Back to Journals » Clinical, Cosmetic and Investigational Dentistry » Volume 14
Lymphangioma Circumscriptum – A Rare Cause of Gingival Enlargement: A Case Report and Review of Literature
Authors Sadasivan A , Ramesh R
Received 21 March 2022
Accepted for publication 24 June 2022
Published 11 July 2022 Volume 2022:14 Pages 199—206
DOI https://doi.org/10.2147/CCIDE.S367281
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Christopher E. Okunseri
Arun Sadasivan,1 Roshni Ramesh2
1Department of Periodontics, SMIDS, Kulashekaram, Tamil Nadu, India; 2Department of Periodontics, Government Dental College, Trivandrum, Kerala, India
Correspondence: Arun Sadasivan, Department of Periodontics, SMIDS, Kulashekaram, Tamil Nadu, India, Tel +91 9847246961, Email [email protected]
Background: Lymphangiomas or microcystic lymphatic malformations (MLM) are hamartomatous formations that occur due to the proliferation of lymphatic vessels. They commonly manifest in the head and neck region with only a few cases reported within the oral cavity. Lymphangioma circumscriptum in the gingiva is a rare condition which presents as asymptomatic pebbly gingival enlargement. They are characterized by lesions that are microscopic thin-walled cysts. Histopathologically, they show multiple dilated lymphatic channels which are lined by endothelial cells. The lumen is filled with lymphatic fluid, red blood cells, and other inflammatory cells. These are dispersed within connective tissue stroma. Multiple modalities of treatment have been reported, including surgical excision, laser therapy, and sclerotherapy.
Case Presentation: This paper reports a rare case of multiple lymphangiomas of the gingiva in a 21-year-old female patient. The clinical picture, surgical treatment, histologic features, and immunohistochemistry [IHC] findings are presented. The histologic findings of lymphangioma were confirmed with IHC being positive for lymphatic marker D2-40. The patient was followed up for a period of 2 years, with no recurrence noted.
Conclusion: Lymphangiomas or microcystic lymphatic malformations presenting as a gingival enlargement are a rare occurrence. Therefore, lymphangiomas may also be considered in the differential diagnosis of gingival enlargements.
Keywords: gingiva, gingival enlargement, immunohistochemistry, lymphangioma, maxilla, oral
Introduction
Superficial lymphangiomas presenting in the mandibular and maxillary gingiva are thought to be developmental anomalies arising from the residue of neonatal lymphangiomas.1 Lymphangioma is a rare hamartomatous tumor of lymphatic vessels. They are also known as microcystic lymphatic malformations (MLM). They have a marked predilection for the head and neck region.2 Lymphangioma circumscriptum (LC) was first described by Fox and Fox in 1879 as groups of vesicle-like elements known as “lymphangiectodes”.3
Intra-oral sites of occurrence include buccal mucosa, tongue, cheeks, lips, palate, floor of mouth, retromolar pads, tonsils, and gingiva.4–7
LC in gingiva usually presents as a soft, slow growing, and painless gingival enlargement with a pebbly surface.8 A few cases of symmetrical bilateral and unilateral presentation in gingiva have been reported.1,5,7,9 They often present cosmetic problems and are therefore surgically removed. Histopathological examination of lesions can help in establishing a diagnosis. Use of monoclonal antibody (D2-40) has been used as a reliable marker to detect lymphatic endothelial cells which helps in confirming the diagnosis.
This article reports a rare case of a 21-year old female patient who presented with multiple LC lesions in the maxillary arch. The aim of this paper is to create awareness among clinicians regarding the possibility of lymphangiomas presenting in the gingiva, thereby improving the diagnosis and proper management of these lesions. This case report has been prepared in accordance with the CARE (Comparison to self and others, Adaptability, Resourcefulness and Emotional well-being) criteria.
Case Report
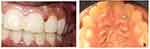
A 21-year-old female patient was referred to the department of Periodontics, Sree Mookambika Institute of Dental Sciences [SMIDS], Kulashekaram, Tamil Nadu, India with a complaint of two gingival enlargements in the maxilla. The first enlargement was in the buccal interdental gingiva between the left maxillary central incisor (#21) and lateral incisor (#22), henceforth called Site A, and the second enlargement was present in the palatal aspect of the maxillary left lateral incisor (#22) and canine (#23), henceforth called Site B, of 2 months duration. Her past dental history revealed frequent oral ulceration, burning sensation, and erythematous gingiva. Hematological examination revealed that lymphocyte count was low. Her medical history revealed a history of asthma for which she was under medication.
