Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 14
Low FEV1 Is Associated With Increased Risk Of Cachexia In COPD Patients
Authors Mokari-Yamchi A , Jabbari M , Sharifi A, Barati M, Kheirouri S
Received 9 July 2019
Accepted for publication 23 September 2019
Published 31 October 2019 Volume 2019:14 Pages 2433—2440
DOI https://doi.org/10.2147/COPD.S221466
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Amin Mokari-Yamchi,1 Masoumeh Jabbari,1 Akbar Sharifi,2 Meisam Barati,3 Sorayya Kheirouri4
1Student Research Committee, Department of Community Nutrition, Faculty of Nutrition and Food Technology, Shahid Beheshti University of Medical Sciences, Tehran, Iran; 2Tuberculosis and Lung Research Center, Tabriz University of Medical Sciences, Tabriz, Iran; 3Department of Cellular and Molecular Nutrition, Faculty of Nutrition Sciences and Food Technology, Shahid Beheshti University of Medical Sciences, Tehran, Iran; 4Department of Nutrition and Food Science, Nutrition Faculty, Tabriz University of Medical Sciences, Tabriz, Iran
Correspondence: Sorayya Kheirouri
Department of Nutrition and Food Science, Nutrition Faculty, Tabriz University of Medical Sciences, Tabriz, Iran
Email [email protected]
Background: Chronic obstructive pulmonary disease (COPD) has been introduced as a major public health problem. It has been suggested that disruption in function or some adipokines and serum proteins’ signaling could play crucial roles in lung diseases. This study’s purpose was to investigate the association between serum levels of S100A1, ZAG, and adiponectin with FEV1 in COPD patients.
Methods: In this cross-sectional study, 90 clinically stable outpatient males with age ranging from 40 to 70 years with COPD diagnosis – FEV1/FVC < 70% – were divided into two groups: mild–moderate COPD patients; FEV1 ≥ 50 (n=52) VS severe and very severe COPD patients; FEV1 < 50 (n=38). The serum levels of ZAG, S100A1, and adiponectin were measured by the use of enzyme-linked immunosorbent assay.
Results: In the present study, lower FEV1 was significantly associated with increased risk of cachexia (OR = 5.76, 95% CI: 2.28–14.54). The serum level of ZAG was significantly higher in the mild–moderate COPD patients in comparison with the severe–very severe COPD patients (p<0.035). However, the resting metabolic rate (RMR) level was significantly higher in FEV1<50 group compared to FEV1≥50 group (p<0.024). Also, strong positive associations between serum S100A1–ZAG, serum adiponectin–ZAG, and serum adiponectin–S100A1 (β>0.800, p<0.001) were shown.
Conclusion: In the present study, we found that low FEV1 was associated with increased risk of cachexia in COPD patients. Additionally, lower serum level of ZAG and higher RMR were observed in patients with severe–very severe COPD as compared to mild–moderate COPD. Therefore, it could be claimed that there is a mechanistic chain of causality between FEV1, serum ZAG, RMR, and cachexia.
