Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 14
Longitudinal change of FEV1 and inspiratory capacity: clinical implication and relevance to exacerbation risk in patients with COPD
Authors Jo YS , Kim SK, Park SJ , Um SJ , Park YB , Jung KS, Kim DK , Yoo KH
Received 2 October 2018
Accepted for publication 28 December 2018
Published 4 February 2019 Volume 2019:14 Pages 361—369
DOI https://doi.org/10.2147/COPD.S189384
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Yong Suk Jo,1 Sung Kyoung Kim,2 Seoung Ju Park,3 Soo-Jung Um,4 Yong-Bum Park,5 Ki Suck Jung,6 Deog Kyeom Kim,7 Kwang Ha Yoo8
1Department of Internal Medicine, Division of Pulmonary and Critical Care Medicine, Kyung Hee University Hospital, Seoul, Republic of Korea; 2Department of Internal Medicine, Division of Pulmonology, St Vincent’s Hospital, The Catholic University of Korea, Seoul, Republic of Korea; 3Department of Internal Medicine, Chonbuk National University Medical School, Jeonju, Republic of Korea; 4Department of Internal Medicine, Division of Pulmonology, Dong-A University Hospital, Busan, Republic of Korea; 5Department of Internal Medicine, Division of Pulmonary, Allergy, and Critical Care Medicine, Hallym University Kangdong Sacred Heart Hospital, Seoul, Republic of Korea; 6Department of Internal Medicine, Division of Pulmonary Medicine, Hallym University Sacred Heart Hospital, Hallym University Medical School, Anyang, Republic of Korea; 7Department of Internal Medicine, Seoul Metropolitan Government, Seoul National University Boramae Medical Center, Seoul, Republic of Korea; 8Department of Internal Medicine, Division of Pulmonary and Allergy Medicine, Konkuk University School of Medicine, Seoul, Republic of Korea
Background and objective: FEV1 is the gold standard for assessment of COPD. We compared efficacy of FEV1, inspiratory capacity (IC), and IC to total lung capacity (TLC) ratio in the evaluation of COPD and their association with exacerbation.
Methods: We analyzed the association of dyspnea severity, quality of life status, and lung function with lung function measurements and exacerbation risk in 982 patients enrolled in the Korea COPD Subgroup Registry and Subtype Research study. Exacerbation and longitudinal lung function change were evaluated in 3 years’ follow-up.
Results: The FEV1, IC, and IC to TLC ratio showed comparable negative correlations with dyspnea severity and quality of life status, and positive correlation with exercise capacity. In patients with >2 events/year, annual rate of change in FEV1 and IC tended to decline more rapidly in those with FEV1 <50% than in those with FEV1 >50% (-14.46±19.40 mL/year vs 12.29±9.24 mL/year, P=0.213; -4.75±17.28 mL/year vs -78.05±34.16 mL/year, P=0.056 for FEV1 and IC, respectively), without significance.
Conclusion: Longitudinal changes in IC and FEV1 were not significantly associated with exacerbation risk.
Keywords: COPD, exacerbation, FEV1, inspiratory capacity
Introduction
COPD, characterized by persistent and progressive airflow limitation, is a leading cause of morbidity and mortality worldwide and its prevalence is increasing. Currently, COPD is ranked the fourth most common cause of mortality in the USA, and is expected to be the third most common in 2020.1 The prevalence of COPD in Korea has recently been reported as 13.1%–14.6%, which is more than the worldwide prevalence; moreover, COPD is reported as one of the major causes of death in Korea.2,3
The goals of COPD assessment are to determine not only the severity of airflow limitation but also the patients’ disease-specific self-perceived health status and the risk of future adverse events, including exacerbations.4 Traditionally, both COPD diagnosis and severity assessment have been based on severity of airflow limitation, represented by FEV1.5 The level of airflow limitation has been regarded as an important index in the prediction of clinical outcomes including exacerbation and mortality, and change in FEV1 over time is still the most widely used index of disease progression.6,7 However, there is a limitation in that FEV1 reflects only one aspect of the disease and does not actually predict the degree of the disease, especially in the early stage.8,9 In addition, correlations of FEV1 with symptoms and impairment of a patient’s health status have been noted as weak.10,11
Lung hyperinflation which is caused by destructive lung parenchymal emphysema and expiratory flow limitation,12 is an important feature of COPD and is known to contribute to symptoms, exercise capacity, exacerbation, and even mortality.13–15 Prognostic impact of an altered inspiratory capacity (IC) and the IC in relation to total lung capacity (TLC) at rest are proven predictors of all-cause and respiratory mortality.14,16 Hence, several studies analyzing the efficacy of inhaler treatment have used IC as an outcome measure.17–20
In the present study, we compared IC, IC to TLC ratio, and FEV1 as clinical measurements in patients with COPD, and analyzed the risk of exacerbation according to the longitudinal change in values through Korean COPD cohort.
