Back to Journals » Cancer Management and Research » Volume 13
Low Density Lipoprotein Receptor (LDLR) and 3-Hydroxy-3-Methylglutaryl Coenzyme a Reductase (HMGCR) Expression are Associated with Platinum-Resistance and Prognosis in Ovarian Carcinoma Patients
Authors Huang X , Wei X, Qiao S, Zhang X, Li R, Hu S, Mao H, Liu P
Received 23 September 2021
Accepted for publication 22 November 2021
Published 6 December 2021 Volume 2021:13 Pages 9015—9024
DOI https://doi.org/10.2147/CMAR.S337873
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Chien-Feng Li
Xueyao Huang,1,2 Xuan Wei,1 Sijing Qiao,1,2 Xue Zhang,1,2 Rui Li,1 Shunxue Hu,3 Hongluan Mao,1,2 Peishu Liu1,2
1Department of Obstetrics and Gynecology, Qilu Hospital of Shandong University, Jinan, Shandong, 250012, People’s Republic of China; 2Shandong University, Jinan, Shandong, 250012, People’s Republic of China; 3Department of Pathology, Qilu Hospital of Shandong University, Jinan, Shandong, 250012, People’s Republic of China
Correspondence: Peishu Liu; Hongluan Mao
Department of Obstetrics and Gynecology, Qilu Hospital of Shandong University, No. 107 Wen Hua Xi Road, Jinan, Shandong, 250012, People’s Republic of China
Tel +8618560081988
; Tel +8618560082027
Email [email protected]; [email protected]
Purpose: The efficacy of post-surgery platinum-based chemotherapy, the primary choice for the treatment of ovarian cancer (OC), is greatly reduced by the development of drug-resistance. In this study, we investigated the association of expression low-density lipoprotein receptor (LDLR) and 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR), two cholesterol metabolism-related proteins, in OC tissues and chemoresistance and patient prognosis.
Methods: Survival analysis using LDLR and HMGCR expression in the ovarian cancer patients using the dataset of Cancer Genome Atlas (TCGA) and Gene Expression Omnibus (GEO) was carried out online. A retrospective study was performed on 65 patients who had undergone surgery for ovarian cancer. In addition, patients were divided into 2 groups: platinum resistance group and platinum sensitivity group. Serum lipid metabolism data were collected and analyzed. Protein expressions of LDLR and HMGCR in ovarian cancer tissue were detected by immunohistochemistry.
Results: Online survival analysis showed that patients with higher LDLR expression had poorer prognosis than those with lower LDLR expression in ovarian cancer cells, while a higher HMGCR expression was associated with better OC prognosis. Overall survival (OS) and disease-free survival (DFS) were lower in patients with higher LDLR levels (OS: P=0.046, DFS: P=0.009). Platinum-resistant patients had higher levels of low-density lipoprotein (LDL) and cholesterol in serum as compared with platinum-sensitive patients (P< 0.001). Immunohistochemistry showed that LDLR expression was high and HMGCR was low in platinum-resistant patients.
Conclusion: The expression of LDLR and HMGCR proteins, involved in the regulation of cholesterol metabolism and the plasma LDL and cholesterol levels were significantly different in platinum-resistant and platinum-sensitive ovarian cancer patients. We postulate that cholesterol metabolic reprogramming might play a role in platinum resistance in ovarian cancer.
Keywords: LDLR, HMGCR, ovarian cancer, platinum resistance, cholesterol metabolism
Introduction
Ovarian cancer is the most lethal gynecological malignancy.1 Despite great advances in surgical techniques and adjuvant therapy, the clinical prognosis of ovarian cancer remains poor, with a 5-year survival rate of only 49%.2 A majority of the OC patients are diagnosed at later stages, where surgery followed by chemotherapy with platinum-based drugs is the primary choice for the treatment.3 Although 60%–90% of OC patients respond well to platinum-based drugs,4 median progression-free survival of patients with advanced disease is only 18 months, for which chemoresistance is largely to be blamed.5 Although PARP inhibitors have shown good clinical efficacy in women with BRCA1/2 mutations, most targeted therapies have still not made substantial progress.6,7 Therefore, elucidating the mechanism behind the development of resistance to platinum-based drugs is important for increasing the efficacy of OC treatment.
