Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Lupus Erythematosus Profundus with Multiple Overlying Cutaneous Ulcerations: A Rare Case
Authors Sutedja E , Widjaya MRH , Dharmadji HP, Suwarsa O , Pangastuti M , Usman HA , Firdaus CP
Received 3 August 2023
Accepted for publication 20 September 2023
Published 28 September 2023 Volume 2023:16 Pages 2721—2726
DOI https://doi.org/10.2147/CCID.S430068
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Endang Sutedja,1 Muhamad Radyn Haryadi Widjaya,1 Hartati Purbo Dharmadji,1 Oki Suwarsa,1 Miranti Pangastuti,1 Hermin Aminah Usman,2 Chaerani Pratiwi Firdaus1
1Department of Dermatology and Venereology, Faculty of Medicine, Universitas Padjadjaran - Dr. Hasan Sadikin Hospital, Bandung, West Java, Indonesia; 2Department of Anatomical Pathology, Faculty of Medicine, Universitas Padjadjaran - Dr. Hasan Sadikin Hospital, Bandung, West Java, Indonesia
Correspondence: Endang Sutedja, Department of Dermatology and Venereology, Faculty of Medicine, Universitas Padjadjaran - Dr. Hasan Sadikin Hospital, Jl. Pasteur 38, Bandung, West Java, 40161, Indonesia, Tel +62222032426 ext. 3449, Fax +62222032426, Email [email protected]
Abstract: Lupus erythematosus profundus (LEP) is a rare subset of chronic cutaneous lupus erythematosus (CCLE), with a reported incidence of 1– 3% in all LE cases. The most common cutaneous clinical presentation includes indurated plaques or subcutaneous nodules with an overlying normal skin. The clinical findings range from skin redness to features of CCLE, such as scaling, follicular plugging, and atrophy. Ulceration is rare and occurs in 28% of all LEP cases. We present a case report of LEP with multiple cutaneous ulcers on the right cheek and scalp accompanied by cicatricial alopecia. No other systemic manifestations were noted. Histopathological examination revealed periadipocyte, perivascular, and perivascular infiltration of lymphocytes, eosinophils, and plasma cells, supporting the diagnosis of LEP. The topical treatments given to the patient were sunscreen, 2% mupirocin cream, and wound dressing with dialkyl carbamoyl chloride (DACC). The patient was also treated systemically with oral corticosteroids and hydroxychloroquine. Clinical improvements were observed in the 3rd month of follow-up, and ulcer healing resulted in atrophic scars and fading erythematous macules. LEP is seldom associated with systemic or discoid lupus erythematosus. This occurs twice as frequently as a distinct entity does. Diagnosis accuracy plays an important role in determining the appropriate wound care, topical, and systemic treatments for LEP patients with multiple overlying cutaneous ulcerations.
Keywords: cutaneous ulcerations, lupus erythematosus profundus, multiple, rare case
Introduction
Lupus erythematosus profundus (LEP) is a rare subset of chronic cutaneous lupus erythematosus (CCLE), predominantly involving the subcutaneous tissue, with a reported incidence of 1–3% of all LE cases.1 This dermatosis is seldomly associated with systemic lupus erythematosus (SLE) ranges form 2–5%.2 It occurs twice as frequently as a distinct entity.1 Most patients with LEP are women aged 20–60 years,2 with a male-to-female ratio of 3:8. The most common cutaneous clinical presentation is indurated plaques or subcutaneous nodules with an overlying normal skin. The findings range from skin redness to features of CCLE such as scaling, follicular plugging, dyspigmentation, telangiectasia, or atrophy, and ulcerations. Skin ulceration is a rare clinical manifestation that affects 28% of all LEP patients.1
Skin ulcer is defined histologically as a break in the epithelial integrity of the skin which may extend to deeper structures.3 Chronic ulcer has a significant impact on the health and quality of life of patients, causing exudate, pain, depression, anxiety, sleep disturbance, and activities restriction.4 This case report presents multiple overlying cutaneous ulcerations as a rare case of LEP.
Clinical Case
A 40-year-old housewife complained of multiple non-healing indurated ulcers on the scalp, forehead, and right cheek, accompanied by hair loss and pus for two months. The patient initially had asymptomatic erythematous patches on the forehead, right cheek, and scalp for three years prior to consultation. One year prior to the consultation, hair loss started, and the erythematous patches became ulcerated, specifically in the last four months. Fatigue, fever, and weight loss were not reported.
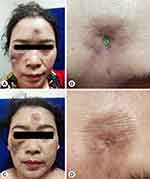
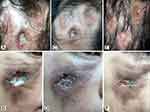
On physical examination, there were multiple, irregular, well-defined, and deep ulcerations with erythematous indurated edges on the scalp, forehead, and right cheek. The diameter of ulcers ranged from 0.5 to 3 cm. Slough and crust were seen covering some lesions (Figures 1A, B and 2A, B). Diffuse and atrophic cicatricial alopecia on the vertex.