On intraoral examination, the lesions were mulberry shaped with a reddish color. The surface of the lesions was found to be pebbly in nature, soft in consistency, with bleeding on probing, pseudopockets were present without any surface ulcerations and was non-tender. The swelling was approximately 5mm X 5 mm in diameter (Figure 1A and B). Intra-oral periapical radiographs of the area did not show any significant bone changes (Figure 2).
 |
Figure 2 Intraoral Periapical radiograph showing minimal bone loss in interdental aspect of teeth 21 and 22 [Site A]. |
The patient was informed about the treatment options and necessity for biopsy to be done. Written informed consent was obtained before starting treatment. A provisional diagnosis of inflammatory gingival enlargement was made. Differential diagnosis included pyogenic granuloma, plasma cell gingivitis, hemangioma, and fibroma.
Case Management
Initial treatment included full mouth scaling and root planing. As the enlargements persisted even after 2 weeks of Phase 1 therapy, excisional biopsy was planned in both sites. Surgical excision was done under local anesthesia and the specimens were sent to the department of oral pathology (SMIDS) for histopathologic evaluation.
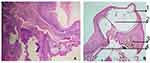
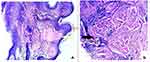
Histopathological examination showed parakeratinized stratified squamous epithelium and underlying connective tissue. The juxtaepithelial connective tissue showed homogeneous eosinophilic areas resembling lymph lined by endothelium with interspersed lymphocytes, occasional plasma cells, tissue macrophages, and red blood cells (RBC). The connective tissue was densely collagenous with interlacing bundles of collagen fibers, fibroblasts, and blood vessels. Areas of focal collection of RBCs and melanin incontinence are also seen at site B (Figures 3 and 4). Both sides had a similar appearance in histologic examination. Immunohistochemistry using D2-40 (Lymphatic marker) was done in the formalin fixed paraffin-embedded (FFPE) tissues which showed a positive staining reaction (Figure 5). The clinical, histopathological, and immunohistochemistry features were suggestive of lymphangioma circumscriptum.
 |
Figure 5 (A) X10 magnification (B) X40 magnification: Immunohistochemical stain (D2-40) highlights the lymphoid spaces and channels. |
Clinical Outcomes
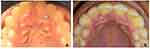
The surgical sites healed uneventfully in 4 weeks. The patient has been followed up for a period of 2 years with no recurrence of the lesion noticed. Periodontal prophylaxis was done at 6 month intervals during this period (Figures 6 and 7).
 |
Figure 6 (A) Pre-operative photograph of Lymphangioma circumscriptum before treatment. [Site A]. (B) 12 months Post-operative photograph after surgical excision, showing complete healing [Site A]. |
Discussion and Literature Review
It was in the year 1828 that Redenbacker gave the first detailed description of a lymphangioma.10 They are rare, benign malformations of the lymphatic system which can either be congenital or acquired in origin. Congenital lymphangiomas are seen associated with genetic disorders like Turner, Edwards, Down, Noonan, and Patau syndromes.11 The acquired origin of lymphangioma or lymphangiectasias may be due to trauma, inflammation, infection, or surgery causing obstruction of lymphatic vessels. Controversy exists as to whether lymphangiomas can been termed as true neoplasms or hamartomas which are usually malformations due to sequestration of lymphatic tissues that fail to establish proper communication with the lymphatic system, which may lead to proliferation.12 In 1976, Whimster13 described LC as a collection of large lymphatic cisterns, lying deep in the subcutaneous plane and which communicate through dilated dermal lymphatics with the superficial vesicles. It is thought that pressure transmitted from the pulsations of cisterns beneath produces saccular dilatations of superficial lymphatics in the form of vesicles.
In 1977, Flanagan and Helwig14 classified cutaneous lymphangioma based on the depth of lesion in skin and size of abnormal lymphatic vessels. They have been grouped into superficial lesions, which include LC, and deeper lesions, which include cavernous lymphangioma and cystic hygroma.
Lymphatic malformations have also been classified as microcystic form (Lymphangioma simplex and LC), macrocystic form (cavernous lymphangioma and cystic hygromas) and mixed (combining these two types).15 In 2001, Weiss and Goldblum12 classified lymphangiomas into three types: simplex (capillary), cavernous, and cystic.