Recent studies have shown that the development of platinum-resistance is a multifactorial phenomenon, involving alterations in the tumor microenvironment, increased DNA repair, cancer stem cells activity, and metabolic reprogramming.8 In a variety of cancer cells, lipid uptake, storage, and lipogenesis are increased and contributed to rapid tumor growth.9 Cholesterol is an essential component of mammalian cell membranes and plays an important role in the regulation of the cell cycle.10 Rapidly growing tumor cells require higher amounts of cholesterol and several studies have suggested that cholesterol metabolism plays an important role in the progression of malignancies and the prognosis of cancer patients.11–13 Reprogramming of cholesterol metabolic pathways has been observed in many cancers,11–14 including ovarian cancer15 and breast cancer caused due to mutations in BRCA1 and BRCA2 genes.16 Recent studies have also shown that reprogramming of cholesterol metabolic pathways might be involved in chemoresistance in ovarian cancer.17,18 Nevertheless, the specific mechanism by which cholesterol metabolic pathway reprogramming causes platinum resistance remains unclear.
Endogenous cholesterol synthesis and exogenous cholesterol uptake synergistically meet the cholesterol requirement of the cells. It is known that chemo-resistant cells down-regulate cholesterol biosynthesis and rely primarily on the exogenous cholesterol for meeting their needs.17 3-Hydroxy-3-methylglutaryl coenzyme A reductase (HMGCR) is a rate-limiting enzyme in endogenous cholesterol biosynthesis and has been implicated in tumor cell growth and proliferation. Cholesterol is transported in the plasma in the form of lipoprotein complexes. Low-density lipoprotein (LDL) contains about 80% lipids, most of which are cholesterol and cholesteryl esters, and 20% apolipoprotein B-100.19 LDL binds to membrane-bound low-density lipoprotein receptor (LDLR) and is internalized. It is released from LDLR in the acidic environment of late endosome/lysosome, where free cholesterol is also released.9 It has been reported that LDLR overexpression in the tumor cells is associated with poor prognosis in patients with epithelial ovarian cancer (EOC) undergoing platinum-based therapy.20 Overall, these studies indicate that lipid metabolism may be involved in drug resistance in cancer cells. In this study, we have investigated the association of LDLR and HMGCR protein expression and the plasma levels of LDL and cholesterol with the clinical prognosis of OC patients.
Methods and Materials
Materials
The data of 1109 patients who underwent surgery for ovarian cancer at Qilu Hospital of Shandong University, China, from December 2013 to December 2018 were screened for the study. Patients who had received neo-adjuvant chemotherapy, diagnosed with borderline ovarian neoplasms, non-high grade serous cancer or secondary ovarian malignant tumor and those with concurrent dyslipidemia were excluded. After screening, a total of 65 patients were selected for inclusion in the study. Reports of laboratory investigations performed pre- and post-surgery and pre- and post-chemotherapy (plasma lipid profile, glucose, CA125), and data of pathological parameters and chemotherapy regimens were obtained. Lipid profile parameters were measured by an automatic biochemistry analyzer (Shandong Yi-Cheng Medical Technology Co. LTD., Ji-Nan, Shandong Province, China). All laboratory tests were performed and reported as per the standard quality control procedures of the laboratory of the Shandong University. Tissue samples of patients were obtained from the Department of Pathology of Qilu Hospital, Shandong University for paraffin sectioning. The study was approved by the Medical Ethics Committee of Qilu Hospital of Shandong University and informed consents were obtained from all patients included in the study. The patients were followed-up by telephone.
Patients were divided into two groups, based on their response to platinum-based chemotherapy: “platinum-resistant” which included 34 patients and “platinum-sensitive” which included 31 patients. Platinum-resistance was defined as progression-free survival lasting less than six months since last round of chemotherapy, while platinum-sensitivity was defined as more than 12 months of progression-free survival since last round of chemotherapy as per Gynecologic Cancer Inter-Group (GCOG) consensus statement.21
Survival Analysis
Online survival analyses were performed to determine the relationship between LDLR and HMGCR gene expression and the prognosis of ovarian cancer patients using the KM Plotter software (http://kmplot.com/analysis/index.php?p=service). The ovarian cancer patients from the Cancer Genome Atlas (TCGA) and the Gene Expression Omnibus (GEO) datasets were analyzed. The treatment groups included the patients who were treated with platinum-based chemotherapy. Survival time was measured by progression-free survival or post-progression survival and other settings in the KM plotter were set at default. Next, Kaplan–Meier survival analysis was performed to assess the association between the expression levels of LDLR and HMGCR protein with overall survival (OS) and disease-free survival (DFS) in ovarian cancer. The OS from the date of diagnosis to death, or last follow-up, was measured. The DFS from the date of diagnosis to recurrence, death or last follow-up was determined.