Methods
Study design and participants
The Korea COPD Subgroup Registry and Subtype Research study (registered on ClinicalTrials.gov with identifier CT02800499) is an ongoing multicenter, prospective observational study at 48 centers in Korea from December 2011 to-date with details previously described.21 Patients with COPD aged ≥40 years and whose bronchodilator response (BDR) FEV1/FVC was <70% were included in this cohort. Among patients diagnosed with COPD, only patients with lung volume measurements were included in the analysis. All study procedures were performed in accordance with the Declaration of Helsinki and relevant guidelines and written informed consent was obtained from all participants before enrollment. The study protocol was approved by the institutional review boards of Kyung Hee University Hospital (IRB no KHUH 2018-04-037).
Clinical measurements
At initial evaluation, demographic data including age and sex, smoking status and pack-years, spirometry results including lung volume, 6MWD, mMRC dyspnea scale, and self-perceived quality of life status assessed by COPD assessment test (CAT) and St George’s Respiratory Disease Questionnaire (SGRQ) were collected by physicians or trained nurses using electronic case-report forms.
Treatment of individual patients depended on their attending pulmonologist; patients were followed-up at least every 6 months, and exacerbation data (ie, sputum, cough, or dyspnea) beyond changes within normal daytime range were collected on every visit. Moderate exacerbation was defined as a response “yes” from the patient to the question “Have you had an aggravation of respiratory symptoms that led to a visit to the outpatient clinic earlier than scheduled?”, and severe exacerbation was defined as “yes” to the question “Have you been hospitalized or visited the emergency department due to deterioration of respiratory symptoms?”.
Pulmonary function tests
Spirometry with a bronchodilator (BD) test was performed according to the recommendations of the American Thoracic Society (ATS) and the European Respiratory Society (ERS) guidelines.22 Lung volume measurement was carried out according to the ATS and ERS guidelines and was conducted before inhalation of a BD. Body plethysmography was used for lung volume measurements.23 The predicted percentage values for the results of spirometry were calculated from the equation developed with Korean populations.24
For assessment of longitudinal changes in the lung volume measurements, spirometry including lung volume follow-up was conducted at least once a year.
Statistical analyses
Baseline characteristics variables were expressed as mean ± SD and absolute number with percentages. Pearson correlation tests were applied to assess the relationships of FEV1, IC, and IC to TLC ratio with other clinical measurements including mMRC, CAT, SGRQ, and 6MWD. All tests were two-sided and a P-value of <0.05 was considered statistically significant.
To eliminate the short-term improvement effects after the start of treatment, we analyzed up to 3 years’ changes in pulmonary function and exacerbation. For longitudinal change of FEV1, IC and IC to TLC ratio were analyzed by mixed-effect linear regressions. The mean annual rates of change in FEV1, IC, and IC to TLC ratio were estimated with a random intercept and a random slope model, with multiple covariates: age, sex, smoking pack-years, body mass index (BMI), previous exacerbation history in the past year and inhaled BD use. To determine the effect of covariates on the annual rates of change in lung function, the interaction of each covariate with time was assessed.
The risk of exacerbation was analyzed by Cox proportional hazards model based on the degree of lung function decline using widely accepted minimal clinically important difference (MCID) of 100 mL and 150 mL for FEV1 and IC, respectively.25–27 Covariates including age, sex, BMI, smoking pack-years, and previous history of exacerbation were adjusted for the multivariate analysis.
Statistical analyses were carried out using STATA software version 14.2 (StataCorp LP, College Station, TX, USA).