Keywords: COPD, FEV1, S100A1, ZAG, adiponectin
Introduction
Chronic obstructive pulmonary disease (COPD) has been introduced as a major public health problem and predicted to take the fifth and third place in the burden of disease and mortality in 2020, respectively1. Despite the increasing consideration that the medical community has paid to this disease, especially in recent years, COPD is still relatively unfamiliar to public health authorities, governments, and societies and has been ignored by them.1 Although COPD could be prevented and treated, it has become a major public health challenge, because there are millions of people who suffer from COPD for years and ultimately die as a result of this pulmonary disorder or its complications. Therefore, COPD has an important contribution to global chronic morbidity and mortality.2
COPD’s typical signs include limitation in airflow and persistent respiratory symptoms that are caused by abnormalities in airways and/or alveoli. It has been identified that the major cause of COPD is remarkable exposure to particles or gases that are poisonous.3
Cachexia has been identified as a result of COPD progression among approximately a quarter of patients. This complication can result in a significant reduction in survival rate of the patients.4 Mechanism of cachexia in COPD has not been completely defined, but it appears that some related metabolic and inflammatory factors such as disruption of resting metabolic rate (RMR) and increase in some inflammatory cytokines' levels may play a crucial role in this regard.5
Exacerbation and disease severity lead to serious health effects, and decreases the quality of life.6 Also, Forced expiratory volume 1 (FEV1), Forced vital capacity (FVC), the spirometric indices, and their ratio are used to define the presence and severity of COPD.7 The rate of decline in FEV1 has a predictive value for morbidity, mortality, and rates of hospitalization in COPD. On the other hand, a rapid decline in FEV1 is associated with specific biomarkers in the plasma of COPD patients.7
S100 proteins are members of a cytosolic calcium-binding protein family, which is composed of 25 members.8 These proteins have wide-spreading intracellular and extracellular biological functions including regulation of proliferation, differentiation, migration, inflammation, apoptosis, etc.9 In addition, S100A1, as a member of the S100 family, is the most highly expressed in striated muscle10 and it is notable that this subtype of S100 protein family plays just intracellular regulatory roles,11 and one of the most important of them is regulating calcium-induced calcium release (CIRC) cascade in sarcoplasmic reticulum (SR).9 It has been suggested that dysregulation of this Ca-related signaling may be contributed to dysfunction of respiratory and locomotor muscles in COPD.9
Zinc-α2-glycoprotein (ZAG) is a glycosylated protein that is soluble, and is also secreted by several types of cells such as secretary epithelial cells in the prostate, breast, pancreas, liver, skeletal muscle, and bowel. It has been proposed that expression and secretion of ZAG occurs in catabolic/cachectic conditions.12 These findings may also be true for the catabolic disease, COPD, and its severity.
Adiponectin, a 30-kDa protein, is an adipokine and its most important identified role is associated with homeostasis of lipid and glucose metabolism. Dissimilar to white adipose tissue, adiponectin secretion has been identified in some other types of cells such as bone marrow, osteoblasts, myocytes, and cardiomyocytes. This suggests a complementary autocrine/paracrine role of this adipokine in various tissues.13 Some human studies suggested that increased levels of serum adiponectin in COPD is associated with the disease severity,14,15 however, the relationship between adipokine levels and COPD outcomes is still unidentified.
Based on above-mentioned documents, in the present study, we hypothesized that serum levels of S100A1, ZAG, and adiponectin may contribute to FEV1 as indicator of COPD disease severity. To the best of our knowledge, the role of S100A1, ZAG, and adiponectin in severity of disease in COPD patients has not been investigated, yet. Moreover, the association between FEV1 and risk of cachexia was unclear, so we conducted this study to examine these associations.
Methods
Study Design And Participants
In the current study, we reanalyzed data from our former work5 to allow us to compare the patient cohort across COPD stages. Ninety clinically stable COPD outpatient males with ages ranging from 40 to 70 years old, were enrolled in the study. COPD was defined with respect to the recommendations of the Global Initiative for Chronic Obstructive Lung Disease (GOLD) based on FEV1/FVC < 70%.3 Patients who met the following criteria were excluded from the study: smoking or using any opioid drugs and alcohol drinking, heart failure, metabolic syndrome, severe endocrine, autoimmune, liver or kidney disorders, diabetes, following a specific diet or using any dietary supplements at the study time or during previous 4 months. Based on GOLD executive guideline for the Diagnosis, Management, and Prevention of COPD, subjects were divided into 4 groups: mild COPD (FEV1 ≥ 80% predicted), moderate COPD (50% ≤ FEV1< 80% predicted), severe COPD (30% ≤ FEV1< 50%), and very severe COPD (FEV1 < 30% predicted). In this study, for better comparison, subjects were divided into 2 groups: mild and moderate COPD; mild–moderate COPD patients; FEV1 ≥ 50 (n=52) VS severe and very severe COPD patients; FEV1 < 50 (n=38).16 This study was conducted in accordance with the Declaration of Helsinki and was approved by the ethics committee (No: IR.TBZMED.REC.1395.779) of Tabriz University of Medical Sciences and all participants signed the written informed consent.
The Measurements
The pulmonary functions, including FVC and FEV1 (% predicted), were assessed by Spirolab III (Medical International Research, Rome, Italy) according to the guidelines of the American Thoracic Society.17 After 15 mins resting, a maximum forced inhalation and then a powerful forced expiration were done by the participant without using a nose clip.