Immunohistochemistry
Expressions of LDLR and HMGCR were determined by immunohistochemistry using 4-μm-thick sections that were stained using a monoclonal anti-LDLR (diluted 1:500 in phosphate buffer saline (PBS)) produced in rabbit (ab52818, Abcam, Cambridge, UK) and monoclonal anti-HMGCR (diluted 1:50 in PBS) produced in mouse (ab242315, Abcam, Cambridge, UK). The slices were dewaxed with xylene and rehydrated in ethanol. The endogenous peroxidase activity was blocked by hydrogen peroxide treatment, and the nonspecific antigens were blocked by goat serum (1:10, Zhongshan Jin-Qiao Biotechnology, Beijing, China). After overnight incubation with primary antibodies at 4°C, the sections were treated with ready-to-use biotin-labeled goat anti-rabbit IgG polymer, followed by horseradish peroxidase-conjugated streptavidin, enabling positive signal detection with DAB substrate. Except for antibodies, all the above reagents were purchased from Zhongshan Jin-Qiao Biotechnology, Beijing, China. The stained tissue specimens were evaluated and scored by two gynecological pathologists in a blind manner. Brown signal was defined as strong positive staining; yellowish brown as moderate staining; and light-yellow reaction as weak staining. The staining intensity was quantified was as follows: 0 = no staining; 1 = weak staining; 2 = moderate staining; 3 = strong staining. The percentage of tumor cells stained was scored as follows: 0 = no staining; 1 = ≤10% of positive tumor cells; 2 = 11–50% positive tumor cells; 3 = 51–80% positive tumor cells; 4 = ≥81% positive tumor cells. The staining score was defined as the sum of staining intensity grading and staining percentage grading and ranged from 0 to 7. For statistical evaluation, the staining score was again classified as: 0 = negative; 1–5 = low expression; 6–7 = high expression.
Statistical Analysis
All experiments were repeated at least three times. Statistical analyses were performed using GraphPad Prism 8 and IBM SPSS 21.0 software. A Chi-squared test was used for categorical data, and Student’s t-tests and one-way ANOVA analyses were used for continuous data. OS and DFS were estimated by Kaplan–Meier method and analyzed with the Log-rank Mantel-Cox test. Data are presented as the mean ± SD unless otherwise stated. Statistical significance was defined as P<0.05.
Results
The Characteristics of Patients
Medical histories of 1109 patients were screened (Figure 1), out of which 621 patients were excluded for various reasons. Ninety-two patients received neoadjuvant chemotherapy, 57 patients had secondary surgery, 186 patients were diagnosed with benign, borderline, or non-epithelial ovarian neoplasms, and the chemotherapy regimen of 286 patients was not available. Out of the remaining 488, only 47 fitted the criteria of exhibiting platinum-sensitivity and 48 platinum-resistance. Further, 16 patients were lost to follow-up, and pathological tissue sections could not be obtained for 14 patients. A total of 65 patients, 31 platinum-sensitive and 34 platinum-resistant were finally included in the study.
The average age at diagnosis for both groups of patients was above 50 years. Interestingly, the mean age (years) of menarche in platinum-sensitive patients was significantly higher (15.93±2.97) than that of platinum-resistant patients (14.02±4.11) (p = 0.013). A majority of cases were diagnosed at FIGO III stage in both platinum-resistant (82.4%) and sensitive group (87.1%). The mortality rate (72.2%) and recurrence rate (50%) of the platinum-resistant group were significantly higher than that of platinum-sensitive group. Other clinical features are presented in Table 1.
