Back to Journals » International Medical Case Reports Journal » Volume 8
Look-alike, sound-alike medication errors: a novel case concerning a Slow-Na, Slow-K prescribing error
Authors Naunton M, Gardiner H, Kyle G
Received 3 December 2014
Accepted for publication 15 January 2015
Published 16 February 2015 Volume 2015:8 Pages 51—53
DOI https://doi.org/10.2147/IMCRJ.S78637
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ronald Prineas
Mark Naunton, Hayley R Gardiner, Greg Kyle
Discipline of Pharmacy, University of Canberra, Bruce, Australian Capital Territory, Australia
Abstract: A 59-year-old man was mistakenly prescribed Slow-Na instead of Slow-K due to incorrect selection from a drop-down list in the prescribing software. This error was identified by a pharmacist during a home medicine review (HMR) before the patient began taking the supplement. The reported error emphasizes the need for vigilance due to the emergence of novel look-alike, sound-alike (LASA) drug pairings. This case highlights the important role of pharmacists in medication safety.
Keywords: LASA, error, sodium, potassium
Case report
Medication errors refer to a failure at some point during a treatment process, which may lead to patient harm.1 Several studies in current literature identify key issues regarding medication errors, specifically, that they are more likely to occur at interfaces of care where there is an element of data transcription.2 The World Health Organization has explicitly identified confusing drug names as one of the most common causes of medication error.3 Factors that contribute to potential confusion between drug names include spelling-related, phonetic, or packaging similarities.4–8 It has been established that confusion, and therefore error, is more likely if medicines appear in the same context or if drugs are being selected from a drop-down list.4 Multiple studies have concluded that look-alike, sound-alike (LASA) errors stem from both human error and the failure of prescribing systems across the health care spectrum to be able to recognize the potential for these errors.4,5,8 One method consistently recommended to decrease LASA errors is Tall Man lettering – a textual format recognized and recommended by the US Federal Drug Administration (FDA), which involves writing the “confusable” parts of LASA drug names in uppercase.9 It has been established that Tall Man lettering does improve accuracy in the perception of drug names.9
The home medicine review (HMR) program was established in Australia in 2001 in order to facilitate quality use of medicines through pharmacist intervention. HMRs are initiated by a patient’s general practitioner and have been established to be an effective tool in medicine safety in a number of patient groups, including those with multiple medications, medication barriers (eg, language barriers or visual impairment), or patients undergoing medication changes – such as a recent discharge from hospital.10–12
A HMR conducted by an accredited community pharmacist in Australia in 2011 revealed that a patient was mistakenly prescribed Slow-Na instead of Slow-K. The patient had a medical history of deep vein thrombosis, right hip fracture, pulmonary embolism, chronic obstructive airway disease, and ischemic heart disease. At the time of the HMR, the patient had current prescriptions for warfarin sodium (5 mg), frusemide (20 mg), fluticasone propionate/salmeterol, tiotropium, and salbutamol sulfate, in addition to the prescription for Slow-Na (600 mg). It was established that the patient was prescribed Slow-K after being diagnosed with hypokalemia following an 8-week hospitalization for pneumonia. During the prescribing process, Slow-Na was mistakenly selected from a drop-down software list (Figure 1). Due to financial reasons, the patient had not filled the incorrect prescription, and the error was identified before the patient obtained Slow-Na. Adverse events that may have eventuated due to untreated hypokalemia include increased risk of arrhythmias, skeletal myopathy, metabolic acidosis, and acute kidney injury.13 Increased sodium intake may have also resulted in altered mental status.13
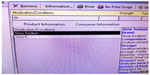  | Figure 1 Image of the prescribing software demonstrating the proximity of the LASA drugs. |
