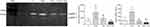Back to Journals » Drug Design, Development and Therapy » Volume 13
Low-dose doxycycline inhibits hydrogen peroxide-induced oxidative stress, MMP-2 up-regulation and contractile dysfunction in human saphenous vein grafts
Authors Saeed M , Arun MZ, Guzeloglu M, Onursal C , Gokce G , Korkmaz CG, Reel B
Received 1 October 2018
Accepted for publication 26 March 2019
Published 23 May 2019 Volume 2019:13 Pages 1791—1801
DOI https://doi.org/10.2147/DDDT.S187842
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Georgios Panos
Mazen Saeed,1 Mehmet Zuhuri Arun,2 Mehmet Guzeloglu,3 Ceylan Onursal,1 Goksel Gokce,2 Ceren Gonen Korkmaz,2 Buket Reel2
1Ege University, Faculty of Pharmacy, Department of Pharmacology, 35100 Bornova, Izmir, Turkey; 2Ege University, Faculty of Pharmacy, Department of Clinical Pharmacy, 35100 Bornova, Izmir, Turkey; 3Optimed Hospital, Department of Cardiovascular Surgery, 59500 Corlu, Tekirdag, Turkey
Background: Cardiopulmonary bypass (CPB) applied during coronary artery bypass grafting (CABG), promotes inflammation, generation of reactive oxygen species (ROS) and up-regulation of matrix metalloproteinases (MMPs). All these complications may lead to contractile dysfunction, restenosis and early graft failure, restricting long-term efficacy of bypass grafts. Low-dose doxycycline is a potent MMP inhibitor and ROS scavenger. In this study, we aimed to investigate the effects of doxycycline on ROS generation, MMP regulation and contractile dysfunction induced by H2O2 in human saphenous vein (HSV) grafts.
Methods: HSV grafts (n=7) were divided into four groups after removing endothelial layer by mechanical scratching and incubated with 10 μM H2O2 and/or 10 μM doxycycline for 16 hrs. Untreated segments served as control. Concentration–response curves to noradrenaline (NA), potassium chloride (KCl), serotonin (5-HT) and papaverine were performed. Superoxide anion and other ROS levels were determined by using lucigenin- and luminol-enhanced chemiluminescence assays, respectively. Expression/activity of gelatinases (MMP-2/MMP-9) was examined by gelatin zymography. MMP-13 expression was evaluated by immunostaining/immunoscoring.
Results: H2O2 incubation increased superoxide anion and other ROS levels. Doxycycline prevented these increments. H2O2 suppressed contractile responses to NA, KCl and 5-HT. Doxycycline ameliorated contractions to NA and KCl but not to 5-HT. H2O2 or doxycycline did not altered relaxation to papaverine. MMP-2 and MMP-13 expression increased with H2O2, but doxycycline inhibited MMP-2 up-regulation/activation.
Conclusion: Low-dose doxycycline may have beneficial effects on increased oxidative stress, MMP up-regulation/activation and contractile dysfunction in HSV grafts.
Keywords: hydrogen peroxide, doxycycline, oxidative stress, matrix metalloproteinase, human saphenous vein
Introduction
Coronary artery bypass grafting (CABG) is a surgical procedure used to treat ruptured or occlusive coronary artery and to improve blood flow to the heart. Human saphenous vein (HSV) graft is one of the most frequently used graft in CABG surgery.1,2 Cardiopulmonary bypass (CPB) is a technique that temporarily takes over the physiologic functions of the heart and lungs during CABG surgery.3 It eminently affects the function of vital organs and clinical outcomes of the patients after CABG.4 However, implementation of CPB and reperfusion after an ischemic period increase drastically the levels of proinflammatory cytokines, mediators and reactive oxygen species (ROS) including hydrogen peroxide (H2O2), superoxide radical (O2•−) and hydroxyl radical (•OH).4,5 Generation of excess ROS acts as a potential toxic messenger. It stimulates inflammation, matrix metalloproteinases (MMPs) and ischemia-reperfusion injury (I/R injury). All these complications of CABG may cause post-operative problems including contractile dysfunction, restenosis, early graft failure and seriously restrict long-term efficacy of bypass graft.2–4
MMPs are a large family of zinc-dependent endopeptidases which mediate degradation of the extracellular matrix. MMP family consists of five subgroups: interstitial collagenases, gelatinases, stromelysins, membrane-type MMPs (MT-MMPs) and others.6,7 MMP activity is regulated by endogenous Tissue Inhibitors of Metalloproteinases (TIMPs-1 to -4).6,7 Alteration of physiologic balance between MMPs and TIMPs on behalf of MMPs contributes to the pathophysiology of vascular diseases such as atherosclerosis, coronary artery disease (CAD) and aneurysms.8,9 MMPs in particular gelatinases (MMP-2 and MMP-9) play major role in neointima formation which is responsible for restenosis after CABG, and plaque rupture which leads to myocardial infarction (MI) or stroke.10 Besides, MMP-13 from interstitial collagenases (MMP-1, -8 and -13) has been implicated in collagen matrix degradation and atherosclerotic plaque vulnerability to rupture.11,12
Low-dose doxycycline is an unique MMP inhibitor which was approved by the US Food and Drug Administration (FDA).13,14 Doxycycline inhibits MMP activity and reduces inflammation in patients with CAD, abdominal aortic aneurysm and post-MI left ventricular remodeling.8,15,16 In a recent clinical trial, doxycycline was reported to decrease serum levels of MMPs and inflammatory burden in CABG patients.17 Furthermore, short-term doxycycline treatment demonstrated to enhance level of TIMP-2 and to reduce infarct size in patients treated with primary percutaneous intervention for the first STEMI (ST-elevation myocardial infarction).18
Low-dose doxycycline has also ROS scavenger and antioxidant activity. Indeed, doxycycline was shown to alleviate hypertension-induced oxidative stress and MMP activity and improve nitric oxide (NO) levels in aortic endothelial cells in 2K-1C hypertensive rats.19 Accordingly, in spontaneously hypertensive rats (SHR), doxycycline was demonstrated to reduce ROS levels and blunt biochemical alterations associated with hypertension.20 In addition, doxycycline treatment was reported to reverse diabetes-induced oxidative stress and prevent MMP-2 activity in diabetic rats.21 Moreover, doxycycline cardioplegia has been shown to reduce oxidative stress and preserve cardiac function against I/R injury in isolated rat heart.22
In the light of these knowledge, in the present study, we aimed to investigate the effects of low-dose doxycycline on ROS generation, MMP regulation and contractile dysfunction induced by H2O2 in HSV grafts.
