Back to Journals » Journal of Pain Research » Volume 14
Low-Dose Methoxyflurane versus Standard of Care Analgesics for Emergency Trauma Pain: A Systematic Review and Meta-Analysis of Pooled Data
Authors Fabbri A , Borobia AM, Ricard-Hibon A, Coffey F, Caumont-Prim A, Montestruc F, Soldi A , Traseira Lugilde S, Dickerson S
Received 17 November 2020
Accepted for publication 6 January 2021
Published 20 January 2021 Volume 2021:14 Pages 93—105
DOI https://doi.org/10.2147/JPR.S292521
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Robert B. Raffa
Andrea Fabbri,1 Alberto M Borobia,2 Agnes Ricard-Hibon,3 Frank Coffey,4 Aurore Caumont-Prim,5 François Montestruc,5 Amedeo Soldi,6 Susana Traseira Lugilde,7 Sara Dickerson8
1Department of Emergency Medicine, Morgagni-Pierantoni Hospital, Forli, Italy; 2Clinical Pharmacology Department, La Paz University Hospital, School of Medicine, Universidad Autónoma de Madrid, IdiPAZ, Madrid, Spain; 3Emergency Department SAMU-SMUR 95, CHG Pontoise-Beaumont/Oise, Pontoise, France; 4DREEAM: Department of Research and Education in Emergency Medicine, Acute Medicine and Major Trauma, Nottingham University Hospitals NHS Trust, Nottingham, UK; 5eXYSTAT, Malakoff, France; 6Mundipharma Pharmaceuticals Srl, Milan, Italy; 7Mundipharma Pharmaceuticals S.L., Madrid, Spain; 8Mundibiopharma Limited, Cambridge, UK
Correspondence: Sara Dickerson
Mundibiopharma Limited, Cambridge Science Park, Milton Road, Cambridge CB4 0AB, UK
Tel +44 1223 397684
Email [email protected]
Purpose: Undertreatment of trauma-related pain is common in the pre-hospital and hospital settings owing to barriers to the use of traditional standard of care analgesics. Low-dose methoxyflurane is an inhaled non-opioid analgesic with a rapid onset of pain relief that is approved for emergency relief of moderate-to-severe trauma-related pain in adults. This analysis was performed to compare the efficacy and safety of low-dose methoxyflurane with standard of care analgesics in adults with trauma-related pain.
Methods: A meta-analysis was performed on pooled data from randomized controlled trials identified via a systematic review. The primary endpoint was the pain intensity difference between baseline and various time intervals (5, 10, 15, 20, and 30 minutes) after initiation of treatment.
Results: The pain intensity difference was statistically superior with low-dose methoxyflurane compared with standard of care analgesics (overall estimated treatment effect=11.88, 95% CI=9.75– 14.00; P< 0.0001). The superiority of low-dose methoxyflurane was demonstrated at 5 minutes after treatment initiation and was maintained across all timepoints. Significantly more patients treated with methoxyflurane achieved response criteria of pain intensity ≤ 30 mm on a visual analog scale, and relative reductions in pain intensity of ≥ 30% and ≥ 50%, compared with patients who received standard of care analgesics. The median time to pain relief was shorter with methoxyflurane than with standard of care analgesics. The findings were consistent in a subgroup of elderly patients (aged ≥ 65 years).
Conclusion: Methoxyflurane can be considered as an alternative to standard of care analgesics in pre-hospital and hospital settings for treatment of adult patients with acute trauma-related pain.
Keywords: acute pain, inhaled analgesic, emergency service, wounds and injury, pain management, analgesia
Plain Language Summary
Many patients with trauma injuries do not receive adequate pain relief, which can lead to unnecessary suffering and a prolonged stay in hospital. This may be because their pain-reliving medicine (analgesic) is not effective enough, or because it is difficult to administer (for example if it has to be given through a vein). Low-dose methoxyflurane is an analgesic that patients can administer themselves using an inhaler. It starts working quickly and is effective for around 30 minutes if used continuously. We analyzed the results from four studies in which adults with trauma-related pain were treated with low-dose methoxyflurane or standard analgesics (including paracetamol, anti-inflammatories, and opioids). Our analysis showed that low-dose methoxyflurane provided better and faster pain relief than the standard analgesics. Satisfaction with treatment was higher among patients who received low-dose methoxyflurane than among those who received standard analgesics. We repeated the analysis in elderly patients (aged ≥65 years) and got similar results. Based on our findings, we recommend that low-dose methoxyflurane is considered as an alternative to standard analgesics for treatment of trauma-related pain in adults.