Given that this particular LASA error has not been reported in the literature to date, it presents a novel drug pair listing in LASA errors. Although this particular LASA error is unlikely to recur in Australia due to the discontinuation of Slow-Na in 2011, it highlights the ongoing need for vigilance with respect to LASA errors, as new drug pairs continue to be identified as potential and actual sources of error. A recent Australian study commissioned by the Australian National Medicines Policy Committee reviewed 32 publications on LASA medication errors and strategies that may be implemented to decrease their incidence.6 The review determined that in order to decrease errors from LASA medications, all stages of the medication process, from manufacture through to administration, must be perused and strategies must be designed to address potential problems.6 This finding is consistent in current literature.1,6 A National Tall Man Lettering Standard in prescribing software was the recommendation provided by the Australian Commission on Safety and Quality in Healthcare following a review.14 The recommended standard would only incorporate a set list of medications associated with orthographic error.14
In the case reported herein, Tall Man lettering may have drawn attention to the LASA error before it perpetuated through the medication process to the patient. Given that LASA errors are well described in the literature, Australia should aspire to develop and apply innovative solutions to decrease the incidence of LASA errors to facilitate quality use of medicines. This may include widespread use of Tall Man Lettering in prescribing and dispensing software. Although the error described in the case herein was identified before the patient commenced treatment with the incorrect drug, it highlights the ongoing need for vigilance with respect to LASA errors, as new drug pairs continue to be identified as potential and actual sources of error. The Slow-Na, Slow-K LASA error reported here was identified during a HMR. The recognition of the error is a significant example of the importance of medication review throughout the entire medication process. Pharmacists in particular have an important role in the community in implementing and undertaking strategies, such as HMRs, to minimize adverse drug events to ensure quality use of medicines. This case highlights the fundamental role of HMRs in the community and these should continue to be utilized as an effective tool in identifying LASA errors.
Disclosure
The authors report no conflicts of interest in this work.
References
Aronson JK. Medication errors: what they are, how they happen, and how to avoid them. QJM. 2009;102(8):513–521. | |
Abdel-Qader DH, Harper L, Cantrill JA, Tully MP. Pharmacists’ interventions in prescribing errors at hospital discharge: an observational study in the context of an electronic prescribing system in a UK teaching hospital. Drug Saf. 2010;33(11):1027–1044. | |
WHO. WHO Collaborating Centre for Patient Safety Solutions, Look-Alike Sound-Alike Medication Names. Patient Safety Solutions. Geneva: WHO; 2007. | |
Emmerton L, Rizk MS. Look-alike and sound-alike medicines: risks and ‘solutions’. Int J Clin Pharm. 2012;34(1):4–8. | |
Hoffman J, Proulx S. Medication errors caused by confusion of drug names. Drug Safety. 2003;26(7):445–452. | |
Ostini R, Roughead EE, Kirkpatrick CMJ, Monteith GR, Tett SE. Quality Use of Medicines – medication safety issues in naming; look-alike, sound-alike medicine names. Int J Pharm Pract. 2012;20(6):349–357. | |
Aronson JK. Medication errors resulting from the confusion of drug names. Expert Opin Drug Saf. 2004;3(3):167–172. | |
Kondrak G, Dorr B. Automatic identification of confusable drug names. Artif Intell Med. 2006;36(1):29–42. | |
Darker IT, Gerret D, Filik R, Purdy KJ, Gale AG. The influence of ‘Tall Man’ lettering on errors of visual perception in the recognition of written drug names. Ergonomics. 2011;54(1):21–33. | |
Castelino RL, Hilmer SN, Bajorek BV, Nishtala P, Chen TF. Drug Burden Index and potentially inappropriate medications in community-dwelling older people: the impact of Home Medicines Review. Drugs Aging. 2010;27(2):135–148. | |
Castelino RL, Bajorek BV, Chen TF. Targeting suboptimal prescribing in the elderly: a review of the impact of pharmacy services. Ann Pharmacother. 2009;43(6):1096–1106. | |
Castelino RL, Bajorek BV, Chen TF. Retrospective evaluation of home medicines review by pharmacists in older Australian patients using the medication appropriateness index. Ann Pharmacother. 2010;44(12):1922–1929. | |
Micromedex® Healthcare Series. Greenwood Village, CO: Thomson Micromedex; 2014. | |
Australian Commission on Safety and Quality in Health Care, National Standard for the Application of Tall Man Lettering. Sydney: WHO Collaborating Centre for Patient Safety Solutions, Look-Alike Sound-Alike Medication Names; 2007. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