Laboratory tests revealed leukopenia (3570/µL), increased erythrocyte sedimentation rate (43 mm/h), and increased C-reactive protein/CRP (0.46 mg/dL). Other specific tests, such as, antinuclear antibody (ANA), anti-double-stranded deoxyribonucleic acid (dsDNA), venereal disease research laboratory (VDRL), and human immunodeficiency virus (HIV) antibodies, were all within normal limits. Skin biopsy was performed at the erythematous indurated edge of the ulcers on the right cheek with the patient’s consent. Differential diagnosis was subcutaneous panniculitis-like T cell lymphoma (SPTCL). The patient was initially treated with topical sunscreen, 2% mupirocin cream, and a modern dressing containing dialkyl carbamoyl chloride (DACC) coated with sterile gauze. The patient also received 24 mg per day methylprednisolone orally (0.5 mg/kg bodyweight of prednisone).
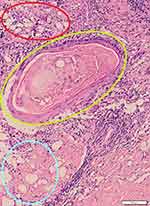
Two weeks later, the amounts of slough and crust decreased (Figure 2C and D). Histopathological changes in hematoxylin and eosin (H&E) staining were observed as perivascular, periadnexal, and periadiposal lymphocytic, eosinophilic, and plasmacytic infiltration in the dermis (Figure 3). Thickening of the basement membrane was evident in the periodic acid-Schiff (PAS) stain (Figure 4). The patient was diagnosed as LEP based on clinicopathological findings.
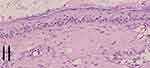 |
Figure 4 PAS-staining revealed thickening of the basement membrane pointed with black arrows. |
After one month period of follow-up, 200 mg of oral hydroxychloroquine twice daily was added for systemic treatment based on ophthalmologic examination. The oral methylprednisolone administration tapered off with 20% reduction every two weeks. The patient responded well to therapy, and during three months of follow-up period, some hair had regrown over the vertex and the skin ulcers had healed, leaving atrophic scars (Figures 1C, D and 2E, F).
Discussion
Lupus erythematosus profundus usually occurs in women aged 20–60 years old,2 with a male-to-female ratio of 3:8. The most common cutaneous clinical presentations are indurated plaques or subcutaneous nodules, ranging from skin redness to features of CCLE, such as scaling, dyspigmentation, follicular plugging, telangiectasia, and atrophy.1 The lesions are sometimes ulcerated. Scarring and lipoatrophy often follow after healing. Scalps, face, shoulders, upper outer arms, trunk, and buttocks are the most commonly involved sites, while the legs involvement is rare.2 Scalp involvement was reported in up to 16.4% of cases, typically resulting in cicatricial alopecia.5 Plaques and nodules in LEP are prone to ulceration due to physical trauma,6 while in some patients ulceration might also be due to vascular changes.7
The gold standard for LEP diagnosis is histopathological findings from a deep skin biopsy of the lesion.3 Epidermal and dermal changes in discoid lupus erythematosus (DLE), including atrophy of the epidermis, thickened basement membrane, vacuolar change at the dermoepidermal junction, superficial and deep perivascular inflammatory infiltrate of lymphocytes involving the dermis, and interstitial mucin between collagen bundles of the dermis were found in more than half of LEP cases.8 The histopathological features of LEP show predominantly lymphocytic lobular or mixed panniculitis with frequent plasma cells and sometimes eosinophils. Lymphoid follicles are present, in 45–78% of cases, sometimes with germinal centers and perilobular distribution in 20% of the cases.7
A subtype of non-Hodgkin lymphoma, namely SPTCL is the most troublesome differential diagnosis of LEP. It presented as solitary or multiple, or deeply seated plaques or nodules.9 Ulcerations, which rarely occur in 6% cases10 mainly involving legs, arms, trunk,9,11 and face,10 might regress as lipoatrophy. The common systemic symptoms are fatigue, fever, and weight loss.5 Histopathologically, rimming of fat lobules by lymphocytes and vascular invasion by atypical lymphocytes have been described in both LEP and SPTCL.9 The plasma cells which are not found in SPCTL could distinguish it from LEP.1 PAS-staining revealed mild and focal thickening of the subepidermal basement membrane in LEP.8 However, that staining did not demonstrate any thickening of the basement membrane in SPTCL.12
To date, no randomized controlled trials have been conducted on LEP. Topical treatment consists of glucocorticosteroids and lubricating ointment. Glucocorticosteroid injections into lesions are generally ineffective and can exacerbate atrophy.3 Antimalarial medications such as hydroxychloroquine (200–400 mg daily dose) or chloroquine (250–500 mg daily dose) have long been used for the systemic, first-line treatment of LEP, and other forms of CLE. The usage of systemic corticosteroids is beneficial for severe cases concurrent with SLE.3,10 Patel et al13 reported a case of a 28-year-old female presented with large non-healing ulcers on the face, trunk, and limbs covered with black hemorrhagic crust. No other systemic manifestation was noted. The establishment of LEP diagnosis was supported by clinical and histopathological findings. After treatment with systemic corticosteroids, hydroxychloroquine, and topical 2% mupirocin, the lesions healed, leaving behind scars.10
The main causes of SLE increased mortality risk are infections, renal disease and cardiovascular diseases.14 LEP is seldomly associated with SLE.2 In this case report, all laboratory tests including ANA were all within normal limits, without any evidence of systemic involvement. Therefore, we determined that the quo ad vitam prognosis of the patient was relatively good. Scarring and lipoatrophy often follow after the healing of LEP lesions.2 The atrophic scars left after the healing of the ulcers in this patient related with worse quo ad functionam prognosis. Regarding the chronic recurrent nature of this dermatosis,7 the quo ad sanationam prognosis of this patient was relatively poor.