Introduction
Despite recommendations that patients with trauma-related pain should receive prompt and effective analgesia during emergency care in both pre-hospital and hospital settings,1 many patients are undertreated.1–4 This results in prolonged hospital stays, unnecessary patient suffering, and dissatisfaction.5–7 A wide range of analgesics is available for use in the pre-hospital and emergency department settings, with a variety of formulations and routes of administration. Healthcare providers can therefore find it challenging to determine the most appropriate treatment.1 In addition, some commonly-used analgesics are associated with factors that contribute, at least in part, to oligoanalgesia in patients with moderate-to-severe trauma pain.1,8 These factors include the limited efficacy of weak analgesics, difficulties with intravenous administration, side-effects, the high administrative burden associated with controlled drugs, and logistical constraints (eg, storage of bulky nitrous oxide canisters).
Low-dose methoxyflurane is a non-opioid analgesic that patients self-administer under medical supervision using a hand-held inhaler device. Self-administration allows the patient to titrate the minimum effective dose to relieve their pain, up to a maximum of two 3 mL vials (maximum 15 mL per week). Low-dose methoxyflurane has a number of features that make it an attractive option for analgesia in the pre-hospital and emergency department settings. Onset of pain relief is rapid (within 6–10 inhalations).9 With continuous use, pain relief lasts for 25–30 minutes; this may be longer with intermittent use.9 Adverse effects are generally mild and transient, resolving quickly after inhalation is stopped.9 Low-dose methoxyflurane does not cause respiratory depression and has no effect on cardiovascular function (systolic blood pressure and pulse rate).10,11 The inhaler is easy to use and allows patients to control their own level of analgesia; it is also compact, making it easy to store.
Low-dose methoxyflurane has been used extensively in Australia for over 40 years to provide short-term relief of acute pain in adults and children.12–14 It is also available in Canada and South Africa, as well as countries in Asia, Latin America, and the Gulf. In the EU, low-dose methoxyflurane is approved for emergency relief of moderate-to-severe trauma-associated pain in conscious adults.9 EU approval was granted based on evidence from the randomized, double-blind, placebo-controlled STOP! trial,15 in which methoxyflurane provided a statistically and clinically significant reduction in pain intensity. Subsequently, low-dose methoxyflurane has been shown to provide superior pain relief to standard of care analgesics in patients with moderate and severe acute trauma pain.16,17
To help healthcare providers make informed treatment decisions, comprehensive, high quality analyses of the efficacy and safety data for analgesics used in acute trauma-related pain are needed. The aim of this systematic review and meta-analysis was to compare the analgesic efficacy and safety of low-dose methoxyflurane with that of standard of care analgesics in emergency care of patients with acute trauma-related pain.
Methods
Methods of the meta-analysis and inclusion criteria for the systematic review were specified in advance and documented in a statistical analysis plan.
Systematic Literature Review
Eligibility Criteria
Eligibility criteria were defined using the PICO (population, interventions, comparisons, and outcome) framework. The population of interest was adults receiving emergency care for acute musculoskeletal, trauma-related pain (defined as a visual analogscale [VAS] score ≥4 and lasting for <48 hours, or defined by the study authors as “trauma”). The intervention was low-dose methoxyflurane and the comparator was standard of care (non-steroidal anti-inflammatory drugs [NSAIDs], paracetamol, weak or strong opioids, or no treatment) ± placebo. For studies in which a placebo inhaler was used, standard of care related to the treatments listed above taken before treatment initiation or in the first minute after the first placebo inhalation; for studies in which a placebo was not used, standard of care related to these treatments taken at treatment initiation or in the first minute after treatment initiation. The primary outcome was pain intensity; secondary outcomes were treatment satisfaction and adverse events (AEs). Only prospective randomized clinical trials published in English were eligible for inclusion.
Search Strategies
MEDLINE and Embase were searched up to June 29, 2020 using the search term methoxyflurane AND emergency AND randomi*.
Study Selection and Data Collection
All titles and abstracts identified in the searches were independently screened; eligible abstracts went forward to full text screening and review. Articles that met the eligibility criteria at full text screening were included in the analysis.