Materials and methods
Materials
L-(−)-Noradrenaline (+)-bitartarate monohydrate (Cat# A9512), serotonin creatinine sulphate monohydrate (Cat# H-7752), papaverine hydrochloride (Cat# P3510), acetylcholine chloride (Cat# A6625), lucigenin (Cat# 2315-97-1) and luminol (Cat# 521-31-3) were purchased from Sigma-Aldrich, Germany. Potassium chloride (Cat# 2538810) and hydrogen peroxide (Cat# 1-08597-1000) were supplied from Merck, Germany. Doxycycline hyclate was kindly given by Eastpharma, Turkey. RPMI 1640 (Cat# 21875-034) and L-Glutamin (Cat# 25030-024) were purchased from Gibco, UK. Penicillin/streptomycin (Cat# 9.A-4061) was supplied from PAA Cell Culture Company, USA. Hepes buffer (Cat# LA-0010E) was obtained from BioWhittaker, Belgium. All drugs were dissolved in distilled water daily. Then, further dilutions of doxycycline were prepared with distilled water. Other drugs were diluted with % 0.9 NaCl from their stock solutions for cumulative concentrations.
Selection of patients and ethics
The remaining segments of HSV from patients (n=7, mean age 63±4.28) undergoing CABG were used in the study. The patients who has diabetes and the patients using beta blocker and/or calcium channel blocker and/or nitrates in last 3 days before the operation were excluded from the study. Additionally, HSV segments from the patients suffering from operative mortality or cardiac morbidity were also excluded from the study.
All patients gave their written informed consent for using the remaining HSV tissue. The experimental protocol was approved by the Ethical Committee of Izmir University (Protocol number is 2015/018). Research was carried out in accordance with Declaration of Helsinki of the World Medical Association.
Preparation of vessels
HSV grafts were harvested and placed immediately into cold (4°C) RPMI 1640 cell culture medium (Gibco, UK) and taken to the laboratory. Following cleaning of adherent connective tissue, endothelial layer of HSVs was gently removed by using curved forceps since many relaxant/contractile factors release from the endothelium and these factors would make it difficult to examine the effects of H2O2 and doxycycline on vascular smooth muscle. Then they were cut into rings of 3 mm in length and the rings were divided into 4 groups as H2O2, doxycycline, H2O2 + doxycycline and control. Thereafter, HSV rings were incubated with H2O2 for 16 hrs to induce oxidative stress. In the present study, a concentration of H2O2 (10 µM) representing human plasma levels in pathological conditions was used.23,24 As for the treatment groups, doxycycline at clinically relevant concentration (10 µM) which inhibits I/R injury was applied based on the literature.25 Briefly, they were incubated in RPMI 1640 medium supplemented with 1% penicillin/streptomycin (5 mg/mL) (Gibco, UK) and 1% L-glutamine (Lonza, Belgium) (200 mM) in the presence of 10 µM H2O2 and/or 10 µM doxycycline for 16 hrs in a humidified atmosphere containing 5% CO2 at 37°C. Untreated HSV segments were kept as control in complete medium for the same period of time.