The height and weight of the patients were measured using standard, calibrated scales to the nearest 0.5 cm and 0.1 kg, respectively. Cachexia was defined as unintentional weight loss > 7.5% of usual body weight in the last 6–12 months, and the patient’s previous weight was acquired from their medical records. The body mass index (BMI) was obtained by dividing the weight in kilogram by the square of the height in meter (kg/m2). By using an ordinary tape measure, with a nearest 0.5 cm, the waist and arm circumference were measured. The bioelectrical impedance analysis (BIA) device (BC-418; Tanita Corp., Tokyo, Japan) was used for analysis of body composition including fat-free mass (FFM), percent of body fat, and total body water (TBW). The ratio of FFM to height in meters squared (kg/m2) was used to calculate the FFM index (FFMI).
Indirect calorimetry (Fitmate MED; COSMED, Rome, Italy) was used to measure RMR. For this purpose, the oxygen consumption and production of carbon dioxide of each participant were measured and converted to energy expenditure using formulae. Fitmate is a desktop metabolic system which is used to measure resting metabolic rate during rest and exercise (kcal/day) via oxygen consumption measurement. First, participants sit in a relaxed supine position. Then a facemask is placed over their face. The facemask was associated with two parts: turbine flow meter which measures the ventilation and a galvanic fuel cell oxygen sensor which analyzes expiration gases in terms of proportion of oxygen. 10–12 hrs overnight fasting, 24 hr avoidance of exercise, and 12 hrs of abstaining from caffeine and smoking were necessary before doing the test.
The 24 hr recall questionnaire was used to assess dietary intake of participants. An expert interviewer interviewed the participants about their food and beverage consumption during the previous day. The Visual Analog Scale (VAS) questionnaire was used to assess hunger and eating habits. By using the VAS questionnaire, appetite was evaluated by a 10 cm analog scale which rated from poor appetite to good appetite.18
For the assessment of the average total physical activity, the Persian long-form version of the International Physical Activity Questionnaire (IPAQ) was used for each participant during the previous 7 days.19 The metabolic equivalent of task (MET) minutes/week was calculated for total physical activity scores. Then the participants were classified into three groups according to physical activity levels including walking, moderate and vigorous activities according to IPAQ guidelines.
For biochemical analysis, fasting blood samples were obtained from the participants, and the serum was separated by centrifugation at 4°C and stored at −70°C until biochemical analysis. For measurement of serum levels of S100A1 (cat no CK-E90946), ZAG (cat no CK-E91945), and adiponectin (cat no CK-E10871), Human ELISA kits (Eastbiopharm, Hangzhou, China) were used. The absorbance was read at 450 nm using an Automatic ELISA Plate Reader (BioTek Instruments, Winooski, VT, USA).
Statistical Analyses
All the statistical analyses were performed using SPSS 19.0 (IBM Corporation, Armonk, NY, USA). To assess variables’ distribution normality, the Kolmogorov–Smirnov test was used. The chi-squared test and the independent samples’ t-test (normal distribution) were used to compare categorical variables and quantitative variables between two groups respectively. Linear regression was used to assess the associations between variables. Statistically significant P-value was considered < 0.05.
Results
Demographic parameters of patients are presented in Table 1. The values of FVC and FEV1/FVC were significantly higher in mild–moderate COPD patients compared to severe–very severe COPD patients (p<0.001). However, these two groups had no significant differences in terms of other variables including age, time elapsed from diagnosis (years), calorie intake, satiety score, physical activity, and drug therapy.
 |
Table 1 Characteristics Of Patients Included In The Study |
As displayed in Table 2, BMI levels were found to be lower in severe–very severe COPD patients in comparison with mild–moderate COPD patients (p= 0.047). Accordingly, there were no statistically significant differences in other body composition and anthropometric variables.
 |
Table 2 Body Composition And Anthropometric Parameters |
The independent sample t-test analysis demonstrated that the serum levels of ZAG were significantly higher in mild–moderate COPD patients compared to severe–very severe COPD patients (p<0.035), also the same trend could be found for serum S100A1 and adiponectin, but the differences were not significant (P > 0.05). Whereas, the RMR level in severe–very severe COPD patients was significantly higher than in mild–moderate COPD patients (p<0.024) (Table 3).