Two reviewers independently extracted the following data from the included trials: demographics, exposure, pain intensity scores, time to pain relief, treatment satisfaction, and AEs. Any discrepancies were resolved by referral to a third reviewer.
Meta-Analysis
The primary endpoint of the analysis was the pain intensity difference between baseline and various time intervals (5, 10, 15, 20, and 30 minutes) after initiation of treatment. Secondary endpoints were: relative pain intensity difference; response criteria (level of pain ≤30 mm, relative reduction in pain intensity [RPID] ≥30% and ≥50%); time to pain relief after first inhalation (patient-declared, time to ≥2-point reduction in VAS score and time to VAS score ≤30); treatment satisfaction (assessed by patient, investigator and nurse on a 5-point Likert scale, where 1=very satisfied and 5=very dissatisfied); safety.
The analysis was carried out using individual patient data with a one-step approach as the primary estimate. The pain intensity difference was compared between low-dose methoxyflurane and standard of care analgesics using a repeated measures analysis of variance (ANOVA) model including the study (as a random effect), treatment, nominal time point, and the interaction between treatment and nominal time point. For the primary endpoint, a two-step approach was also used to estimate heterogeneity between the studies and consolidate results: each study data set was analyzed separately using repeated measures ANOVA and the treatment effects across studies were combined using the weighted average of treatment effects (where weights correspond to the inverse of treatment effect variance) with a random effects model (as it is assumed that intervention effects follow a distribution across studies).
Study Populations
Efficacy analyses were carried out on the meta-analysis population (MAP1), which included all patients aged ≥18 years who were randomized to low-dose methoxyflurane or standard of care analgesics ± placebo (patients receiving methoxyflurane plus standard of care analgesics were excluded from the analysis). Safety analyses were carried out on the safety population, ie, all MAP1 patients who received at least one dose of study medication.
Risk of Bias Across Studies
Most studies reported pain intensity on a 100 mm VAS, where 0=no pain and 100=worst pain imaginable. One study used an 11-point numerical rating scale (NRS) (where 0=no pain and 10=unbearable pain);16 pain intensity data from this study were multiplied by 10 to allow comparison with the other studies. In addition, this study did not report NRS scores at randomization so the score at T0 (ie, just before the first dose of study medication) was used in place of the score at randomization. To evaluate potential bias regarding use of NRS, a correlation between NRS at randomization and the closest value in the VAS was carried out.
Additional Analysis
All analyses were also carried out in a subpopulation of elderly patients (aged ≥65 years). A sensitivity analysis was carried out that considered standard of care as analgesics taken before treatment initiation or in the first 20 minutes after treatment initiation. The results of this sensitivity analysis are not reported here.
Results
Study Selection
The systematic literature review yielded 56 articles. After initial screening, 23 went forward for full text review. Eighteen of these were excluded; the most common reasons for exclusion were that the studies reported were retrospective or were not randomized controlled trials. The analysis therefore included five publications describing four RCTs: STOP!,15,19 InMEDIATE,16 MEDITA,17 and PenASAP.18 A flowchart of study identification and selection is shown in Figure 1.
 |
Figure 1 PRISMA flow diagram for study selection. Abbreviation: RCT, randomized controlled trial. |
STOP! (NCT01420159) was a randomized, double-blind, multicenter study comparing low-dose methoxyflurane with placebo in patients who presented at UK emergency departments with acute minor trauma.15,19 The primary outcome of the study was change in pain intensity from baseline to 5, 10, 15, and 20 minutes after first inhalation. The study included 300 patients, 90 of whom were adolescents and therefore outside the scope of this meta-analysis.15 A subgroup analysis of 204 adult patients included in STOP! has been published.19
The randomized open-label InMEDIATE study (NCT03256903) compared low-dose methoxyflurane with standard of care in patients with mild-to-severe trauma pain in Spanish prehospital and emergency department settings.16 The study had coprimary endpoints of change from baseline in pain intensity during the first 20 minutes of treatment and time to first pain relief. MEDITA (NCT03585374) was a randomized open-label study comparing methoxyflurane and standard of care for moderate-to-severe acute trauma pain in Italian prehospital and emergency department settings.17 The primary outcome was change in pain intensity from baseline at 3, 5, and 10 minutes after initiation of treatment. The PenASAP study (NCT03798899) was a randomized, double-blind study comparing methoxyflurane (plus standard of care) with placebo (plus standard of care) in patients presenting to the French emergency department with moderate-to-severe trauma-related pain.18 The primary outcome was time to pain relief. A summary of the key outcomes from these studies is given in Table 1.
