the umbilical region for two days with associated fever of 38°C and nausea without vomiting. He denied any melena, hematochezia or diarrhea, except for delayed bowel habit for a day. The patient reported no chronic abdominal pain or transit disorders. He did not have any significant personal history or family history. At the time of the initial visit, his vital signs were unremarkable. The abdomen was soft and non-tender with no mass or organomegaly on palpation. Laboratory parameters upon admission showed a white blood cell count of 12.7 G/l (neutrophil 83.4%). The other laboratory tests, including those measuring amylase and tumor markers (CEA and CA19-9), were within normal limits.
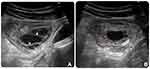
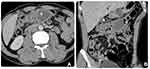
For diagnosis, abdominal X-ray, US and CT were used. The abdominal X-ray displayed no specific findings. The abdominal US showed no abnormalities of the visceral abdomen as well as no peritoneal fluid, however, in the supra umbilicus, there was a focal lesion involving the transverse colon wall. This lesion manifested as a multilocular anechoic cystic mass with internal septa of varying thickness, and no hypervascularity on echocardiography (Figure 1). An abdominal CT scan revealed a low-density multilocular mass, with a low contrast-enhanced wall and internal septa located in the transverse colon; the proximal transverse colon showed thickened submucosal edema (Figure 2). The rest of the abdomen revealed no remarkable abnormalities. Although CT was suggestive of cystic lymphangioma with colonic segmental inflammation upstream of this cyst, it was not sufficient to rule out other more frequent diagnoses. Therefore, the patient was taken for endoscopy to better define the characteristics of the lesion.
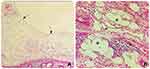
Flexible colonoscopy documented a broad-based smooth polypoid mass with glossy pinkish mucosal coating in the middle part of the transverse colon (Figure 3). Because this lesion was covered by near-normal mucosa and was suggestive of a submucosal tumor, a biopsy was not performed. Based on the diagnosis of submucosal tumor of the transverse colon, laparoscopic surgery with right hemicolectomy was performed, which showed a 4 x 6 cm, soft and spongy submucosal polypoid lesion on palpation, confined to intramural colon and non-invasion out of the serosa (Figure 4). Macroscopically, the cystic mass had multiple septa fluid-filled compartments. Histopathological examination revealed thin-walled dilated spaces consisting of only a single layer of endothelial cells (Figure 5). These findings were consistent with the diagnosis of cystic lymphangioma. The patient recovered well and was discharged on the 6th post-operative day. The 6-months subsequent follow-up was unremarkable.
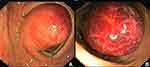 |
Figure 3 Colonoscopic view (A and B) demonstrated a large smooth-surfaced sub-pedunculated polyp with glossy, pinkish mucosal coating located in the transverse colon presenting as a submucosal tumor. |
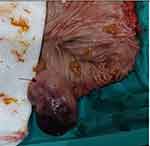 |
Figure 4 Gross morphology during surgery revealed a 4×6 cm, soft and spongy submucosal polypoid mass (arrow) in the transverse colon. |
Discussion
Colorectal lymphangioma used to be considered an extremely rare disease. Geographically, they have been reported mainly in Eastern countries (95%), with Japan alone accounting for 85%.2 The most common age at diagnosis is in the 40s to 60s; and slight predominance in men with 1.4 to 1.6 times more than women.2,7 In cases of lymphangioma presenting in late adulthood and old age, the occurrence of the lesion may have developed secondary to local disturbance of lymphatic circulation.7 Factors such as certain intestinal inflammations, surgical procedures, and radiation may be responsible for the appearance of these tumors.1
Common sites of colorectal lymphangiomas are the transverse colon, ascending colon, and cecum; and most of them are single lesions.2,7 The size of the mass is variable, up to 23 cm, and not related to the patient’s age nor to colonic location.2,7 Lymphangioma of colon is usually located in the submucosa with the overlying mucosa remaining intact. Depending on the size of the lymphatic spaces, this tumor has been classified into simple (or capillary), cavernous, and cystic types.3 Of these, cystic types are the most common - 70%,7 with 80% being multilocular.2
Cystic lymphangiomas of colon (CLCs) are often asymptomatic with small tumors. Symptoms, when present, are usually nonspecific and, depending on their size and location, may be acute abdominal pain with or without bloody stools, diarrhea or constipation.7 There have also been several cases reported of complicated acute abdomen such as infection,8 intussusception,9,10 anemia1,3,11 and protein-losing enteropathy.12,13 To date, to our knowledge, there have been no reported cases in which these tumors transformed into malignancy.