Isolated organ chamber experiments
At the end of the incubation period, one HSV ring (n=7) from each group was mounted on L-shaped stainless-steel hooks and suspended in the organ chamber (PanLab, Spain) filled with 10 mL 37°C Krebs solution continuously gassed with 95% O2–5% CO2. Krebs solution (pH 7.4) was composed (in mM) of NaCl, 118; KCl, 4.7; CaCl2, 2.5; KH2PO4, 1.2, MgSO4, 1.2; NaHCO3, 25 and glucose, 11.1. HSV rings were gradually stretched to resting tension of 2 g and allowed to equilibrate for 1 hr at their optimal length. During resting period, Krebs solution was changed every 15 mins. The contraction and relaxation responses were measured by an isometric force transducer (AD Instruments, USA) and recorded by a computer-based data acquisition system (Lab Chart 7.0, AD Instruments, USA). After equilibration period, HSV rings were contracted with 80 mM KCl to assess their viability. Subsequently, HSV rings were returned to basal tone by washing three times within a half-hour period. Following this period, the rings were precontracted by a submaximal concentration of NA (10−6 M) and unresponsiveness of the rings to acetylcholine (10−6 M) was assessed in order to confirm the absence of endothelium. Concentration-dependent contraction responses to NA (10−9–10−4M), 5-HT (10−9–3×10−5M) and relaxation responses to papaverine (10−4 M) after precontraction with NA (10−6 M) were examined in each preparation. Each agonist was washed out by changing the Krebs solution three times within 30 mins before further experimentation.
Measurements of ROS levels
In parallel experiments, 4 rings from the remaining HSV graft segments of same patients (n=7) were cut and incubated with H2O2, doxycycline or H2O2 + doxycycline for 16 hrs. One ring was kept untreated control for the same period of time. Then these rings from 4 groups were frozen under liquid nitrogen and stored at −80°C until use. Superoxide and other ROS levels in these rings were determined by using lucigenin- and luminol-enhanced chemiluminescence method as previously described.26 Briefly, HSV rings were placed into OptiPlate solid black 96 well plates containing 200 μL HEPES-buffered PBS solution (pH 7.4). Chemiluminescence enhancers, lucigenin or luminol was added to different wells containing tissue samples (final concentration 5 µmol/L). The resulting photonic activity between reactive oxygen species and luminol or lucigenin was recorded at 10-second intervals for 10 mins by a multimodal plate reader (Varioscan Flash, Thermo Fisher Scientific, USA) and corrected for wet tissue weight. The area under curve (AUC) plotted against time using the saved values was calculated by using GraphPad Prism (version 5.03, MacOS, USA) software. Results were expressed as relative light units (rlu)/mg wet tissue.
Immunohistochemistry
In parallel rings cut from HSV grafts of the same patients (n=7), after incubation period applied for 16 hrs to represent 4 groups as explained above, immunohistochemical staining procedure was performed by the avidin-biotin peroxidase method. HSV rings were immediately placed in 10% neutral buffered formalin for 24 hrs, routinely dehydrated in a graded series of isopropyl alcohol (60–100%) and followed by xylol before being embedded in paraffin. Immunostaining was performed using monoclonal antibody specific for MMP-13 (Cat # sc-101564, Santa Cruz Biotechnology) in 5 µm sections from the paraffinized samples as previously described.27 Appropriate positive controls were also stained. Following immunostaining procedure, images of the rings were captured by using a BX-51 light microscope (Olympus, Tokyo, Japan) equipped with a high-resolution video camera (Olympus DP-71). The intensity of immunopositivity for each MMP protein in intima, media and adventitia layers was evaluated semi-quantitatively using immunoscoring scale. Immunoscoring was performed by assessing the intensity of positive cytoplasmic staining of cells and by scoring according to the following scale: negative (0), weak (1), moderate (2) and strong (3) immunoreactivity.28 A mean score was calculated for each sample.
Tissue homogenization and protein extraction
In a separate and parallel experiment serial, 4 rings from the same HSV grafts (n=5) were cut and incubated as described above to represent 4 groups. Then they were frozen under liquid nitrogen and stored at −80°C until use. On the day of analysis, these rings were finely diced with scalpels and pulverized under liquid nitrogen. Then, the samples were homogenized on ice in homogenization buffer (50 mM tris base, 0.5% Triton, ddH2O, pH 7.4) at a ratio of 1/4 for 30 s using an ultrasonic homogenizator (Bandelin Sonopuls, Bandelin Electronic, Berlin, Germany). The homogenates were centrifuged (Beckman Coulter Microfuge 22 R Centrifuge, CA, USA) at 10,000 g for 10 mins at +4°C. Thereafter, supernatants were stored at −20°C for further analyses.
Total protein measurement
Total protein content was determined in tissue supernatants using bicinchoninic acid protein assay kit (BCA-1; Pierce Cat# 23225, Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s protocol. The absorbance was read at 562 nm on a spectrophotometer (Varioscan Flash, Thermo Fisher Scientific, Finland). Bovine serum albumin was used as standard. After protein measurement, all supernatants were stored at −80°C until for using in zymographic analyses.