 |
Table 1 Clinicopathologic Characteristics of Platinum-Resistant and Platinum-Sensitive Patients |
Upregulated LDLR and Downregulated HMGCR Were Associated with Poor Prognosis
To determine the relationship between LDLR and HMGCR expression and prognosis (measured by progression-free survival or post-progression survival), online survival analyses were performed using the OC patients’ dataset of the Cancer Genome Atlas (TCGA) and Gene Expression Omnibus (GEO) datasets. The two datasets with relatively large numbers of patients treated with platinum-containing chemotherapy (GSE14764 and GSE26193) were selected for survival analysis. In TCGA dataset, patients with tumors expressing high levels of LDLR had poorer survival (Figure 2C). In contrast, high expression of HMGCR was associated with better prognosis (Figure 2F). Similarly, in GEO dataset, patients with tumors expressing high levels of LDLR and low levels of HMGCR had poorer survival (Figure 2A, B, D and E). Kaplan–Meier analysis and Log rank test were performed to assess the relationship of LDLR and HMGCR protein expression with OS and DFS in OC patients. OC patients expressing high levels of LDLR had poorer DFS than patients expressing low levels of LDLR (Figure 3B, p = 0.009). A similar tendency was observed for OS (Figure 3A, p = 0.046). However, no significant association between the expression of HMGCR and DFS or OS was found (Figure 3C and D).
 |
Figure 3 High levels of LDLR were associated with poorer DFS and OS (A and B). Expression levels of HMGCR were not associated with DFS and OS (C and D). |
Serum LDL and Cholesterol Levels Were Elevated in Platinum-Resistant Patients
The t-test was used to determine significant differences in the levels of LDL and cholesterol in platinum-resistant and platinum-sensitive patients (Figure 4). The mean LDL and cholesterol levels (mmol/L) of 3.01±0.67 and 5.01±0.82, respectively, in platinum-sensitive patients were significantly lower than the corresponding values of 3.34±1.01 and 5.38±1.11 in platinum-resistant patients (p < 0.001) (Figure 4A and B, Table 2). The mean TG level (mmol/L) of 1.44±0.84 in platinum-sensitive patients was also significantly different from the TG level of 1.69±0.91 in platinum-resistant patients (Figure 4C, p = 0.001). However, the levels of HDL were not statistically different between the two group (Figure 4D).
 |
Table 2 Serum Lipid Levels (mmol/L) in Platinum-Resistant and Platinum-Sensitive OC Patients |
Immunohistochemical Analysis of LDLR and HMGCR Protein Expression Levels
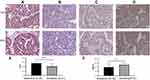
The LDLR and HMGCR staining was observed in both resistant and sensitive cancer tissues. LDLR was located on the plasma membrane and HMGCR in the cytoplasm with slightly higher staining in the endoplasmic reticulum. The LDLR was highly expressed (Figure 5A), whereas HMGCR expression was low in patients with platinum resistance (Figure 5C). A total of 22 (64.7%) resistant patients showed high expression of LDLR and 27 (79.4%) showed low expression of HMGCR. In platinum-sensitive patients, the results of immunohistochemistry were the opposite (Figure 5B and D). Statistical analysis showed that the staining score of LDLR was higher in platinum-resistant patients (Figure 5E, p < 0.0001), and that of HMGCR was lower in platinum-resistant patients (Figure 5F, p = 0.0012).
Discussion
Ovarian cancer is the most lethal malignancy of the female reproductive system.1 Although 60–90% of ovarian cancer patients respond well to first-line platinum chemotherapy,4 the prognosis is still poor due to high incidence of drug resistance.5 The studies conducted in the past three decades have implicated factors such as altered tumor microenvironment, increased DNA repair, defects in the execution of apoptotic pathways, and cell death programs in the development of platinum resistance.8 However, our understanding of the development of platinum-resistance is still incomplete and effective means of countering platinum resistance are still not available.8,22 Therefore, it is of great significance to explore the mechanisms of platinum resistance for improving the prognosis of cancer patients.
Recent studies have shown an association between hypercholesterolemia and poor prognoses of various malignant tumors, such as breast cancer, thyroid cancer, and liver cancer.11–13 Gustbee et al reported that high HMGCR expression is associated with less aggressive breast cancer.23 He et al showed that treatment of hepatocellular carcinoma (HCC) with lipopolysaccharide (LPS) increased the intracellular cholesterol concentrations by upregulating LDLR and HMGCR, and higher cholesterol concentrations further promoted LPS/NF-κB induced pro-inflammatory state.13 Revilla et al showed that LDLR was up-regulated, while HMGCR was down-regulated in more aggressive thyroid tumors, increasing LDL uptake and intracellular cholesterol concentration.12 These studies support the idea that cholesterol metabolic reprogramming is involved in tumorigenesis and tumor progression. In this study, we found that high expression of LDLR was associated with poor prognosis, while high expression of HMGCR was associated with better prognosis in OC patients, which is similar to the results of the above study. Based on the observations of this study, we speculate that altered cholesterol metabolism is related to platinum resistance in ovarian cancer. However, the sample number of our study is small, and further large-sample studies are still needed to clarify the relationship between the expression of LDLR and HMGCR and the prognosis of patients.