Practically, US is often the initial examination used for the symptomatic abdomen. CLCs typically show a multilocular cystic mass with septa, thin wall, containing anechoic fluid which are highly suggestive of the diagnosis10 (Figure 1A). Some lesions may be complicated by an intracystic infection or hemorrhage, causing internal echoes or sedimentation, with fluid–fluid levels caused by debris.14 CLCs are mostly avascular masses, but Doppler imaging may depict seemingly normal tiny flow signals of veins and arteries in the margin and/or septa without solid components associated with vascularity14 (Figure 1B). Although US is considered the first level of imaging investigation for a suspicious mass suggestive of a cystic mass, confirmation of a cystic lesion within or outside the bowel wall needs to be supplemented by CT or MRI because of the need for panoramic views and also to obtain additional information such as structural features, contrast enhancement, as well as to exclude locoregional extent of a malignant lesion.12
In an emergency setting, CT scans play a central role in the diagnosis. On CT lymphangiomas are cystic masses, typically attenuation values of water if the content is serious, limited and continuous with the colonic wall. This is further confirmed after administration of intravenous contrast and multiplane reconstructions showed slow or non-enhancement of the capsule and fibrous septations of the lesion as well as of the colonic wall with which it was involved1,14,15 (Figure 2). This cystic lesion may contain a low- to water-density content of the chylous fluid.16 High-density CT manifestations of intracystic bleeding and cystic wall calcification are uncommon in intra-abdominal lymphangiomas;5 and there seem to be no reports of these features in CLCs. Furthermore and for non-urgent settings, MRI better clarifies the cystic nature of masses and provides a better differentiation from other cyst-like masses,17 which we will discuss in the endoscopic ultrasound paragraph. On MRI, lymphangiomas are similar to signal intensity of fluid with low-signal intensity on T1-weighted images and high signal intensity on T2-weighted images. In the presence of chylous fluid, a signal drop may be seen in the opposed-phase chemical shift MR images.14
Most CLCs are preoperatively diagnosed as submucosal tumors using colonoscopy, which is the most commonly used diagnostic modality. Colonoscopic features of CLCs are characterized by a steep rising margin and a somewhat narrow base, covered with normal colon mucosa but pinkish color, translucent, tense and lustrous surface7 (Figure 3). The shape is altered by postural change and compression with forceps, and the fluctuation is called “cushion sign”.15,16,18,19
The role of endoscopic ultrasound (EUS) in the definitive and differential diagnosis of CLCs has been widely reported.11,15,18–20 EUS with the advantage of direct access to the surface of the colonic mucosa, can provide precise information about the layer of origin and contour of the submucosal tumor, especially with high accuracy in the diagnosis. On EUS, CLCs are confined to the submucosal layer with well-defined margins containing anechoic cystic spaces with septa and intact muscularis propria. These features are important for differential diagnosis of CLCs from other cystic-like masses located in submucosa of the colon such as leiomyomas, schwannomas, GISTs, lymphoma, and carcinoid tumor; these lesions are usually hypoechoic structures.20,21 Also based on these characteristic features of CLCs on EUS, Bhutani et al consider that endoscopic biopsies are unnecessary for diagnosis; moreover, they pose a risk of infection for these multicystic lesions.19