Gelatin zymography
Zymography samples (n=5) were prepared from tissue supernatants by normalizing with equal protein concentration (25 µg) of each sample. After the samples were loaded into pre-cast 10% zymogram protein gels (Cat# EC6175, Thermo Fisher Scientific, Waltham, MA, USA) including 2 mg/mL gelatin, the gels were subjected to electrophoresis with SDS running buffer (Cat # LC2675, Thermo Fisher Scientific, Waltham, MA, USA) using electrophoresis system including XCell SureLock™ Mini-Cell apparatus (Hu X 2010) and power supply (Labnet Powerstation 200, Labnet International Inc., NJ, USA). Following electrophoresis, the gels were incubated in renaturing buffer (Cat # LC2670, Thermo Fisher Scientific, Waltham, MA, USA) for 30 mins at room temperature with gentle agitation. Then, the gels were equilibrated in developing buffer (Cat # LC 2671, Thermo Fisher Scientific, Waltham, MA, USA) for 30 mins at room temperature and incubated in fresh developing buffer overnight at 37°C. After the incubation period, gels were stained in SimplyBlue Safe stain solution (Cat # LC600, Thermo Fisher Scientific, Waltham, MA, USA) for 1 hr at room temperature. Gelatinase activity was detected as clear bands on a dark background. Images of the gels were photographed by using scanner (Epson WF-7525, CA, USA) and densitometric analysis of bands was performed using ImageJ software (ImageJ 1.46r, National Institute of Health, USA). Gelatin substrate digestion levels were quantified as relative proteinase activity (area × optical density/µg protein).
Statistical analyses
All contraction responses were normalized with dry weight (mg) of the rings. Values of maximum effect (Emax) and 50% effective concentration (EC50) were calculated for each cumulative concentration–response curve by nonlinear curve fitting GraphPad Prism (GraphPad Prism, 5.03, San Diego, CA, USA). Papaverine-induced relaxations were expressed as percentage (%) relaxation of single dose NA pre-contraction. Friedman test followed by Wilcoxon signed rank test was conducted using GraphPad Prism software to analyze the results from isolated organ chamber experiments and immunohistochemical analyses. Friedman test followed by Conover test was applied using R software (version 3.4.3) to compare the data from ROS measurements and zymography experiments. All data were expressed as median and range (min-max). p≤0.05 was considered statistically significant.
Results
Effects H2O2 and doxycycline on KCl (80 mM)-induced contractions
H2O2 (10 µM) incubation for 16 hrs resulted in a statistically significant reduction in KCl (80 mM)-induced maximum (Emax) contraction responses in HSV rings (Figure 1A). However, concomitant incubation of HSV rings with H2O2 (10 µM) and doxycycline (10 µM) normalized reduced Emax responses to KCl (Figure 1A).
Effects of H2O2 and doxycycline on cumulative noradrenaline (10−9–10−4M)-induced contractions
Incubation of HSV rings with 10 µM H2O2 for 16 hrs induced a statistically significant decrease in cumulative NA Emax contraction responses (Figure 1B). In contrast, incubation with 10 µM doxycycline plus 10 µM H2O2 significantly increased the Emax contractions to NA in HSV rings (Figure 1B). Incubation with neither H2O2 nor accompanying doxycycline caused any significant change in sensitivity to NA in HSV rings (Figure 1B, Table 1).
 | Table 1 Effects of H2O2 and doxycycline on pD2 values of contractile agents in HSV rings |
Effects of H2O2 and doxycycline on cumulative 5-HT (10−9–3×10−5M)-induced contractions
Incubation of HSV rings with 10 µM H2O2 for 16 hrs significantly reduced Emax contraction responses to cumulative 5-HT (Figure 1C). Incubation with 10 µM doxycycline did not change decreased contractile response to 5-HT by H2O2 (Figure 1C). Sensitivity to 5-HT was not altered by either H2O2 or H2O2 plus doxycycline incubation (Figure 1C, Table 1).
Effects of H2O2 and doxycycline on acetylcholine (10−6 M)-induced relaxations
To confirm endothelial denudation, Ach (10−6 M)-induced relaxations were assessed in HSV rings which were precontracted with 10−6 M NA. Endothelial denudation almost completely abolished relaxation response to acetylcholine in HSV rings. This evidence confirmed the denudation of endothelium (Figure 2A).
Effects of H2O2 and doxycycline on papaverine (10−4 M)-induced relaxations
Incubation with 10 µM H2O2 and/or 10 µM doxycycline for 16 hrs did not significantly affect maximum relaxation responses to papaverine in HSV rings which were precontracted with 10−6 M NA (Figure 2B).
Effects of H2O2 and doxycycline on ROS production
Superoxide anion and other ROS levels were detected by using lucigenin- and luminol-enhanced chemiluminescence, respectively. H2O2 incubation increased lucigenin-enhanced chemiluminescence and superoxide anion levels up to 4 times compared to control (Figure 3A). H2O2-incubation also increased luminol-enhanced chemiluminescence and ROS levels up to 5 times higher than in that of control group (Figure 3B). Incubation with H2O2 plus doxycycline significantly reduced superoxide anion and other ROS levels (Figure 3A and B).
Effects of H2O2 and doxycycline on MMP regulation
Gelatinase enzyme (MMP-2 and MMP-9) expressions were investigated by gelatin zymography in HSV grafts after H2O2 incubation and doxycycline treatment for 16 hrs. Zymography analyses revealed that H2O2 incubation increased both pro and active MMP-2 expression. However, H2O2 accompanied by doxycycline significantly attenuated pro MMP-2 expression and MMP-2 activity (Figure 4). Either expression or activity of MMP-9 enzyme were not observed in zymogram.