To support this hypothesis, we have compared the differences in plasma lipid profiles between platinum-resistant and platinum-sensitive ovarian cancer patients. The results of this study showed that serum LDL and cholesterol levels in platinum-resistant patients were significantly higher than those in platinum-sensitive patients, indicating that cholesterol accumulation in the cancer cells might be one of the risk factors for the development of platinum-resistance in ovarian cancer patients. Cholesterol is an essential component of the mammalian cell membrane required for maintaining the integrity and fluidity of the cell membrane.24 A study by Wu et al has shown that lung adenocarcinoma patients with hypercholesterolemia are prone to developing resistance to cisplatin.25 The mechanism might involve a decrease in the permeability and fluidity of the membranes of the cancer cells due to high cholesterol content, blocking the entry of drugs.26,27 Studies have also suggested that chemoresistance may be attributed to cholesterol-induced ATP binding cassette subfamily G member 2 (ABCG2) overexpression,25 which allows cells to escape from the apoptosis induced by endoplasmic reticulum stress.28 We also found that triglyceride levels in platinum-resistant patients were higher than those in platinum-sensitive patients. Hypertriglyceridemia has been shown to be associated with an increased risk for disease recurrence in prostate cancer,29 and a positive association has been found between triglycerides and the risk of ovarian cancer.30 These observations indicate that TG may play an important role in the progression of various types of cancers, but their role in platinum resistance is not clear, and further studies are needed in this area.
Intracellular cholesterol homeostasis is maintained by cholesterol synthesis, uptake, transportation and conversion.31 HMGCR is a key rate-limiting enzyme in cholesterol biosynthesis pathway. Mammalian cells absorb LDL from the blood via LDLR and the cholesteryl ester in the LDL is hydrolyzed to release the free cholesterol in late endosomes/lysosome.9 The rapid growth of tumors require a large amount of cholesterol, and tumor cells reprogram cholesterol metabolism pathways to meet the cholesterol requirement for rapid tumor growth.15 Our results indicate that cholesterol metabolism reprogramming may be involved in the development of platinum-resistance in OC. Immunohistochemistry showed a high LDLR expression and a low HMGCR expression in the tumor cells of drug-resistant patients. It is likely that the upregulation of LDLR expression in platinum-resistant ovarian cancer cells leads to increased uptake of exogenous cholesterol, while downregulation of HMGCR expression decreases endogenous cholesterol synthesis. This allows the tumor cells to obtain the cholesterol required for rapid growth without expending large amounts of cellular energy. Higher levels of cholesterol and LDL were observed in the plasma of platinum-resistant patients compared with platinum-sensitive patients. LDL is synthesized by the liver and is absorbed via LDLR on the liver and extrahepatic cells.32 Circulating cholesterol mainly comes from intestinal absorption and endogenous synthesis (hepatic and peripheral tissues).33 Although platinum-resistant ovarian cancer cells express LDLR at higher levels, the increased uptake of cholesterol by these cells is not expected to significantly affect the concentration of LDL cholesterol in plasma. Further studies are needed to understand the mechanism and significance of higher cholesterol levels in the plasma of platinum-resistant OC patients.
Conclusion
The expression of LDLR and HMGCR proteins, which are involved in the regulation of cholesterol metabolism and the levels of LDL and cholesterol were significantly different in platinum-resistant and platinum-sensitive ovarian cancer patients. We postulate that cholesterol metabolic reprogramming might play a role in platinum resistance in ovarian cancer. This metabolic reprogramming may allow the cancer cells to meet the elevated energy and cholesterol requirements associated with rapid tumor growth.
Ethics Statement
The study was planned in accordance with the Declaration of Helsinki and approval from the Medical Ethics Committee of Qilu Hospital of Shandong University was obtained (Ethics Approval No. is KYLL-2021107-066-1). All patients provided informed consent before data collection, and we ensured that the data were anonymized before analysis.