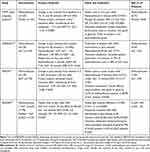 |
Table 1 Key Outcomes from the Included Studies |
Patients
In total, the four studies included 1,260 patients. Of these, 1,090 were included in MAP1 and 1,102 were included in the safety population. The most common reasons for exclusion from MAP1 were for being a pediatric patient and for being randomized to methoxyflurane but receiving standard of care before or in the first minute after inhalation. Twenty-four patients were excluded from MAP1 because they had VAS scores <30 mm at T0; these patients were included in the safety population. Overall, 96 pediatric patients were excluded from MAP1; all these patients were in the STOP! study. Table 2 shows patient disposition in the MAP1 and safety populations by treatment group.
 |
Table 2 Patient Disposition (MAP1 and Safety Populations) |
Of the 1,090 patients in MAP1, 536 had received low-dose methoxyflurane and 554 had received standard of care ± placebo. Patient demographics were similar between the two groups (Table 3). The mean (SD) age was 43.3 (17.9) years in the methoxyflurane group and 42.2 (18.0) years in the standard of care group. In both treatment groups, most primary injuries were to the lower limb; contusions were the most common type of primary injury (Table 3). Most patients who used either a methoxyflurane or placebo inhaler in the studies needed just one inhaler: 81% of the 536 patients who used a methoxyflurane inhaler and 68% of the 272 who used a placebo inhaler.
 |
Table 3 Patient Demographics (MAP1) |
Eighty-two patients (15.3%) in the methoxyflurane group and 72 (13.0%) in the standard of care group were aged ≥65 years; patient demographics and disposition for this elderly subgroup population are shown in the Supplementary Table 1.
Meta-Analysis Results
Figure 2 shows the analysis of pain intensity difference. The overall estimated treatment effect was statistically significant and in favor of low-dose methoxyflurane versus pooled standard of care analgesics ± placebo (estimated treatment effect=11.88, 95% CI=9.75–14.00; P<0.0001). The superiority of low-dose methoxyflurane was demonstrated at 5 minutes after treatment initiation and was maintained across all timepoints. A similar pattern was seen when low-dose methoxyflurane was compared with each individual standard of care treatment category; the only instance when methoxyflurane did not show statistical superiority was in the comparison with opioids at T30, although the treatment difference remained in favor of methoxyflurane.
The results for pain intensity difference were similar in the elderly subgroup. The estimated overall treatment effect (95% CI) for methoxyflurane versus pooled standard of care analgesics ± placebo was 8.61 (3.27–13.95); P=0.0016. As in the overall population, superiority of methoxyflurane was seen at 5 minutes and was maintained at all timepoints.
For RPID, the estimated overall treatment effect (95% CI) for methoxyflurane versus pooled standard of care analgesics ± placebo was 17.43 (14.71– 20.54); P<0.0001 in the overall population. In the elderly subgroup, this was 10.57 (3.20–17.94); P=0.0050.
Low-dose methoxyflurane was also statistically superior to pooled standard of care analgesics ± placebo in terms of response criteria across all time points (Table 4). By T20, approximately half the patients (51.9%) treated with low-dose methoxyflurane achieved pain intensity <30 mm (considered a marker for effective pain relief in emergency departments), compared with less than one-third (29.8%) who received standard of care analgesics. Consistent results were seen when low-dose methoxyflurane was compared with each individual standard of care treatment category; the only instances when methoxyflurane did not show statistical superiority were for relative reductions in pain intensity ≥30% and ≥50% compared with opioids at T30, and pain intensity ≤30 mm compared with paracetamol at T15 and T20. A similar pattern was seen in the elderly subgroup, although treatment differences were less marked (see Supplementary Table 2; Supplementary Figure 1). By T20, 42.5% of the 82 elderly patients treated with low-dose methoxyflurane achieved pain intensity <30 mm, compared with 28.6% of the 154 who received standard of care analgesics.