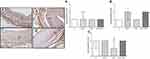
Expression of MMP-13 which is a MMP from the group of collagenases was examined in intima, media and adventitia layers of the HSV grafts immunohistochemically and evaluated by immunoscoring (Figure 5).
MMP-13 expression significantly increased in media layer of the HSV grafts from H2O2-induced group compared to control group. MMP-13 expression levels did not alter in any layer of HSV grafts from H2O2 plus Dox treatment groups (Figure 5). Only in Dox treatment group, MMP-13 expression significantly decreased compared to control group.
Discussion
In the present study, we provided evidence first time that doxycycline inhibited H2O2-induced oxidative stress, MMP-2 up-regulation/activation and contractile dysfunction in HSV grafts.
The results of our study revealed that incubation of HSV grafts with H2O2 for 16 hrs caused 5-fold and 4-fold increments in ROS and superoxide anion levels, respectively. Doxycycline significantly reduced ROS and superoxide anion levels. This result is consistent with the previous studies. In an earlier study, in 2K-1C renovascular hypertension model, doxycycline has been demonstrated to improve oxidative stress, endothelium-derived relaxation and to inhibit MMP-2 activation, vascular smooth muscle (VSM) cell proliferation.29 In another study, doxycycline was shown to normalize contractile dysfunction and impaired endothelium-dependent relaxation in diabetic rats. The authors suggested that these beneficial effects of doxycycline is related with its antioxidant features.21 Interestingly, a study conducted with Langendorff-isolated perfusion system in rat hearts, doxycycline contained cardioplegic solution was notified to decrease oxidant parameters, MMP-2 upregulation/activation and to improve cardiac function.22 Supportingly, our findings suggest that doxycycline may be a protective agent against to H2O2-induced oxidative stress damage by decreasing production of ROS in HSV grafts.
Oxidative stress can cause up-regulation/activation of MMPs in cardiovascular diseases.30,31 We have previously demonstrated that increased ROS levels may cause to increase MMP-13 expression in VSMCs.32
On the other hand, doxycycline has been shown to inhibit oxidative stress-induced MMP expression and activity in numerous studies. Zeydanli et al reported that doxycycline ameliorated vascular endothelial and contractile dysfunction in thoracic aorta by inhibiting oxidative stress and up-regulation of gelatinases in diabetic rats.21 Recently, inhibitory effects of doxycycline in mice model of abdominal aortic aneurysm (AAA) were attributed to antioxidant and MMP inhibitory effects of this agent.33 Similarly in a clinical study, doxycycline treatment was shown to cause higher plasma levels of TIMP-2 which, in turn, inversely correlate with 6-month infarct size and severity as well as LV remodelling.18 Consistently, we found that doxycycline inhibits H2O2-induced MMP-2 up-regulation/activity in HSV grafts. However, increased MMP-13 expression due to H2O2 was not changed by doxycycline. In contrast, doxycycline treatment was shown to decrease MMP-13 expression in rat osteoarthritis model.34 This discrepancy may be due to the fact that doxycycline treatment period for 16 hrs may not be sufficient to determine the alteration in MMP-13 expression. Furthermore, we have not observed visible pro or active MMP-9 bands in zymogram. This finding suggests that MMP-2 and -9 may have different time-dependent activity pattern from each other. Indeed, Katsu et al pointed out that MMP-9 expression/activity peaked at the 8th hour after induction with hemoglobin, but decreased to the 24th hour and returned to basal levels at the 72th hour, whereas MMP-2 up-regulation showed significant change at the 72th hour but not at the 24th hour.35
In our study, H2O2 incubation leads to a significant contractile dysfunction to contractile agents including KCl, NA and 5-HT in HSV grafts. A previous study suggested that H2O2-induced relaxation in bovine pulmonary arteries is due to NO releasing from endothelium, guanylate cyclase activation and increased sGMP.36 Also, it has been reported that H2O2 caused to increase the formation of arachidonic acid products, which may stimulate large conductance Ca2+-activated potassium channels (BKCa) and thus exert relaxing effects.37
On the other hand, the increased levels of ROS in the H2O2-induced HSV grafts suggest that the contractile dysfunction may cause H2O2-induced oxidative stress damage. Up-regulation of MMP-2 due to oxidative stress may contribute to this contractile dysfunction. Chew et al reported that gelatinases reduced contractile responses to phenylephrine and KCl by inhibiting the Ca2+ entry mechanism in rat aorta.38 In addition, MMPs may inhibit Ca2+ entry by affecting Ca2+ channels directly.9 MMP-2 may also directly activate K+ channels and cause VSM hyperpolarization. This may finally result in reduced Ca2+ influx through voltage-gated channels and contractile dysfunction.39 Alternatively, MMPs may degrade collagen and produce Arg-Gly-Asp (RGD)-containing peptides which may bind to αvβ3 integrin receptors and inhibit Ca2+ entry into VSM cells.9,40 Furthermore, MMPs may stimulate protease-activated receptors (PARs) and trigger signaling pathways that could lead to blockade of Ca2+ channels.9,41
On the other hand, the effects of doxycycline on vascular reactivity in HSV grafts were investigated for the first time herein. In our study, doxycycline ameliorated vascular dysfunction to KCl and NA but not 5-HT. Similarly, Zeydanli et al reported that oxidative stress decreased contractile responses to KCl and phenylephrine in diabetic rats. They also reported that doxycycline enhanced decreased contractile responses by exerting antioxidant, cell protective and free radical scavenging effects.21 Moreover, Cena et al reported that doxycycline inhibits LPS-induced MMP-2 activity and contractile dysfunction to phenylephrine and KCl in rat aorta.42 The researchers also propounded that MMP-2 proteolyzed calponin-1 and this may be responsible for contractile dysfunction in LPS-induced endotoxemic rats.43 In all these studies, the beneficial effects of doxycycline were based on MMP inhibition.