Funding
This work was supported by the National Natural Science Foundation of China (grant nos. 81702559 and 81902657), China Postdoctoral Science Fund (nos. 21510077311145 and 21300076311047) and Science Foundation of Qilu Hospital of Shandong Province.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Stanojevic Z, Djordjevic B, Pajovic SB, Zivanov-Curlis J, Najman S. Molecular pathogenesis of borderline and invasive ovarian tumors. J Buon. 2009;14(1):7–18.
2. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN Estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
3. Agarwal R, Kaye SB. Ovarian cancer: strategies for overcoming resistance to chemotherapy. Nat Rev Cancer. 2003;3(7):502–516. doi:10.1038/nrc1123
4. Markman M, Rothman R, Hakes T, et al. Second-line platinum therapy in patients with ovarian cancer previously treated with cisplatin. J Clin Oncol. 1991;9(3):389–393. doi:10.1200/JCO.1991.9.3.389
5. Zhou F, Yang X, Zhao H, et al. Down-regulation of OGT promotes cisplatin resistance by inducing autophagy in ovarian cancer. Theranostics. 2018;8(19):5200–5212. doi:10.7150/thno.27806
6. Oza AM, Matulonis UA, Malander S, et al. Quality of life in patients with recurrent ovarian cancer treated with niraparib versus placebo (ENGOT-OV16/NOVA): results from a double-blind, Phase 3, randomised controlled trial. Lancet Oncol. 2018;19(8):1117–1125. doi:10.1016/S1470-2045(18)30333-4
7. Friedlander M, Gebski V, Gibbs E, et al. Health-related quality of life and patient-centred outcomes with olaparib maintenance after chemotherapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT Ov-21): a placebo-controlled, phase 3 randomised trial. Lancet Oncol. 2018;19(8):1126–1134. doi:10.1016/S1470-2045(18)30343-7
8. Binju M, Padilla MA, Singomat T, et al. Mechanisms underlying acquired platinum resistance in high grade serous ovarian cancer - a mini review. Biochim Biophys Acta Gen Subj. 2019;1863(2):371–378. doi:10.1016/j.bbagen.2018.11.005
9. Cheng C, Geng F, Cheng X, Guo D. Lipid metabolism reprogramming and its potential targets in cancer. Cancer Commun. 2018;38(1):27. doi:10.1186/s40880-018-0301-4
10. Sarkar P, Rao BD, Chattopadhyay A. Cell cycle dependent modulation of membrane dipole potential and neurotransmitter receptor activity: role of membrane cholesterol. ACS Chem Neurosci. 2020;11(18):2890–2899. doi:10.1021/acschemneuro.0c00499
11. Dos Santos CR, Domingues G, Matias I, et al. LDL-cholesterol signaling induces breast cancer proliferation and invasion. Lipids Health Dis. 2014;13:16. doi:10.1186/1476-511X-13-16
12. Revilla G, Pons MP, Baila-Rueda L, et al. Cholesterol and 27-hydroxycholesterol promote thyroid carcinoma aggressiveness. Sci Rep. 2019;9(1):10260. doi:10.1038/s41598-019-46727-2
13. He M, Zhang W, Dong Y, et al. Pro-inflammation NF-kappaB signaling triggers a positive feedback via enhancing cholesterol accumulation in liver cancer cells. J Exp Clin Cancer Res. 2017;36(1):15. doi:10.1186/s13046-017-0490-8
14. Guo D, Reinitz F, Youssef M, et al. An LXR agonist promotes glioblastoma cell death through inhibition of an EGFR/AKT/SREBP-1/LDLR-dependent pathway. Cancer Discov. 2011;1(5):442–456. doi:10.1158/2159-8290.CD-11-0102
15. Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11(2):85–95. doi:10.1038/nrc2981
16. Ramadan S, Arm J, Silcock J, et al. Lipid and metabolite deregulation in the breast tissue of women carrying BRCA1 and BRCA2 Genetic Mutations. Radiology. 2015;275(3):675–682. doi:10.1148/radiol.15140967