 |
Table 3 Comparison Of serum S100A1, ZAG, Adiponectin, And RMR Between The Study Groups |
According to Table 4, the result of linear regression analysis indicated that in the group with FEV1<50, there was a strong positive association between serum S100A1–ZAG (β=0.836, p<0.001), serum adiponectin–ZAG (β=0.794, p<0.001), and serum adiponectin–S100A1 (β=0.815, p<0.001). On the other hand, there was a strong positive association between serum levels of S100A1–ZAG (β=0.891 p<0.001), serum adiponectin–ZAG (β=0.849, p<0.001), and serum adiponectin–S100A1 (β=0.889, p<0.001) in the group with FEV1≥50 (Table 4).
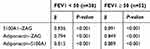 |
Table 4 Comparison Of Serum S100A1, ZAG, Adiponectin, And RMR Between The Study Groups |
 |
Table 5 Frequency Of Cachexia Between The Patients With FEV1 < 50 And FEV1 ≥ 50 |
 |
Table 6 Risk Of Cachexia In The Study Population |
With respect to chi-squared test, frequency of cachexia was significantly higher in the group with FEV1<50 (73.7%) compared to the group with FEV1≥50 (32.7%) (Table 5).
As shown in Table 6, lower FEV1 was significantly associated with increased risk of cachexia based on binary logistic regression test (OR = 5.76, 95% CI: 2.28–14.54).
Discussion
In the present study, the BMI level was significantly higher in mild–moderate COPD patients in comparison with the severe and very severe COPD patients. Also, there were no statistically significant differences in other body composition and anthropometric variables between these two groups.
In a study by Richard L. et al, the effective and significant impact of BMI (especially under 30 kg/m2) on all of the lung volumes and capacities was indicated.20 They reported a significant and positive relationship between lung total and vital capacity and BMI.20 Celli BR et al retrospectively examined 400 COPD patients, and reported the association of low BMI (<25 kg/m2) with a significant elevation of mortality risk.21 Sajal DE, in his study reported that, along with COPD severity progression, the mean BMI reduces significantly.22 Researchers suggested the routine evaluation of body composition such as body weight, BMI, fat mass, and fat free mass for assessing COPD patients for its beneficial properties.23 There is increasing evidence about this issue that BMI evaluation is a useful and important parameter for assessing the functional status in COPD patients.22,24 While obesity results in reduction in lung volumes, low body weight and low FFMI predict undesirable effects on COPD patients' health status, exercise capacity, mortality, and survival.23 Therefore, the result of this study indicated that patients with better lung function (FEV1≥50), have significantly higher BMIs in comparison with those patients with lower lung function (FEV1<50). As FEV1 could be an indicator of COPD severity,3 these results are in accordance with previous studies in this field.
Our findings indicated that COPD patients with lower FEV1 level had higher RMR, also, previous researchers have demonstrated similar results.25,26 Creutzberg et al investigated 172 patients with COPD and 92 healthy, age-matched subjects. They found that RMR was significantly higher in COPD patients compared to control group.25 Energy expenditure in COPD patients increases because of infection and respiratory impairment. Breathing, with healthy lungs, requires 36 to 72 kcal/day; however, this amount increases by 10 fold in patients with COPD.27
In the present study, frequency of cachexia was significantly higher in severe–very severe COPD patients (73.7%) compared to the mild–moderate COPD patients (32.7%). In addition, there was a significant association between lower FEV1 and increased risk of cachexia.
Based on GOLD, FEV1 is an indicator of COPD stage and disease severity.3 Molen, in his review article, claimed that decreasing FEV1 is associated with increased malignancy and lung cancer risk in COPD patients.28
Although there are increasing research and reviews on the mechanism of cachexia in COPD patients,4,29 there are some unclear aspects in this regard that still have not been elucidated. In the present study, there was a significant difference between these two groups in BMI and cachexia prevalence – the group with higher FEV1 (FEV1≥50) had the lower prevalence of cachexia. Also, in the present study, FEV1≥50 group had significantly higher BMIs than the other group (FEV1<50). Accordingly, the body fat mass index in our population was between normal ranges and there was no significant difference between these two groups in terms of this variable, so these results could support the protective effect of higher FFMI in preventing COPD exacerbation. Although, we should not neglect the importance of better functional lungs regarding cachexia frequency.