 |
Table 4 Response Criteria (MAP1): Changes in Pain Intensity Over Time |
Time to pain relief was shorter with low-dose methoxyflurane than with any of the standard of care treatments (Table 5). The median time to patient-declared pain relief was 10 minutes with methoxyflurane vs 18 minutes with pooled standard of care treatments (hazard ratio=2.03; 95% confidence interval=1.75–2.36; P<0.0001). The median time to a ≥30% reduction in pain intensity was 10 minutes with methoxyflurane, compared with 20 minutes with pooled standard of care analgesics (hazard ratio=1.93; 95% confidence interval=1.68–2.23; P<0.0001). Patients in the “no treatment” group had the longest median time to pain relief: not evaluable for patient declared pain relief and 31 minutes for ≥30% reduction in pain intensity. A similar pattern was seen in the elderly subgroup (see Supplementary Table 3), although the hazard ratios were only statistically significant for methoxyflurane vs NSAIDs (time to patient declared pain relief) and methoxyflurane vs no treatment (time to ≥reduction in pain intensity).
 |
Table 5 Time to Pain Relief (MAP1) |
Compared with standard of care analgesics, low-dose methoxyflurane was associated with a higher proportion of patients who were “satisfied” or “very satisfied” with treatment: 63.5% vs 49.2% (Figure 3). A similar pattern of treatment satisfaction was seen among nurses, study investigators, and the subgroup of elderly patients (see Supplementary Table 4; Supplementary Figure 2).
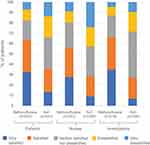 |
Figure 3 Treatment satisfaction. Abbreviation: SoC, standard of care. |
The incidence of AEs was low and generally comparable between methoxyflurane and standard of care analgesics. However, low-dose methoxyflurane was associated with a higher incidence of dizziness (16.7% vs 3.6%), somnolence (5.9% vs 0.9%), and feeling drunk (4.0% vs 0.5%) than standard of care analgesics. Serious and severe (ie, Grade 3 or 4) AEs were reported by less than 1% of patients, regardless of whether they received methoxyflurane or standard of care analgesics. The safety profile in the elderly population reflected that observed in the overall population.
Discussion
This meta-analysis based on four randomized clinical trials showed that low-dose methoxyflurane provided superior efficacy to a range of standard of care analgesics when used for emergency care of adults with acute musculoskeletal, trauma-related pain. The analysis confirmed the fast onset of pain relief with low-dose methoxyflurane: superior analgesia was demonstrated in the primary endpoint (pain intensity difference) from 5 minutes post-initiation and was maintained throughout the 30-minute assessment period. The superior analgesic effect of low-dose methoxyflurane was consistent across a range of other endpoints, including time to pain relief and various response criteria. The improvements in pain measures were supported by greater treatment satisfaction with low-dose methoxyflurane compared with standard of care analgesics, as reported by patients, nurses, and study investigators. All the analgesics included in the analysis were well tolerated; mild, transient dizziness and somnolence were more frequent with low-dose methoxyflurane than with standard of care analgesics, which is consistent with its known safety profile.
There was no evidence that the efficacy or safety of low-dose methoxyflurane differs in elderly patients: outcomes in patients aged ≥65 years were comparable with the overall population. Some caution is needed when interpreting data in the elderly subgroup given the low patient numbers (82 in the methoxyflurane group and 72 in the standard of care group). Nevertheless, we consider the data to be sufficiently robust as the calculated a posteriori power was 80% for the elderly subgroup.
To our knowledge, this is the first review of pooled data comparing low-dose methoxyflurane with a range of standard of care analgesics used to manage acute trauma-related pain. Coupled with additional benefits that methoxyflurane has over other short-acting analgesics such as ease of use, a non-opioid mechanism of action, and the ability for patients to self-titrate to pain control, this meta-analysis provides reassurance to healthcare providers that low-dose methoxyflurane is also a highly effective short-term treatment option in this setting.
It is important that patients are carefully coached in use of the inhaler. Methoxyflurane is added to the inhaler via a one-way valve and absorbed by a polypropylene wick. Once absorbed, methoxyflurane vaporizes and the patient can inhale the vapor through the mouthpiece. Patients should be told to take gentle breaths at first, and then to inhale intermittently to achieve adequate analgesia. As patients exhale into the inhaler, any exhaled methoxyflurane is captured in an activated carbon chamber, which reduces the risk of exposure. Non-interventional and observational studies show that occupational exposure to methoxyflurane among healthcare providers who are supervising patients using the inhaler is very low.20,21
This meta-analysis only included data from adults with acute trauma pain. This is a limitation but is in line with the current EU approval for methoxyflurane. A recent systematic review found no evidence that the efficacy, onset of action, or safety of low-dose methoxyflurane differ between adults and children.22
Another limitation is that the included studies were limited to hospital emergency departments in the UK, France, Italy, and Spain; however, the standard of care analgesics included in the analysis broadly reflect treatments that are commonly used in other countries for pain relief in patients with musculoskeletal trauma injuries.23 Our literature review did not identify any randomized controlled trials in the pre-hospital setting.