Other cystic lesions, although rare, that should be noted for differential diagnosis are duplication cysts and colitis cystica profunda. Cross-sectional modalities such as MRI and transrectal US are capable of demonstrating the cystic nature of these lesions. Both are more common anorectal site. Duplication cysts of colon generally have a significant exoenteric location, and the demonstration of mural layers in the cyst wall on US.22
Treatment of CLCs is nonconsensual and depends on their various presentations, sizes and locations. Traditionally, CLCs presenting asymptomatic or <2 cm in size can be managed conservatively by observation.19 However, many lesions need to be confirmed via histological diagnosis by removal of tumor due to ineffective noninvasive diagnostic techniques. Endoscopic polypectomy described in the literature so far has been done for lesions 2–3.5 cm in size.8,10 Moreover, there are also cases where the mass size up to 5cm has been successfully removed by this method.23 For cases that are difficult to manage endoscopically or require a differential diagnosis from other malignancies or are symptomatic, laparoscopic surgery should be considered as one choice to avoid complications such as superinfection, progressive growth, rupture or bleeding.15,16
The final diagnosis requires histological examination. Macroscopically, CLCs present as yellow, greyish, or yellow-pink, multilocular cysts or spongy masses, containing watery/milky fluids at sectioning18 (Figure 4). Microscopically, they consist of cysts lined by flat endothelial cells devoid of atypia, similar to those of normal lymphatic tissue. The surrounding stroma is usually irregular enlarged lymphatic spaces and aggregates of lymphoid tissue. The presence of smooth muscle bundles and adipocytes are additional features4,6 (Figure 5). In the case of atypical histology, the diagnosis might require immunohistochemistry. Endothelial cells stain positive with lymphatic endothelial markers such as D2-40 marker and also for vascular markers including CD34-, CD31-, factor VIII–related antigen and VEGFR3.11,13
Conclusion
Cystic lymphangiomas are very uncommon and benign lesions of the colon. The clinical presentation varies from incidental findings on imaging to presentation with acute abdomen. Typical features are usually multilocular cyst located in intramural colon on cross-sectional imaging and submucosal mass on endoscopy. The low frequency and acute abdominal condition can make it difficult for physicians to diagnose colonic lymphangiomas even with typical imaging features. Resection is essential to prevent complications, confirm the diagnosis histologically, and definitively exclude malignancy.
Ethics Approval and Consent to Participate
The case report received approval for publication from the Ethics Committee of Saint Paul Hospital of Ha Noi.
Consent for Publication
Written informed consent for publication of the clinical details and clinical images was obtained from the patient.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Lepre L, Costa G, Baldini D, et al. Emergency presentation of cystic lymphangioma of the colon: a case report and literature review. Int J Surg Case Rep. 2016;24:162–165. doi:10.1016/j.ijscr.2016.05.045
2. Huguet KL, Metzger PP, Menke DM. Colorectal lymphangioma. Am Surg. 2007;73(4):414–416. doi:10.1177/000313480707300423
3. Bonhomme A, Broeders A, Oyen RH, Stas M, De Wever I, Baert AL. Cystic lymphangioma of the retroperitoneum. Clin Radiol. 2001;56(2):156–158. doi:10.1053/crad.2000.0162
4. Allen JG, Riall TS, Cameron JL, Askin FB, Hruban RH, Campbell KA. Abdominal lymphangiomas in adults. J Gastrointest Surg. 2006;10(5):746–751. doi:10.1016/j.gassur.2005.10.015
5. Makni A, Chebbi F, Fetirich F, et al. Surgical management of intra-abdominal cystic lymphangioma. Report of 20 cases. World J Surg. 2012;36(5):1037–1043. doi:10.1007/s00268-012-1515-2