In the present study, serum level of ZAG was significantly higher in mild–moderate COPD patients in comparison with severe–very severe COPD patients. Also, there were strong positive associations between serum levels of S100A1, ZAG, and adiponectin in both groups.
Adiponectin is a type of adipokine that has anti-inflammatory effects.30 Results from several studies that compared COPD patients with healthy controls, indicated that serum/plasma adiponectin levels were elevated in patients compared to controls.14,15,31 In the present study, we observed that serum adiponectin level was higher in COPD patients with FEV1≥50 compared to FEV1<50, however, that was not significant. Consistent with our results, Yoon et al demonstrated that there were no significant differences in FEV1 level in comparison to serum adiponectin quartiles.32
Differences between results of different studies may be caused by various reasons including: comparing COPD patients with healthy controls, different disease stages, study on underweight or obese patients and presence of other complications such as cancer and cardiovascular disease which could create confounding and different results.
We reported in our previous paper that the serum level of ZAG was higher in cachectic patients with COPD in comparison with non-cachectic group.5 We suggested that this may be related to the correlation between ZAG and cachexia development.5 Also, in the current study, at the time that we categorized the COPD patients based on FEV1, the serum level of ZAG was significantly higher in the group with better clinical signs such as FEV1, FVC, and FEV1/FVC. The fame of ZAG is caused by its cachectic and lipolytic effects.12 Several studies reported low level of ZAG in obese subjects and also its relationship with higher levels of inflammatory cytokines such as IL-6, IL-12, and TNF-α.33–35 To the best of our knowledge, there is no published work in literature with the purpose of comparing serum ZAG levels in COPD stages and categories. With respect to the characteristics of our study population, we suggested that the higher levels of ZAG in the patients with FEV1≥50 could be associated with the inflammatory condition. However, the nature of this change is still unclear to us, and consequently requires more detailed studies.
Moreover, the role of S100A1 in pulmonary diseases and COPD is less documented. It has been suggested that various members of S100 family of proteins are overexpressed in pulmonary diseases,36 cachexia,5 and lung cancers.37 However, evidence of A1 member of this family in pulmonary diseases has not been identified yet. Accordingly, studies suggested that S100A1 is a modulator of muscle excitation-contraction coordination throughout the well-known mechanism.38 Furthermore, several research has indicated that S100A1 over-expression would result in delayed myocardial remodeling, improved contractile efficiency, and also increment of myocardial infarction survival.39
As S100A1 plays a well-known role in modulating endothelial cells' contraction/relaxation because of Ca+ - binding characteristics39 and NO production,40 it appears that elevation of this protein level in patients with FEV1≥50 could be due to those characteristics of S100A1 compared to patients with FEV1<50. This means that more attention should be paid when we know that patients with higher FEV1 have more functional pulmonary and alveolar endothelial cells than others.
Positive strong correlation between these serum factors (ZAG, S100A, and adiponectin) in the present study may signify a biologic message in this field. It appears that these three peptides act in a similar physiological path at least in COPD patients, and this similarity and correlation reinforce the singular effect of these peptides.
The present study also had some limitations. Firstly, we did not measure some inflammatory factors such as IL-6, IL-12, and TNF-α in the study participants. The second point may be small sample size. As sample size could affect statistical significance, we may have found more significant associations if we had a larger sample size. On the other hand, we measured biochemical variables only in serum samples. If future studies can measure local status of alveoli and bronchi in terms of inflammation in the study population, it could have more informative data for interpreting the results. In the present study, we accomplished measurements only on male participants, because of the small sample size in each group. If the analysis was performed on females or the two gender groups were compared, we may have proposed different results and interpretations. Moreover, having a cross-sectional design may be another limitation of the present study, which did not allow for the interpretation of the causal relationships between variables.