Although the mean age was similar between the methoxyflurane and overall standard of care groups (43.3 years vs 42.2 years, respectively), patients who received opioids were older (56.8 years). These patients also had more severe pain at randomization than patients receiving methoxyflurane or any of the other standard of care treatments. However, this should be interpreted with caution given the low number of patients who received opioids (n=56).
Further limitations include differences in the study designs (ie, some were double-blind and others were open label) and in the way pain scores were captured (eg, via tablet, on paper, or using a sliding scale ruler).
Conclusion
Low-dose methoxyflurane provides superior and more rapid pain relief to standard of care analgesics commonly used in the pre-hospital and emergency room settings. It should therefore be considered in these settings for treatment of adult patients with acute trauma-related pain.
Acknowledgments
Dr Joanna Todd (Stellar Medical Communications Limited, Ely, UK) provided medical writing and editorial support; this was funded by Mundibiopharma Limited.
Funding
This systematic review and meta-analysis was funded by Mundibiopharma Limited, which was involved in all stages from study design to manuscript submission.
Disclosure
Dr Fabbri has received fees from Mundipharma as a member of the scientific committee for the MEDITA study, and was corresponding author for that study. Dr Borobia has received fees from Mundipharma as a member of the scientific committee for InMEDIATE, as well as speaker fees from Mundipharma and Menarini. Dr Borobia is Principal Investigator on clinical trials sponsored by GSK, Daiichi Sankyo, Janssen and Farmalider. Dr Ricard-Hibon has served as an advisory board member for Mundipharma. Professor Coffey was chief investigator for the STOP! Study and has received honoraria to present at symposia sponsored by Mundipharma. Aurore Caumont-Prim and François Montestruc are employees of eXYSTAT, a contract research organization which received fees from Mundibiopharma Limited to perform the meta-analysis. Amedeo Soldi is an employee of Mundipharma Srl. Susana Traseira Lugilde is an employee of Mundipharma S.L. Sara Dickerson is an employee of Mundibiopharma Limited. The authors report no other conflicts of interest in this work.
References
1. European Society for Emergency Medicine. Guidelines for the management of acute pain in emergency situations; 2020. Available from: https://www.eusem.org/images/EUSEM_EPI_GUIDELINES_MARCH_2020.pdf.
2. Albrecht E, Taffe P, Yersin B, Schoettker P, Decosterd I, Hugli O. Undertreatment of acute pain (oligoanalgesia) and medical practice variation in prehospital analgesia of adult trauma patients: a 10 yr retrospective study. Br J Anaesth. 2013;10:96–106. doi:10.1093/bja/aes355
3. Dale J, Bjørnsen LP. Assessment of pain in a Norwegian Emergency Department. Scand J Trauma Resusc Emerg Med. 2015;23:86. doi:10.1186/s13049-015-0166-3
4. Mura P, Serra E, Marinangeli F, et al. Prospective study on prevalence, intensity, type, and therapy of acute pain in a second-level urban emergency department. J Pain Res. 2017;10:2781–2788. doi:10.2147/JPR.S137992
5. Sokoloff C, Daoust R, Paquet J, Chauny J-C. Is adequate pain relief and time to analgesia associated with emergency department length of stay? A retrospective study. BMJ Open. 2014;4(3):e004288. doi:10.1136/bmjopen-2013-004288
6. Tanabe P, Buschmann M. A prospective study of ED pain management practices and the patient’s perspective. J Emerg Nurs. 1999;25:171–177. doi:10.1016/S0099-1767(99)70200-X
7. Todd KH, Ducharme J, Choiniere M; for PEMI Study Group. Pain in the emergency department: results of the pain and emergency medicine initiative (PEMI) multicenter study. J Pain. 2007;8:460–466. doi:10.1016/j.jpain.2006.12.005
8. Dißmann PD, Maignan M, Cloves PD, Gutierrez Parres B, Dickerson S, Eberhardt A. A review of the burden of trauma pain in emergency settings in Europe. Pain Ther. 2018;7:179–192. doi:10.1007/s40122-018-0101-1
9. Penthrox® (methoxyflurane) 3 mL inhalation vapour liquid [Summary of Product Characteristics]. Craigavon, UK: Galen Limited; 2016. Available from: http://www.medicines.org.uk/emc/medicine/31391.