In cerebral artery of spontaneously hypertensive stroke-prone rats which is considered as an oxidative stress model, doxycycline treatment decreased MMP-2 expression/activity but did not change impaired contractile responses to 5-HT.44 Similarly, our result also suggests that doxycycline did not affect significantly the vascular elasticity and contraction mediated by 5-HT receptors in our experimental conditions.
We also investigated papaverine-induced relaxation response in HSV grafts and we found that neither H2O2 nor doxycycline changed relaxant response to papaverine. This results may indicate that contractile dysfunction and beneficial effects of doxycycline are directly related with contractile mechanisms of VSM. Consistently, in renovascular hypertensive rats while VSM cell proliferation, hyperplasia and MMP-2 up-regulation were observed, relaxation responses to sodium nitroprusside were not affected by doxycycline treatment.29
Our study has some limitations. Organ chamber experiments were performed with the small sample size (n=5) per group. Besides, inhibition of contractile and relaxant factors (PGI2, TXA2, EDHF, etc.) releasing from endothelium by mechanical endothelial denudation is one of the factors that limit the clinical translation of this study. Besides, since the model we applied in our study is an ex vivo tissue culture model, the HSV tissue is isolated from leukocyte and platelets in the circulation and activation of complement system and immune response. For this reason, the model excludes mentioned important factors in the clinic. Furthermore, HSV grafts were used in our study since it was the most widely used graft in CABG operations and allowed to obtain larger vessel segment. However, it may be useful to examine the reproducibility of similar results with left internal mammary artery (LIMA) grafts which are considered the gold standard in CABG operations in the context of further studies.
On the other hand, in our ex vivo study, oxidative stress was induced by H2O2 in HSV grafts for 16 hrs. HSV grafts were also treated with doxycycline for the same period of time. But, during CABG operation, oxidative stress may be induced so faster than that in our experimental conditions. Therefore, it can not be claimed that ex vivo and in vivo treatment periods should be the same.
To conclude, our results suggest that low-dose doxycycline may have beneficial effects on increased oxidative stress, MMP up-regulation/activation and contractile dysfunction in HSV grafts. Further studies on the effects of this agent especially focused on cardiovascular-renal system diseases and acute coronary syndromes related with increased oxidative stress and MMP expression would help to develop new therapeutical strategies.
Acknowledgments
The authors would like to thank to Prof. Bekir Ugur Ergur for contributions to immunohistochemical analyses, to Hatice Uluer for valuable statistical consultancy and to Scientific Research Foundation of Ege University for the financial support.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Al-Sabti HA, Al Kindi A, Al-Rasadi K, Banerjee Y, Al-Hashmi K, Al-Hinai A. Saphenous vein graft vs. radial artery graft searching for the best second coronary artery bypass graft. J Saudi Heart Assoc. 2013;25(4):247–254. doi:10.1016/j.jsha.2013.06.001
2. Wadey K, Lopes J, Bendeck M, George S. Role of smooth muscle cells in coronary artery bypass grafting failure. Cardiovasc Res. 2018;114(4):601–610. doi:10.1093/cvr/cvy021
3. Day J, Taylor K. The systemic inflammatory response syndrome and cardiopulmonary bypass. Int J Surg. 2005;3(2):129–140. doi:10.1016/j.ijsu.2005.04.002
4. Zakkar M, Guida G, Suleiman M, Angelini GD. Cardiopulmonary bypass and oxidative stress. Oxid Med Cell Longev. 2015;2:1–8. doi:10.1155/2015/189863
5. Orhan G, Yapici N, Yuksel M, et al. Effects of N-acetylcysteine on myocardial ischemia–reperfusion injury in bypass surgery. Heart Vessels. 2006;21(1):42–47. doi:10.1007/s00380-005-0873-1
6. Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006;69(3):562–573. doi:10.1016/j.cardiores.2005.12.002
7. Newby AC. Dual role of matrix metalloproteinases (matrixins) in intimal thickening and atherosclerotic plaque rupture. Physiol Rev. 2005;85(1):1–31. doi:10.1152/physrev.00048.2003