Results
Characteristics of patients
A total of 982 COPD patients with lung volume measurement were included in the analysis (Figure 1). Table 1 shows baseline characteristics of patients. Most of the patients were male individuals with mean age of 72.7 years and 21.6% (210 of 982) experienced exacerbation in the past year before enrollment. Mean post-BD FEV1 was 62.7%±19.3% of the predicted value and mean post-BD FEV1 to FVC ratio was 49.8%±11.7% of the predicted value. More than half of the patients were in GOLD stage II. Summary of lung volume measurements are described in Table 2. Mean values of IC and IC to TLC ratio were 1.9±0.6 L (74.6%±22.9% of the predicted value) and 31.3%±9.9%, respectively, and each showed moderate positive correlation with post-BD FEV1 (r=0.44 and 0.51 for IC and IC to TLC ratio; P<0.001 in both) (Figure S1).
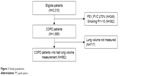  | Figure 1 Study population. |
Correlation between the lung function and other clinical measurements
Figure 2 shows the correlation between IC, IC to TLC ratio and dyspnea scale, disease specific quality of life status, and exercise capacity. The IC and IC to TLC ratio had slightly negative correlations with mMRC (r=−0.24 and −0.29, respectively; P<0.001 in both), SGRQ (r=−0.19 and −0.24, respectively; P<0.001 in both), and CAT (r=−0.22 and −0.24, respectively; P<0.001 in both), and a positive correlation with 6MWD (r=0.11 and 0.14, respectively; P=0.001 and <0.001 for IC and IC/TLC, respectively). FEV1 showed similar correlation results with IC and IC to TLC ratio; r=−0.36, −0.24, 0.31, and 0.16 for mMRC, SGRQ, CAT, and 6MWD, respectively, and P<0.001 for all.
Longitudinal changes in lung function
Mean change of FEV1 and IC from enrollment are presented in Figure 3. In patients who had mild to moderate airflow obstruction (FEV1 ≥50% of predicted value), FEV1 decreased significantly compared with baseline at 3-year follow-up (−20.35±21.82 mL, P<0.001) and in those with more than severe airflow obstruction (FEV1 <50% of predicted value), FEV1 was significantly increased during the first 2 years of treatment, and IC decreased significantly at 3 years (−76.86±71.69 mL, P=0.040). During the 3 years’ follow-up period, mean ratio of IC to TLC decreased gradually from 32.5% to 31.5%, without significance considering the basal IC to TLC ratio of 31.3%.
  | Figure 3 Mean change of FEV1 and IC from enrollment. |
Baseline demographic characteristics, smoking status, and pulmonary function had no significant effect on the annual rates of change in FEV1, IC, and IC to TLC ratio. Both dyspnea severity and quality of life status did not significantly affect lung function change. The occurrence of exacerbation seemed to affect the decline in IC but not FEV1 over time (Figure S2). However, the exacerbation did not significantly affect the annual decline of FEV1, IC, and IC to TLC ratio after adjusting for multiple covariates even after dividing patients according to the severity of airflow limitation (mild to moderate, FEV1 ≥50% of predicted value; and more than severe, FEV1 <50% of predicted value) (Table 3). Even in patients with frequent exacerbation, no significant reductions in FEV1, IC, and IC to TLC ratios were observed compared with patients who did not.
Risk of exacerbation depending on lung function decline
Whether the exacerbation occurred was identified in 194 out of 544 subjects (35.7%) in the first, 144 out of 386 (37.3%) in the second, and 114 out of 248 (46.0%) in the third year, with 0.42±1.17, 0.29±0.89, and 0.24±0.10 events/year incidence rate, respectively. On adjusting for multiple covariates, for the 3 years’ follow-up, clinically important difference defined as FEV1 change more than 100 mL and IC change more than 150 mL did not relate to increased risk of exacerbation. In addition, based on multivariable analysis, low IC to TLC ratio was not a significant influencing factor for risk of exacerbation (Table 4).