Conclusion
In the present study, we found that low FEV1 increased the risk of cachexia. Additionally, low FEV1 was associated with lower serum ZAG and higher RMR in COPD patients. We also found a strong positive association between ZAG, S100A, and adiponectin in both study groups. Therefore, it could be claimed that there is a mechanistic chain of causality between FEV1, serum ZAG, RMR, and cachexia. However, more in vitro and in vivo studies and clinical trials are required to further refine this idea.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–365. doi:10.1164/rccm.201204-0596PP
2. López‐Campos JL, Tan W, Soriano JB. Global burden of COPD. Respirology. 2016;21(1):14–23. doi:10.1111/resp.12660
3. Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am J Respir Crit Care Med. 2017;195(5):557–582. doi:10.1164/rccm.201701-0218PP
4. Wagner P. Possible mechanisms underlying the development of cachexia in COPD. Eur Respir J. 2008;31(3):492–501. doi:10.1183/09031936.00074807
5. Mokari-Yamchi A, Sharifi A, Kheirouri S. Increased serum levels of S100A1, ZAG, and adiponectin in cachectic patients with COPD. Int J Chron Obstruct Pulmon Dis. 2018;13:3157.
6. Seemungal TA, Donaldson GC, Paul EA, Bestall JC, Jeffries DJ, Wedzicha JA. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157(5):1418–1422.
7. Burge S, Wedzicha JA. COPD exacerbations: definitions and classifications. Eur Respir J Suppl. 2003;41(41 suppl):46s–53s.
8. Moore BW. A soluble protein characteristic of the nervous system. Biochem Biophys Res Commun. 1965;19(6):739–744.
9. Xia C, Braunstein Z, Toomey AC, Zhong J, Rao X. S100 proteins as an important regulator of macrophage inflammation. Front Immunol. 2018;8:1908.
10. Zimmer DB, Landar A. Analysis of S100A1 expression during skeletal muscle and neuronal cell differentiation. J Neurochem. 1995;64(6):2727–2736. doi:10.1046/j.1471-4159.1995.64062727.x
11. Lagasse E, Weissman I. Mouse MRP8 and MRP14, two intracellular calcium-binding proteins associated with the development of the myeloid lineage. Blood. 1992;79(8):1907–1915.
12. Cabassi A, Tedeschi S. Zinc-α2-glycoprotein as a marker of fat catabolism in humans. Curr Opin Clin Nutr Metab Care. 2013;16(3):267–271. doi:10.1097/MCO.0b013e32835f816c
13. Meier U, Gressner AM. Endocrine regulation of energy metabolism: review of pathobiochemical and clinical chemical aspects of leptin, ghrelin, adiponectin, and resistin. Clin Chem. 2004;50(9):1511–1525. doi:10.1373/clinchem.2004.032482
14. Uzum AK, Aydin MM, Tutuncu Y, Omer B, Kiyan E, Alagol F. Serum ghrelin and adiponectin levels are increased but serum leptin level is unchanged in low weight chronic obstructive pulmonary disease patients. Eur J Intern Med. 2014;25(4):364–369. doi:10.1016/j.ejim.2013.02.012
15. Kırdar S, Serter M, Ceylan E, Şener AG, Kavak T, Karadağ F. Adiponectin as a biomarker of systemic inflammatory response in smoker patients with stable and exacerbation phases of chronic obstructive pulmonary disease. Scand J Clin Lab Invest. 2009;69(2):219–224. doi:10.1080/00365510802474400
16. Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176(6):532–555. doi:10.1164/rccm.200703-456SO
17. Crapo RO, Hankinson JL, Irvin C, et al. Standardization of spirometry: 1994 update. Am J. Respir Crit Care Med. 1995;152(3):1107–1136. doi:10.1164/ajrccm.152.3.7663792
18. Flint A, Raben A, Blundell J, Astrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes. 2000;24(1):38. doi:10.1038/sj.ijo.0801083
19. Vasheghani-Farahani A, Tahmasbi M, Asheri H, Ashraf H, Nedjat S, Kordi R. The Persian, last 7-day, long form of the International Physical Activity Questionnaire: translation and validation study. Asian J Sports Med. 2011;2(2):106. doi:10.5812/asjsm
20. Jones RL, Nzekwu -M-MU. The effects of body mass index on lung volumes. Chest. 2006;130(3):827–833. doi:10.1378/chest.130.3.827
21. Celli BR, Cote CG, Lareau SC, Meek PM. Predictors of survival in COPD: more than just the FEV1. Respir Med. 2008;102:S27–S35. doi:10.1016/S0954-6111(08)70005-2
22. De S. Body mass index among patient with chronic obstructive pulmonary diseases. Indian J Physiol Pharmacol. 2012;56(4):353–358.