Lymphangiomas are predominantly seen in the head and neck region (in more than 75% of cases). The majority of the cases (about 90%) have been seen in children below 2 years of age.2 Oral lymphatic malformations are most frequently reported on the anterior two thirds of the tongue, other sites include the lips, cheek, palate, and alveolar ridge.16 Gingival presentations have been rarely reported in the literature. The clinical appearance of LC of gingiva has been variously described as papillary lesions with a pebbly surface, which is due to the presence of several translucent vesicles with a reddish hue, thereby producing a frog eggs or tapioca pudding like appearance.17
Josephson and van Wyk, McDaniel and Adcock, and Motahhary et al have reported symmetrical bilateral lymphangiomas in gingiva.1,5,7 Kalpidis et al6,9 in 2006 and Maboudi and Seyedmajidi in 2014 have reported a unilateral lesion in gingiva. In our case we also found a somewhat similar appearance, the lesions presented in the interdental gingiva and had a mulberry like appearance. The two lesions were seen in the interdental gingiva between teeth in the maxillary arch. Cases of lymphangiomas presenting in the gingiva reported in the literature have been compiled in a table (Table 1). In a case series published in 2022, Ahmadian et al23 reported 20 of these rare solitary gingival lymphangiomas. Their cases showed a 2:1 female-to-male ratio with a predilection for the first two decades of life. The cases showed almost equal distribution between the maxillary and mandibular gingiva with a notable predisposition for the anterior gingival tissue which presented as pebbly, hyperplastic, and vesicular lesions.
 |
Table 1 Summary of the Cases That Have Reported Lymphangioma/Lymphatic Malformations of the Gingiva in Literature |
Microscopically, LC is characterized by dilated lymph channels forming solitary or multiple cystic spaces. The vessels will often diffusely infiltrate the adjacent soft tissues. The presence of benign lymphoid aggregates in the vessel walls is said to be another distinctive feature of lymphangioma.24 The channels are lined by endothelial cells. The lumen is seen filled with lymphatic fluid, but may also contain red blood cells, neutrophils, macrophages, and lymphocytes. Dilated lymphatic vessels with thickened muscular walls may be seen in deeper layers of the dermis. Similar histopathologic features have been seen in our case too.
Using immunohistochemistry techniques, specific antibodies for lymphatic endothelium including D2-40, Anti-Homeobox prospero-like protein 1 (Prox1), podoplanin, lymphatic vessel endothelial HA receptor-1 (LYVE-1), and vascular endothelial growth factor receptor 3 (VEGR3) have been used. It has been shown that D2-40 monoclonal antibody and podoplanin can be used as lymphatic endothelial cell markers to differentiate between lymphatic vessels and blood vessels.25,26 The monoclonal antibody D2-40 is said to be a highly specific and sensitive marker for lymphatic endothelium. It is an antibody to a Mr 40 000 O-linked sialoglycoprotein which is said to react with a fixation-resistant epitope in lymphatic endothelium.27 In the present case, immunohistochemistry using D2-40 (Lymphatic marker) was done which showed a positive staining reaction.
Histopathological and immunohistochemistry findings confirm the diagnosis of lymphangioma. The absence of any injury, infections, or surgery ruled out the possibility of an acquired lymphangioma. The absence of lesions at birth, limited growth, and multiple sites could suggest a developmental origin with a hamartomatous nature. The diagnosis of LC in this case is similar to those reported in the literature.1,5,7 Most of the time LC lesions are asymptomatic and present just a cosmetic problem. However, rupture with recurrent bacterial infection and formation of thick plaques or undergoing verrucous changes have been infrequently reported. Tempero et al28 reported lymphocytopenia in children presenting with lymphatic malformations.
A careful consideration of technique chosen for treatment should take into consideration the type, size of lesion, involvement of adjacent anatomical structures, and infiltration into surrounding tissues. Microcystic lesions are diffuse, are present in multiple tissue planes, and are difficult to remove. Macrocystic lesions are more localized and are easier to excise.29
The treatment of choice for LC is surgical excision of lesions either by gingivectomy or flap surgery.1,5,7 Various sclerosing agents, including 25% dextrose, hypertonic saline, ethanol, sodium morrhuate, and doxycycline have been tried in the management of lymphangiomas. Caliskan et al30 have recently used topical sirolimus (at 0.75 mg/mL concentration) twice daily on MLM lesions on the left trunk of an 8-year-old child for a period of 3 months with no recurrence noted at 8 months of follow-up. However, the occurrence of perilesional fibrosis, after effects such as fever and swelling at the site of application, have limited the use of this treatment modality. Other treatment modalities done include radiation therapy, laser therapy, cryotherapy, electrocautery, steroid administration, laser surgery, embolization, ligation, and radiofrequency tissue ablation techniques.31 The recurrence of LC lesions after surgical excision have not been reported in follow-up periods ranging from 13– years in the literature.1,5,7,9
Differential diagnosis for LC includes hemangioma, amyloidosis, congenital hypothyroidism, neurofibromatosis, granular cell tumor, pyogenic granuloma, malignant melanoma, and herpes infection. The definitive diagnosis should be made by biopsy and histopathological examination.4,32 The rarity of this case presentation of MLM in the gingiva is that it occurred at a later age (90% lesions are said to develop by 2 years of age), presented as multiple lesions in the maxilla (the majority of the cases have been reported in the mandible and as solitary lesions) and the gingival location of the lesion is a very rare site.