10. Johnston S, Wilkes GJ, Thompson JA, Ziman M, Brightwell R. Inhaled methoxyflurane and intranasal fentanyl for prehospital management of visceral pain in an Australian ambulance service. Emerg Med J. 2011;28:57–63. doi:10.1136/emj.2009.078717
11. Oxer HF. Effects of Penthrox® (methoxyflurane) as an analgesic on cardiovascular and respiratory functions in the pre-hospital setting. J Mil Veterans Health. 2016;24:14–20.
12. Bendall JC, Simpson PM, Middleton PM. Prehospital analgesia in New South Wales, Australia. Prehosp Disaster Med. 2012;26:422–426. doi:10.1017/S1049023X12000180
13. Grindlay J, Babl FE. Review article: efficacy and safety of methoxyflurane analgesia in the emergency department and prehospital setting. Emerg Med Australas. 2009;21:4–11. doi:10.1111/j.1742-6723.2009.01153.x
14. Porter KM, Dayan AD, Dickerson S, Middleton PM. The role of inhaled methoxyflurane in acute pain management. Open Access Emerg Med. 2018;10:149–164. doi:10.2147/OAEM.S181222
15. Coffey F, Wright J, Hartshorn S, et al. STOP!: A randomised, double-blind, placebo-controlled study of the efficacy and safety of methoxyflurane for the treatment of acute pain. Emerg Med J. 2014;31:613–618. doi:10.1136/emermed-2013-202909
16. Borobia AM, Garcia Collado S, Carballo Cardona C; for InMEDIATE Investigators Group. Inhaled methoxyflurane provides greater analgesia and faster onset of action versus standard analgesia in patients with trauma pain: inMEDIATE: a randomized controlled trial in emergency departments. Ann Emerg Med. 2019;75:315–328. doi:10.1016/j.annemergmed.2019.07.028
17. Mercadante S, Voza A, Serra S; for MEDITA Study Group. Analgesic efficacy, practicality and safety of inhaled methoxyflurane versus standard analgesic treatment for acute trauma pain in the emergency setting: a randomised, open-label, active-controlled, multicentre trial in Italy (MEDITA). Adv Ther. 2019;36:3030–3046. doi:10.1007/s12325-019-01055-9
18. Ricard-Hibon A, Lecoules N, Savary D, et al. Inhaled methoxyflurane for the management of trauma related pain in patients admitted to hospital emergency departments: a randomised, double-blind placebo-controlled trial (PenASAP study). Eur J Emerg Med. 2020. doi:10.1097/MEJ.0000000000000686
19. Coffey F, Dissmann P, Mirza K, Lomax M. Methoxyflurane analgesia in adult patients in the Emergency Department: a subgroup analysis of a randomized, double-blind, placebo-controlled study (STOP!). Adv Ther. 2016;33:2012–2031. doi:10.1007/s12325-016-0405-7
20. Frangos J, Belbachir A, Dautheville S, et al. Non-interventional study evaluating exposure to inhaled, low-dose methoxyflurane experienced by hospital emergency department personnel in France. BMJ Open. 2020;10:e034647. doi:10.1136/bmjopen-2019-034647
21. Ruff R, Kerr S, Kerr D, Zalcberg D, Stevens J. Occupational exposure to methoxyflurane administered for procedural sedation: an observational study of 40 exposures. Br J Anaesth. 2018;120:1435–1437. doi:10.1016/j.bja.2018.01.029
22. Hartshorn S, Middleton PM. Efficacy and safety of inhaled low-dose methoxyflurane for acute paedatric pain: a systematic review. Trauma. 2019;21:94–102. doi:10.1177/1460408618798391
23. Xia AD, Dickerson SL, Watson A, Nokela M, Colman S, Szende A. Evaluation of pain relief treatment and timelines in emergency care in six European countries and Australia. Open Access Emerg Med. 2019;11:219–240. doi:10.2147/OAEM.S214396
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