23. Gologanu D, Ionita D, Gartonea T, Stanescu C, MA B. Body composition in patients with chronic obstructive pulmonary disease. Maedica. 2014;9(1):25.
24. Wu Z, Yang D, Ge Z, Yan M, Wu N, Liu Y. Body mass index of patients with chronic obstructive pulmonary disease is associated with pulmonary function and exacerbations: a retrospective real world research. J Thorac Dis. 2018;10(8):5086. doi:10.21037/jtd.2018.08.67
25. Creutzberg E, Schols A, Bothmer-Quaedvlieg F, Wouters E. Prevalence of an elevated resting energy expenditure in patients with chronic obstructive pulmonary disease in relation to body composition and lung function. Eur J Clin Nutr. 1998;52(6):396. doi:10.1038/sj.ejcn.1600571
26. Brúsik M, Ukropec J, Joppa P, et al. Circulatory and adipose tissue leptin and adiponectin in relationship to resting energy expenditure in patients with chronic obstructive pulmonary disease. Physiol Res. 2012;61(5):469–480.
27. Hill K, Vogiatzis I, Burtin C. The importance of components of pulmonary rehabilitation, other than exercise training, in COPD. Eur Respiratory Soc. 2013;22:405–413. doi:10.1183/09059180.00002913
28. van der Molen T. Co-morbidities of COPD in primary care: frequency, relation to COPD, and treatment consequences. Prim Care Respir J. 2010;19(4):326. doi:10.4104/pcrj.2010.00053
29. Sanders KJ, Kneppers AE, van de Bool C, Langen RC, Schols AM. Cachexia in chronic obstructive pulmonary disease: new insights and therapeutic perspective. J Cachexia Sarcopenia Muscle. 2016;7(1):5–22.
30. Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005;115(5):911–919.
31. Chan KH, Yeung SC, Yao TJ, et al. Elevated plasma adiponectin levels in patients with chronic obstructive pulmonary disease. Int J Tuberc Lung Dis. 2010;14(9):1193–1200.
32. Yoon HI, Li Y, Man SP, et al. The complex relationship of serum adiponectin to COPD outcomes. Chest. 2012;142(4):893–899.
33. Bao Y, Bing C, Hunter L, Jenkins JR, Wabitsch M, Trayhurn P. Zinc‐α2‐glycoprotein, a lipid mobilizing factor, is expressed and secreted by human (SGBS) adipocytes. FEBS Lett. 2005;579(1):41–47.
34. Mracek T, Ding Q, Tzanavari T, et al. The adipokine zinc‐α2‐glycoprotein (ZAG) is downregulated with fat mass expansion in obesity. Clin Endocrinol. 2010;72(3):334–341.
35. Marrades M, Martinez J, Moreno-Aliaga M. ZAG, a lipid mobilizing adipokine, is downregulated in human obesity. J Physiol Biochem. 2008;64(1):61–66.
36. Schneider M, Hansen JL, Sheikh SP. S100A4: a common mediator of epithelial–mesenchymal transition, fibrosis and regeneration in diseases? J Mol Med. 2008;86(5):507–522.
37. Wang T, Huo X, Chong Z, Khan H, Liu R. A review of S100 protein family in lung cancer. Clinica Chimica Acta. 2018;476:54–59.
38. Prosser BL, Wright NT, Hernãndez-Ochoa EO, et al. S100A1 binds to the calmodulin-binding site of ryanodine receptor and modulates skeletal muscle excitation-contraction coupling. JBC. 2008;283(8):5046–5057.
39. Cannon BR, Zimmer DB, Weber DJ. S100A1 (S100 calcium binding protein A1). Atlas Genet Cytogenet Oncol Haematol. 2011;15(10):873–876.
40. Pleger ST, Harris DM, Shan C, et al. Endothelial S100A1 modulates vascular function via nitric oxide. Circ Res. 2008;102(7):786–794.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