8. Bench TJ, Jeremias A, Brown DL. Matrix metalloproteinase inhibition with tetracyclines for the treatment of coronary artery disease. Pharmacol Res. 2011;64(6):561–566. doi:10.1016/j.phrs.2011.05.002
9. Benjamin MM, Khalil RA. Matrix metalloproteinase inhibitors as investigative tools in the pathogenesis and management of vascular disease. EXS. 2012;103:209–279. doi:10.1007/978-3-0348-0364-9_7
10. Newby AC. Metalloproteinases promote plaque rupture and myocardial infarction: a persuasive concept waiting for clinical translation. Matrix Biol. 2015;44:157–166. doi:10.1016/j.matbio.2015.01.015
11. Libby P. Mechanisms of acute coronary syndromes and their implications for therapy. N Engl J Med. 2013;368(21):2004–2013. doi:10.1056/NEJMra1216063
12. Quillard T, Tesmenitsky Y, Croce K, et al. Selective inhibition of matrix metalloproteinase-13 increases collagen content of established mouse atherosclerosis. Arterioscler Thromb Vasc Biol. 2011;31(11):2464–2472. doi:10.1007/s12012-013-9231-1
13. Golub L, Lee H-M, Ryan M, Giannobile W, Payne J, Sorsa T. Tetracyclines inhibit connective tissue breakdown by multiple non-antimicrobial mechanisms. Adv Dent Res. 1998;12(1):12–26. doi:10.1177/08959374980120010501
14. Peterson JT. Matrix metalloproteinase inhibitor development and the remodeling of drug discovery. Heart Fail Rev. 2004;9(1):63–79. doi:10.1023/B:HREV.0000011395.11179.af
15. Golledge J, Norman PE. Current status of medical management for abdominal aortic aneurysm. Atherosclerosis. 2011;217(1):57–63. doi:10.1016/j.atherosclerosis.2011.03.006
16. Villarreal FJ, Griffin M, Omens J, Dillmann W, Nguyen J, Covell J. Early short-term treatment with doxycycline modulates postinfarction left ventricular remodeling. Circulation. 2003;108(12):1487–1492. doi:10.1161/01.CIR.0000089090.05757.34
17. Kormi I, Alfakry H, Tervahartiala T, Pussinen PJ, Sinisalo J, Sorsa T. The effect of prolonged systemic doxycycline therapy on serum tissue degrading proteinases in coronary bypass patients: a randomized, double-masked, placebo-controlled clinical trial. Inflamm Res. 2014;63(5):329–334. doi:10.1007/s00011-013-0704-2
18. Cerisano G, Buonamici P, Gori AM, et al. Matrix metalloproteinases and their tissue inhibitor after reperfused ST-elevation myocardial infarction treated with doxycycline. Insights from the TIPTOP trial. Int J Cardiol. 2015;197:147–153. doi:10.1016/j.ijcard.2015.06.024
19. Castro MM, Rizzi E, Ceron CS, et al. Doxycycline ameliorates 2K-1C hypertension-induced vascular dysfunction in rats by attenuating oxidative stress and improving nitric oxide bioavailability. Nitric Oxide. 2012;26(3):162–168. doi:10.1016/j.niox.2012.01.009
20. Antonio RC, Ceron CS, Rizzi E, Coelho EB, Tanus-Santos JE, Gerlach RF. Antioxidant effect of doxycycline decreases MMP activity and blood pressure in SHR. Mol Cell Biochem. 2014;386(1–2):99–105. doi:10.1007/s11010-013-1848-7
21. Zeydanli EN, Kandilci HB, Turan B. Doxycycline ameliorates vascular endothelial and contractile dysfunction in the thoracic aorta of diabetic rats. Cardiovasc Toxicol. 2011;11(2):134–147. doi:10.1007/s12012-011-9107-1
22. Ozcinar E, Okatan EN, Tuncay E, Eryilmaz S, Turan B. Improvement of functional recovery of donor heart following cold static storage with doxycycline cardioplegia. Cardiovasc Toxicol. 2014;14(1):64–73. doi:10.1007/s12012-013-9231-1
23. Banerjee D, Madhusoodanan UK, Nayak S, Jacob J. Urinary hydrogen peroxide: a probable marker of oxidative stress in malignancy. Clin Chim Acta. 2003;334(1–2):205–209. doi:10.1016/S0009-8981(03)00236-5
24. Lacy F, Kailasam MT, O’Connor DT, Schmid-Schonbein GW, Parmer RJ. Plasma hydrogen peroxide production in human essential hypertension: role of heredity, gender, and ethnicity. Hypertension (Dallas, Tex: 1979). 2000;36(5):878–884.
25. Hummitzsch L, Zitta K, Berndt R, et al. Doxycycline protects human intestinal cells from hypoxia/reoxygenation injury: implications from an in-vitro hypoxia model. Exp Cell Res. 2017;353(2):109–114. doi:10.1016/j.yexcr.2017.03.017
26. Gokce G, Arun M. Ergothioneine produces relaxation in isolated rat aorta by inactivating superoxide anion. Eur Rev Med Pharmacol Sci. 2014;18(21):3339–3345.