Discussion
The severity of airflow obstruction, represented with FEV1, is regarded as the most widely acceptable index in assessing patients with COPD. However, IC is also regarded as a potential index of COPD in relation to hyperinflation and exercise capacity. In the present study, FEV1, IC, and IC to TLC ratio showed similar significant correlation with dyspnea, quality of life status, and exercise capacity. FEV1 was significantly decreased at 3-year follow-up in patients with mild to moderate airflow limitation, and IC in patients with more than severe airflow limitation, but overall, longitudinal change did not show significant association with exacerbation risk.
The goal of COPD management is to improve symptoms, exercise tolerance, and health status, and to alleviate progression as well as prevent exacerbation. Severity of airflow limitation assessed by FEV1 is considered as the most powerful biomarker of COPD, which not only reflects disease severity, but is also as a predictor of exacerbation and mortality, and a tool for the evaluation of treatment response.28–30 However, FEV1 could not provide a guideline for treatment. In addition, although the FEV1 values were the same, the degree of dyspnea and quality of life were different in each patient, and the agreement rate was not high.10,11 In the GOLD guideline revised in 2017,4 FEV1 was excluded from the COPD classification due to limitations of disease activity, exacerbation, and mortality prediction. Instead, patients were classified simply by dyspnea severity and exacerbation history. However, there is a debate on the exclusion of FEV1 in predicting the prognosis of COPD patients, and several studies disprove it.28–30 In addition to being a strong predictor of mortality and new hospital admission in COPD patients,31 baseline pulmonary function has been reported to be associated with increased exacerbation risk.32,33 In contrast, it was reported that the exacerbation incidence measured by patients’ self-report was inaccurate.34 This suggests a need for an objective indicator other than FEV1 in patients’ outcome prediction rather than simply self-reporting exacerbation.
The IC and IC to TLC ratio were also taken as an index of inspiratory restraint resulting from hyperinflation of the lung.14 Moreover, evaluation of IC can be reliably performed by recording flow-volume loop at rest.35 Thus, there is an advantage of immediate measurement without repeated spirometry for BDR. Hyperinflation of the lung is an important feature that causes exertional dyspnea and reduced exercise capacity in COPD.27 Besides, decrement of lung hyperinflation correlates well with improvement in dyspnea after BD therapy.36 For this reason, several clinical studies have assessed the efficacy of inhaler treatment by change in IC.17–20 However, IC has not yet been considered as a standard spirometry index of COPD assessment, and there are few studies comparing the usefulness of FEV1 and IC as an indicator of COPD.
In this study, FEV1, IC, and IC to TLC ratio showed negative correlation with dyspnea severity and quality of life status but positive correlation with exercise capacity in both. Although all correlations had statistical significance, the degree of association was not high but similar. It might be suggested that based on a single lung function measurement, it would be difficult to accurately assess breathlessness or quality of life status. In the analysis related to exacerbation, annual rate of change in FEV1 and IC did not significantly differ from patients who experienced exacerbation or those who did not. Although, frequent exacerbators who experience more than two events per year, showed faster annual decline rates of FEV1 and IC compared to those who did not, but, without statistical significance. During the 3 years’ follow-up, IC decreased more rapidly than FEV1, but until the second year, both values improved compared with those at the time of enrollment. Therefore, it is necessary to pay attention to the interpretation of the lung function decline and its relationship with exacerbation. Severity of airflow limitation is a known predictor of exacerbation,31,33 but increased frequency of exacerbation is also a contributing factor to lung function decline.6,37 We did not find a significant association between lung function changes and exacerbation. Actually, a 3-year follow-up is a relatively short period considering the course of the disease, and also, the reduction of FEV1 and IC became clear only in the third year. Thus, further longitudinal analysis for identifying the relationship between change in lung function and exacerbation is needed.
In order to analyze the risk of exacerbation in each year, depending on changes in lung function, the effect of changes in MCID of each lung function index on exacerbation was evaluated. In patients with decreased FEV1 and IC by more than MCID, the risk of exacerbation increased at 3 years, without statistical significance. In fact, since lung function was reduced after 3 years, analysis over a longer period would be needed. Moreover, a single value estimate may be difficult to use and may not present meaningful results. This is mainly due to heterogeneity of COPD patients. The same MCID estimate cannot be applied in both patients with mild airflow obstruction and with severe airflow obstruction.