17. Criscuolo D, Avolio R, Calice G, et al. Cholesterol Homeostasis Modulates Platinum Sensitivity in Human Ovarian Cancer. Cells. 2020;9:4. doi:10.3390/cells9040828
18. Kim S, Lee M, Dhanasekaran DN, Song YS. Activation of LXRɑ/β by cholesterol in malignant ascites promotes chemoresistance in ovarian cancer. BMC Cancer. 2018;18(1):1232. doi:10.1186/s12885-018-5152-5
19. Klein-Szanto AJP, Bassi DE. Keep recycling going: new approaches to reduce LDL-C. Biochem Pharmacol. 2019;164:336–341. doi:10.1016/j.bcp.2019.04.003
20. Chang WC, Wang HC, Cheng WC, et al. LDLR-mediated lipidome-transcriptome reprogramming in cisplatin insensitivity. Endocr Relat Cancer. 2020;27(2):81–95. doi:10.1530/ERC-19-0095
21. Stuart GC, Kitchener H, Bacon M, et al. 2010 Gynecologic Cancer InterGroup (GCIG) consensus statement on clinical trials in ovarian cancer: report from the Fourth Ovarian Cancer Consensus Conference. Int J Gynecol Cancer. 2011;21(4):750–755. doi:10.1097/IGC.0b013e31821b2568
22. Galluzzi L, Senovilla L, Vitale I, et al. Molecular mechanisms of cisplatin resistance. Oncogene. 2012;31(15):1869–1883. doi:10.1038/onc.2011.384
23. de Gonzalo-calvo D, Lopez-Vilaro L, Nasarre L, et al. Intratumor cholesteryl ester accumulation is associated with human breast cancer proliferation and aggressive potential: a molecular and clinicopathological study. Bmc Cancer;2015. 15. doi:10.1186/s12885-015-1014-6
24. Espinosa G, Lopez-Montero I, Monroy F, Langevin D. Shear rheology of lipid monolayers and insights on membrane fluidity. Proc Natl Acad Sci U S A. 2011;108(15):6008–6013. doi:10.1073/pnas.1018572108
25. Wu Y, Si R, Tang H, et al. Cholesterol reduces the sensitivity to platinum-based chemotherapy via upregulating ABCG2 in lung adenocarcinoma. Biochem Biophys Res Commun. 2015;457(4):614–620. doi:10.1016/j.bbrc.2015.01.035
26. Rivel T, Ramseyer C, Yesylevskyy S. The asymmetry of plasma membranes and their cholesterol content influence the uptake of cisplatin. Sci Rep-Uk. 2019;2:9.
27. Kopecka J, Trouillas P, Gasparovic AC, Gazzano E, Assaraf YG, Riganti C. Phospholipids and cholesterol: inducers of cancer multidrug resistance and therapeutic targets. Drug Resist Updat. 2020;49:100670. doi:10.1016/j.drup.2019.100670
28. Hsu -H-H, Chen M-C, Baskaran R, et al. Oxaliplatin resistance in colorectal cancer cells is mediated via activation of ABCG2 to alleviate ER stress induced apoptosis. J Cell Physiol. 2018;233(7):5458–5467. doi:10.1002/jcp.26406
29. Allott EH, Howard LE, Cooperberg MR, et al. Serum lipid profile and risk of prostate cancer recurrence: results from the SEARCH database. Cancer Epidemiol Biomarkers Prev. 2014;23(11):2349–2356. doi:10.1158/1055-9965.EPI-14-0458
30. Zhang D, Xi Y, Feng Y. Ovarian cancer risk in relation to blood lipid levels and hyperlipidemia: a systematic review and meta-analysis of observational epidemiologic studies. Eur J Cancer Prev. 2021;30(2):161–170. doi:10.1097/CEJ.0000000000000597
31. Chang TY, Chang CC, Ohgami N, Yamauchi Y. Cholesterol sensing, trafficking, and esterification. Annu Rev Cell Dev Biol. 2006;22:129–157. doi:10.1146/annurev.cellbio.22.010305.104656
32. Hegele RA. Plasma lipoproteins: genetic influences and clinical implications. Nat Rev Genet. 2009;10(2):109–121. doi:10.1038/nrg2481
33. Santosa S, Varady KA, AbuMweis S, Jones PJH. Physiological and therapeutic factors affecting cholesterol metabolism: does a reciprocal relationship between cholesterol absorption and synthesis really exist? Life Sci. 2007;80(6):505–514. doi:10.1016/j.lfs.2006.10.006
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.