Conclusion
Lupus erythematosus profundus occurs twice, as frequently as a distinct entity. It is seldom associated with SLE or DLE. Accurate diagnosis is important to determine appropriate wound care, as well as topical and systemic treatment for patients with multiple ulcers.
Ethical Statement
Publication of images was included in the patient’s consent for case publication. Institutional approval from The Research Ethic Committee of Dr. Hasan Sadikin General Hospital Bandung, Indonesia has been obtained to publish the case details (approval number: LB.02.01/X.6.5/257/2023).
Consent Statement
The authors certify that they have obtained all appropriate patient consent forms. The patient signed a consent form for the publication of the case and images.
Acknowledgments
The authors would like to thank all staff of the Department of Dermatology and Venereology, Faculty of Medicine, Universitas Padjadjaran, Bandung, West Java, Indonesia.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Thapa DP, Kubba R, Kubba A. Lupus erythematosus profundus: a case series and review of literature. Lupus Open Acces. 2018;3(1):1–5. doi:10.35248/2684-1630.18.3.133
2. Zhang R, Dang X, Shuai L, He Q, He X, Yi Z. Lupus erythematosus panniculitis in a 10-year-old female child with severe systemic lupus erythematosus. Medicine. 2018;97(3):1–8. doi:10.1097/MD.0000000000009571
3. Suliman YA, Kafaja S, Fitzgerald J, et al. Ultrasound characterization of cutaneous ulcers in systemic sclerosis. Clin Rheumatol. 2018;37(6):1555–1561. doi:10.1007/s10067-018-3986-5
4. Klein TM, Andrees V, Kristen N, Portz K, Augustin M, Blome C. Social participation of people with chronic wounds: a systematic review. Int Wound J. 2021;18(3):287–311. doi:10.1111/iwj.13533
5. Udompanich S, Chanprapaph K, Suchonwanit P. Hair and scalp changes in cutaneous and systemic lupus erythematosus. Am J Clin Dermatol. 2018;19(5):679–694. doi:10.1007/s40257-018-0363-8
6. Cruz CG, Espanol GA, Fabrega BF, Calderon VC, Pichel MG, Briones VG. Lupus panniculitis: clinicopathological features of a series of 12 patients. Med Clin. 2018;151(11):444–449. doi:10.1016/j.medcli.2018.06.024
7. Castrillon MA, Murrell DF. Lupus profundus limited to a site of trauma: case report and review of the literature. Int J Women Dermatol. 2017;3(2017):117–120. doi:10.1016/j.ijwd.2017.03.002
8. Narges A, Rana R, Alireza M, Sara NR. Lupus erythematosus panniculitis. Our Dermatol Online. 2017;8(4):427–430. doi:10.7241/ourd.20174.121
9. Morita TCAB, Tres GFS, Garcia MSJ, Halpern I, Criado PR, Carvalho JF. Panniculitides of particular interest to rheumatologist. Adv Rheumatol. 2019;59(63):1–12. doi:10.1186/s42358-019-0077-5
10. Bednarek A, Bartoszak L, Samborski W. Case report on a patient with lupus panniculitis. Postepy Dermatol Alergol. 2015;32(1):59–62. doi:10.5114/pdia.2014.40958
11. Wang BH, Xu XL, Sun JF. Subcutaneous panniculitis-like T-cell lymphoma: a case report. Int J Dermatol Venereol. 2019;2(2):109–111. doi:10.1097/01.JD9.0000559521.46675.2c
12. Kao GF, Resh B, McMahon C, Gojo I, Sun CC, Philips D. Fatal subcutaneous panniculitis-like T-cell lymphoma γ/δ subtype (cutaneous γ/δ T-cell lymphoma): report of a case and review of the literature. Am J Dermatopathol. 2008;30(6):593–599. doi:10.1097/DAD.0b013e318182c7bf
13. Patel RM, Marfatia YS. Lupus panniculitis as an initial manifestation of systemic lupus erythematosus. Indian J Dermatol. 2010;55(1):99–101. doi:10.4103/0019-5154.60364
14. Essouma M, Nkeck JR, Endomba FT, Bigna JJ, Ngandeu MS, Hachulla E. Systemic lupus erythematosus in Native sub-Saharan Africans: a systematic review and meta-analysis. J Autoimm. 2020;106:1–8. doi:10.1016/j.jaut.2019.102348
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.