6. Levy AD, Cantisani V, Miettinen M. Abdominal lymphangiomas: imaging features with pathologic correlation. AJR Am J Roentgenol. 2004;182(6):1485–1491. doi:10.2214/ajr.182.6.1821485
7. Matsuda T, Matsutani T, Tsuchiya Y, et al. A clinical evaluation of lymphangioma of the large intestine: a case presentation of lymphangioma of the descending colon and a review of 279 Japanese cases. J Nippon Med Sch. 2001;68(3):262–265. doi:10.1272/jnms.68.262
8. Rebai W, Ben Mahmoud A, Yacine O, Haddad A, Atri S, Kacem M. Unusual location and complication of a cystic lymphangioma: a case report. Ann Med Surg. 2020;58:41–43. doi:10.1016/j.amsu.2020.08.029
9. Ly MMG, De Robles MS, McKenzie C, Young CJ. Colonic lymphangioma presenting with intermittent pain and intussusception. J Surg Case Rep. 2019;2019(1):rjy336. doi:10.1093/jscr/rjy336
10. Matsuba Y, Mizuiri H, Murata T, Niimi K. Adult intussusception due to lymphangioma of the colon. J Gastroenterol. 2003;38(2):181–185. doi:10.1007/s005350300030
11. Lu G, Li H, Li Y. Lymphangiomatosis of the sigmoid colon - a rare cause of lower gastrointestinal bleeding: a case report and review of the literature. Oncol Lett. 2017;13(1):339–341. doi:10.3892/ol.2016.5399
12. Kim J, Han D, Hong CH, et al. Colonic lymphangiomatosis associated with protein-losing enteropathy. Dig Dis Sci. 2005;50(9):1747–1753. doi:10.1007/s10620-005-2929-6
13. Ding XL, Yin XY, Yu YN, Chen YQ, Fu WW, Liu H. Lymphangiomatosis associated with protein losing enteropathy: a case report. World J Clin Cases. 2021;9(15):3758–3764. doi:10.12998/wjcc.v9.i15.3758
14. Francavilla ML, White CL, Oliveri B, Lee EY, Restrepo R. Intraabdominal lymphatic malformations: pearls and pitfalls of diagnosis and differential diagnoses in pediatric patients. AJR Am J Roentgenol. 2017;208(3):637–649. doi:10.2214/AJR.16.17008
15. Zhuo CH, Shi DB, Ying MG, et al. Laparoscopic segmental colectomy for colonic lymphangiomas: a definitive, minimally invasive surgical option. World J Gastroenterol. 2014;20(26):8745–8750. doi:10.3748/wjg.v20.i26.8745
16. Nabeshima K, Machimura T, Wasada M, Ogoshi K, Makuuchi H. A case of colon lymphangioma treated with laparoscopy-assisted ileocecal resection. Tokai J Exp Clin Med. 2008;33(1):61–64.
17. Romeo V, Maurea S, Mainenti PP, et al. Correlative imaging of cystic lymphangiomas: ultrasound, CT and MRI comparison. Acta Radiol Open. 2015;4(5):2047981614564911. doi:10.1177/2047981614564911
18. Katsuno H, Maeda K, Hanai T, Mizuno M, Kurashita T, Tsukamoto T. Gigantic lymphangioma with marked extraluminal progression of the ascending colon: report of a case. Surg Today. 2015;45(7):919–923. doi:10.1007/s00595-014-1055-5
19. Bhutani MS, Annangi S, Koduru P, Aggarwal A, Suzuki R. Diagnosis of cystic lymphangioma of the colon by endoscopic ultrasound: biopsy is not needed! Endosc Ultrasound. 2016;5(5):335–338. doi:10.4103/2303-9027.191668
20. Zhou PH, Yao LQ, Zhong YS, He GJ, Xu MD, Qin XY. Role of endoscopic miniprobe ultrasonography in diagnosis of submucosal tumor of large intestine. World J Gastroenterol. 2004;10(16):2444–2446. doi:10.3748/wjg.v10.i16.2444
21. Barbeiro S, Martins C, Goncalves C, et al. Schwannoma-A rare subepithelial lesion of the colon. GE Port J Gastroenterol. 2015;22(2):70–74. doi:10.1016/j.jpge.2015.01.006
22. Pickhardt PJ, Kim DH, Menias CO, Gopal DV, Arluk GM, Heise CP. Evaluation of submucosal lesions of the large intestine: part 2. Nonneoplastic causes. Radiographics. 2007;27(6):1693–1703. doi:10.1148/rg.276075028
23. Badipatla KR, Chandrala C, Ayyadurai P, et al. Cystic lymphangioma of the colon: endoscopic removal beyond the frontiers of size. Case Rep Gastroenterol. 2017;11(1):178–183. doi:10.1159/000462966
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.