Among all enrolled patients, only 4.7% had frequent exacerbation, defined as more than two exacerbations during follow-up period, and >70% of patients were mild to moderate disease status according to FEV1 criteria, considering this, the overall burden of exacerbations may be underestimated with milder disease. However, in our study, occurrence of frequent exacerbations was similar in patients with non-severe and severe status according to FEV1 criteria.
The main strength of this analysis is the use of a large cohort of patients with COPD and lung volume measurement. Although we did not extract a significant relationship between exacerbation risk and lung function decline, we found that the longitudinal decline of FEV1 and IC were different according to severity of airflow limitation, which reflects that both indices could complement each other in patient assessment. However, there are several limitations concerning interpretation of the findings. First, there were limited patients with annual follow-up of lung volume. Second, longer follow-up duration and prolonged volume measurement were needed for meaningful relevance between lung function change and exacerbation. Third, in our study, exacerbation events occurred only in male subjects, consequently, we could not analyze the sex-based differences in exacerbation risk. In addition, our study did not include a population sample but a sample of symptomatic patients known to respiratory clinicians and only Korean COPD patients were included. Moreover, we assessed exacerbation based on patients’ self-reporting but other large cohort studies collected exacerbation data in the same way.38,39 Lastly, we could not compare COPD patients by phenotype due to the absence of an integrated radiologic test.
Conclusion
In summary, we showed that the exacerbation risk did not relate to significant change of either FEV1 or IC over time, although the results of pulmonary function tests showed significant correlation with symptom and exercise performance indicators. This finding supports the hypothesis that assessing and predicting outcome in COPD patients by pulmonary function criteria alone is not appropriate and is consistent with current guidelines. We suggest that variation of FEV1 and IC should be interpreted differently according to severity of airflow limitation, and follow-up of longer duration is needed to evaluate the relationship between lung function alteration and risk of exacerbation because of marked lung function deterioration from year 3.
Data sharing statement
Data sharing is limited due to the lack of consent from research participants. Thus, no further data will be available.
Acknowledgment
This research was supported by funds (2016ER670102 and 2018ER670100) from Research of Korea Centers for Disease Control and Prevention.
Disclosure
The authors report no conflicts of interest in this work.
References
Mannino DM, Braman S. The epidemiology and economics of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2007;4(7):502–506. | ||
Hwang YI, Park YB, Yoo KH. Recent trends in the prevalence of chronic obstructive pulmonary disease in Korea. Tuberc Respir Dis (Seoul). 2017;80(3):226–229. | ||
Yoon HK, Park YB, Rhee CK, Lee JH, Oh YM; Committee of the Korean COPD Guideline 2014. Summary of the chronic obstructive pulmonary disease clinical practice guideline revised in 2014 by the Korean Academy of tuberculosis and respiratory disease. Tuberc Respir Dis (Seoul). 2017;80(3):230–240. | ||
Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: 2017 update. Available from: http://www.goldcopd.org. Accessed June 14, 2017. | ||
Celli BR, MacNee W, Agusti A; ATS/ERS Task Force. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23(6):932–946. | ||
Donaldson GC, Seemungal TA, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002;57(10):847–852. | ||
Cao Z, Ong KC, Eng P, Tan WC, Ng TP. Frequent hospital readmissions for acute exacerbation of COPD and their associated factors. Respirology. 2006;11(2):188–195. | ||
Nishimura K, Izumi T, Tsukino M, Oga T. Dyspnea is a better predictor of 5-year survival than airway obstruction in patients with COPD. Chest. 2002;121(5):1434–1440. | ||
Franciosi LG, Page CP, Celli BR, et al. Markers of disease severity in chronic obstructive pulmonary disease. Pulm Pharmacol Ther. 2006;19(3):189–199. | ||