Conclusion
Microcystic lymphatic malformations in the gingiva are rare. We have reported this case with multiple LC to highlight the fact that this lesion should also be considered in the differential diagnosis of gingival enlargements. An early identification and treatment can prevent further complications from occurring. Histopathological and immunohistochemistry evaluation will provide an accurate diagnosis in the lesions.
Abbreviations
MLM, Microcystic lymphatic malformations; IHC, Immunohistochemistry; LC, Lymphangioma circumscriptum; RBC, Red blood cell; FFPE, Formalin fixed paraffin embedded; Prox1, Anti- Homeobox prospero-like protein 1; LYVE-1, Lymphatic vessel endothelial HA receptor-1; VEGR3, vascular endothelial growth factor receptor 3; H & E staining, Haematoxylin & Eosin staining.
Consent for Publication
The authors certify that they have obtained written informed consent from the patient while treating her. In the form, the patient was told about the disease condition and treatment options available were explained to her. Consent was obtained for reporting the clinical details and images in the journal. She understood that her name will not be published and due efforts will be made to conceal her identity. Approval has been obtained from the institution [Sree Mookambika Institute of Dental Sciences (SMIDS)] for publishing the case details.
Funding
This study was conducted without external funding.
Disclosure
The authors declare that they have no competing interests.
References
1. McDaniel RK, Adcock JE. Bilateral symmetrical lymphangiomas of the gingiva. Oral Surg Oral Med Oral Pathol. 1987;63(2):224–227. doi:10.1016/0030-4220(87)90317-3
2. Neville BW, Damm DD, Allen CM, Chi A. Soft Tissue Tumors. In: Oral and Maxillofacial Pathology First South Asia Edition. Philadelphia: W.B. Saunders; 2015:510–512.
3. Fox T, Fox TC. On the case of lymphangiectodes with an account of the histology of the growth. Trans Path Soc. 1879;30:470–476.
4. Brennan TD, Miller AS, Chen SY. Lymphangiomas of the oral cavity: a clinicopathologic, immunohistochemical, and electron-microscopic study. J Oral Maxillofac Surg. 1997;55(9):932–935. doi:10.1016/s0278-2391(97)90062-8
5. Motahhary P, Sarrafpour B, Abdirad A. Bilateral symmetrical lymphangiomas of the gingiva: case report. Diagn Pathol. 2006;1(9). doi:10.1186/1746-1596-1-9
6. Kalpidis CD, Lysitsa SN, Kolokotronis AE, Samson J, Lombardi T. Solitary superficial microcystic lymphatic malformation (lymphangioma circumscriptum) of the gingiva. J Periodontol. 2006;77(10):1797–1801. doi:10.1902/jop.2006.060067
7. Josephson P, van Wyk CW. Bilateral symmetrical lymphangiomas of the gingiva. A Case Report. J Periodontol. 1984;55(1):47–48. doi:10.1902/jop.1984.55.1.47
8. Sunil S, Gopakumar D, Sreenivasan BS. Oral lymphangioma - Case reports and review of literature. Contemp Clin Dent. 2012;3(1):116–118. doi:10.4103/0976-237X.94561
9. Maboudi A, Seyedmajidi M. Unilateral gingival lymphangioma: a first case report. J Indian Soc Periodontol. 2014;18(6):794–796. doi:10.4103/0972-124X.147442
10. Redenbacker EAH. De ranula sublingua, speciali, cum casa congenita. Munich, Germany: Monachii Lindhauer; 1828.
11. Shulman LP, Emerson DS, Felker RE, Phillips OP, Simpson JL, Elias S. “High frequency of cytogenetic abnormalities in fetuses with cystic hygroma diagnosed in the first trimester”. Obstet Gynecol. 1992;80(1):80–82.
12. Weiss SW, Goldblum IR. Tumors of Lymph Vessels. In: Weiss SW, Goldblum IR, editors. Enzinger and Weiss’s Soft Tissue Tumors.