27. Guzeloglu M, Reel B, Atmaca S, Bagrıyanık A, Hazan E. The effects of PPARγ agonist rosiglitazone on neointimal hyperplasia in rabbit carotid anastomosis model. J Cardiothorac Surg. 2012;57(7):1–8. doi:10.1186/1749-8090-7-57
28. Ogut D, Reel B, Korkmaz CG, Arun MZ, Micili SC, Ergur BU. Doxycycline down-regulates matrix metalloproteinase expression and inhibits NF-κB signaling in LPS-induced PC3 cells. Folia Histochem Cytobiol. 2016;54(4):171–180. doi:10.5603/FHC.a2016.0022
29. Castro MM, Rizzi E, Figueiredo-Lopes L, et al. Metalloproteinase inhibition ameliorates hypertension and prevents vascular dysfunction and remodeling in renovascular hypertensive rats. Atherosclerosis. 2008;198(2):320–331. doi:10.1016/j.atherosclerosis.2007.10.011
30. Newby AC. Matrix metalloproteinases regulate migration, proliferation, and death of vascular smooth muscle cells by degrading matrix and non-matrix substrates. Cardiovasc Res. 2006;69(3):614–624. doi:10.1016/j.cardiores.2005.08.002
31. Kelly PJ, Morrow JD, Ning M, et al. Oxidative stress and matrix metalloproteinase-9 in acute ischemic stroke: the Biomarker Evaluation for Antioxidant Therapies in Stroke (BEAT-Stroke) study. Stroke. 2008;39(1):100–104. doi:10.1161/STROKEAHA.107.488189
32. Arun MZ, Reel B, Sala-Newby GB, et al. Zoledronate upregulates MMP-9 and -13 in rat vascular smooth muscle cells by inducing oxidative stress. Drug Des Devel Ther. 2016;10:1453–1460. doi:10.2147/DDDT.S103124
33. Yu M, Chen C, Cao Y, Qi R. Inhibitory effects of doxycycline on the onset and progression of abdominal aortic aneurysm and its related mechanisms. Eur J Pharmacol. 2017;811:101–109. doi:10.1016/j.ejphar.2017.05.041
34. Lee -H-H, Lin T-H, Su H-M, et al. Recurrent thrombosis in a case of coronary ectasia with large thrombus burden successfully treated by adjunctive warfarin therapy. Acta Cardiol Sin. 2013;29(5):462–466.
35. Katsu M, Niizuma K, Yoshioka H, Okami N, Sakata H, Chan PH. Hemoglobin-induced oxidative stress contributes to matrix metalloproteinase activation and blood–brain barrier dysfunction in vivo. J Cereb Blood Flow Metab. 2010;30(12):1939–1950. doi:10.1038/jcbfm.2010.45
36. Burke TM, Wolin MS. Hydrogen peroxide elicits pulmonary arterial relaxation and guanylate cyclase activation. Am J Physiol Heart Circ Physiol. 1987;252(4):H721–H732. doi:10.1152/ajpheart.1987.252.4.H721
37. Barlow RS, White RE. Hydrogen peroxide relaxes porcine coronary arteries by stimulating BKCa channel activity. Am J Physiol Heart Circ Physiol. 1998;275(4):H1283–H1289. doi:10.1152/ajpheart.1998.275.4.H1283
38. Chew DK, Conte MS, Khalil RA. Matrix metalloproteinase-specific inhibition of Ca2+ entry mechanisms of vascular contraction. J Vasc Surg. 2004;40(5):1001–1010. doi:10.1016/j.jvs.2004.08.035
39. Raffetto JD, Ross RL, Khalil RA. Matrix metalloproteinase 2-induced venous dilation via hyperpolarization and activation of K+ channels: relevance to varicose vein formation. J Vasc Surg. 2007;45(2):373–380. doi:10.1016/j.jvs.2006.10.041
40. Waitkus-Edwards KR, Martinez-Lemus LA, Wu X, et al. α4β1 Integrin activation of L-type calcium channels in vascular smooth muscle causes arteriole vasoconstriction. Circ Res. 2002;90(4):473–480. doi:10.1161/hh0402.105899
41. Macfarlane SR, Seatter MJ, Kanke T, Hunter GD, Plevin R. Proteinase-activated receptors. Pharmacol Rev. 2001;53(2):245–282.
42. Cena JJ, Lalu MM, Cho WJ, et al. Inhibition of matrix metalloproteinase activity in vivo protects against vascular hyporeactivity in endotoxemia. Am J Physiol Heart Circ Physiol. 2009;298(1):H45–H51. doi:10.1152/ajpheart.00273.2009
43. Castro MM, Cena J, Cho WJ, Walsh MP, Schulz R. Matrix metalloproteinase-2 proteolysis of calponin-1 contributes to vascular hypocontractility in endotoxemic rats. Arterioscler Thromb Vasc Biol. 2012;32(3):662–668. doi:10.1161/ATVBAHA.111.242685
44. Pires PW, Rogers CT, McClain JL, Garver HS, Fink GD, Dorrance AM. Doxycycline, a matrix metalloprotease inhibitor, reduces vascular remodeling and damage after cerebral ischemia in stroke-prone spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2011;301(1):H87–H97. doi:10.1152/ajpheart.01206.2010
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.