13. Whimster IW. The pathology of lymphangioma. Br J Dermatol. 1976;94(5):473–486. doi:10.1111/j.1365-2133.1976.tb05134.x
14. Flanagan BP, Helwig EB. Cutaneous lymphangioma. Arch Dermatol. 1977;113(1):24–30. PMID: 831620. doi:10.1001/archderm.1977.01640010026002
15. Aciole GT, Aciole JM, Soares LG, Santos NR, Santos JN, Pinheiro AL. Surgical treatment of oral lymphangiomas with CO2 laser: report of two uncommon cases. Braz Dent J. 2010;21(4):365–369. PMID: 20976390. doi:10.1590/s0103-64402010000400014
16. Stănescu L, Georgescu EF, Simionescu C, Georgescu I. Lymphangioma of the oral cavity. Rom J Morphol Embryol. 2006;47(4):373–377. PMID: 17392986.
17. Rathan JJ, Vardhan BH, Muthu MS, Saraswathy K, Sivakumar N, Sivakumar N. Oral lymphangioma: a case report. J Indian Soc Pedo Prev Dent. 2005;23:185–189. doi:10.4103/0970-4388.19007
18. Grenier C, Bouchard C, Avon SL. Solitary gingival lesion in an adolescent. J Can Dent Assoc. 2013;79:d88.
19. González Morales CD, Herrera Herrera A, Diaz Caballero A. Exeresis of gingival lymphangioma with an electric scalpel. Rev Cubana Estomatol. 2015;52:1.
20. Chakravarthy, YSHSC, Reddy GP, Pallavi T, Chandra PA. Lymphangioma affecting gingiva Unilaterally: a rare case report. J Res Adv Dent. 2017;6:93–95.
21. Aggarwal Shivani AS, Anchal V, Aggarwal Ashim AA, Dimple A. Lymphangioma of gingiva: a rare case report: a rare case of lymphangioma of the gingiva. Niger J Med. 2021;62(1):46–48.
22. Xiao H, Pradhan B, Pradhan S, et al. A rare case of extensive gingival lymphatic malformation. Oral Oncol. 2022;124:105459. doi:10.1016/j.oraloncology.2021.105459
23. Ahmadian M, Wolk R, Freedman P, Russell A. Solitary gingival lymphangioma: case series and review of literature. Oral Surg Oral Med Oral Pathol Oral Radiol. 2022;133(5):e137. doi:10.1016/j.oooo.2021.12.047
24. McAlvany JP, Jorizzo JL, Zanolli D, et al. Magnetic resonance imaging in the evaluation of lymphangioma circumscriptum. Arch Dermatol. 1993;129(2):194–197. doi:10.1001/archderm.1993.01680230078009
25. Xuan M, Fang YR, Wato M, Hata S, Tanaka A. Immunohistochemical co-localization of lymphatics and blood vessels in oral squamous cell carcinomas. J Oral Pathol Med. 2005;34(6):334–339. PMID: 15946180. doi:10.1111/j.1600-0714.2005.00316.x
26. Ordóñez NG. D2-40 and podoplanin are highly specific and sensitive immunohistochemical markers of epithelioid malignant mesothelioma. Hum Pathol. 2005;36(4):372–380. PMID: 15891998. doi:10.1016/j.humpath.2005.01.019
27. Fukunaga M. Expression of D2-40 in lymphatic endothelium of normal tissues and in vascular tumours. Histopathology. 2005;46(4):396–402. PMID: 15810951. doi:10.1111/j.1365-2559.2005.02098.x
28. Tempero RM, Hannibal M, Finn LS, et al. Lymphocytopenia in children with lymphatic malformation. Arch Otolaryngol Head Neck Surg. 2006;132(1):93–97. doi:10.1001/archotol.132.1.93
29. Dogan N, Durmaz CE, Sencimen M, et al. The treatment of recurrent lymphangioma in the oral buccal mucosa by cryosurgery: a case report. OHDMBSC. 2010;9(1):7–10.
30. Caliskan E, Altunel CT, Ozkan CK, Tunca M. A case of microcystic lymphatic malformation successfully treated with topical sirolimus. Dermatol Ther. 2018;31(5):e12673. doi:10.1111/dth.12673
31. Kheur SM, Routray S, Ingale Y, Desai R. Lymphangioma of tongue: a rare entity. Indian J Dent Adv. 2011;3(3):635–638. doi:10.5866/3.3.635
32. Martin SG, Michael G, Jonathan AS. Burket’s Oral Medicine. In: Hamilton: BC Decker Inc.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.