Han MK, Muellerova H, Curran-Everett D, et al. Gold 2011 disease severity classification in COPDGene: a prospective cohort study. Lancet Respir Med. 2013;1(1):43–50. | ||
Jones PW. Health status and the spiral of decline. COPD. 2009;6(1):59–63. | ||
Rossi A, Aisanov Z, Avdeev S, et al. Mechanisms, assessment and therapeutic implications of lung hyperinflation in COPD. Respir Med. 2015;109(7):785–802. | ||
O’Donnell DE, Guenette JA, Maltais F, Webb KA. Decline of resting inspiratory capacity in COPD: the impact on breathing pattern, dyspnea, and ventilatory capacity during exercise. Chest. 2012;141(3):753–762. | ||
Casanova C, Cote C, de Torres JP, et al. Inspiratory-to-total lung capacity ratio predicts mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;171(6):591–597. | ||
Zaman M, Mahmood S, Altayeh A. Low inspiratory capacity to total lung capacity ratio is a risk factor for chronic obstructive pulmonary disease exacerbation. Am J Med Sci. 2010;339(5):411–414. | ||
Tantucci C, Donati P, Nicosia F, et al. Inspiratory capacity predicts mortality in patients with chronic obstructive pulmonary disease. Respir Med. 2008;102(4):613–619. | ||
O’Donnell DE, Casaburi R, Frith P, et al. Effects of combined tiotropium/olodaterol on inspiratory capacity and exercise endurance in COPD. Eur Respir J. 2017;49(4):1601348. | ||
Fujimoto K, Yamazaki H, Ura M, Kitaguchi Y. Efficacy of tiotropium and indacaterol monotherapy and their combination on dynamic lung hyperinflation in COPD: a random open-label crossover study. Int J Chron Obstruct Pulmon Dis. 2017;12:3195–3201. | ||
Salomon J, Stolz D, Domenighetti G, et al. Indacaterol and glycopyrronium versus indacaterol on body plethysmography measurements in COPD-a randomised controlled study. Respir Res. 2017;18(1):13. | ||
Calzetta L, Ora J, Cavalli F, Rogliani P, O’Donnell DE, Cazzola M. Impact of LABA/LAMA combination on exercise endurance and lung hyperinflation in COPD: a pair-wise and network meta-analysis. Respir Med. 2017;129:189–198. | ||
Lee JY, Chon GR, Rhee CK, et al. Characteristics of patients with chronic obstructive pulmonary disease at the first visit to a pulmonary medical center in Korea: the Korea COPD subgroup Study Team cohort. J Korean Med Sci. 2016;31(4):553–560. | ||
Wanger J, Clausen JL, Coates A, et al. Standardisation of the measurement of lung volumes. Eur Respir J. 2005;26(3):511–522. | ||
Criée CP, Sorichter S, Smith HJ, et al. Body plethysmography – its principles and clinical use. Respir Med. 2011;105(7):959–971. | ||
Choi JK, Paek D, Lee JO. Normal predictive values of spirometry in Korean population. Tuberc Respir Dis. 2005;58(3):230–242. | ||
Donohue JF. Minimal clinically important differences in COPD lung function. COPD. 2005;2(1):111–124. | ||
Cazzola M, MacNee W, Martinez FJ, et al. Outcomes for COPD pharmacological trials: from lung function to biomarkers. Eur Respir J. 2008;31(2):416–469. | ||
O’Donnell DE, Revill SM, Webb KA. Dynamic hyperinflation and exercise intolerance in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164(5):770–777. | ||
Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(10):1005–1012. | ||
Cote CG. Surrogates of mortality in chronic obstructive pulmonary disease. Am J Med. 2006;119(10):54–62. | ||
Donaldson GC, Wedzicha JA. COPD exacerbations 1: Epidemiology. Thorax. 2006;61(2):164–168. | ||
Flattet Y, Garin N, Serratrice J, Perrier A, Stirnemann J, Carballo S. Determining prognosis in acute exacerbation of COPD. Int J Chron Obstruct Pulmon Dis. 2017;12:467–475. | ||
Donaldson GC, Müllerova H, Locantore N, et al. Factors associated with change in exacerbation frequency in COPD. Respir Res. 2013;14(1):79. | ||
Hurst JR, Vestbo J, Anzueto A, et al; Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Investigators. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363(12):1128–1138. | ||
Frei A, Siebeling L, Wolters C, et al. The inaccuracy of patient recall for COPD exacerbation rate estimation and its implications: results from central adjudication. Chest. 2016;150(4):860–868. | ||
Yan S, Kaminski D, Sliwinski P. Reliability of inspiratory capacity for estimating end-expiratory lung volume changes during exercise in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1997;156(1):55–59. | ||
Belman MJ, Botnick WC, Shin JW. Inhaled bronchodilators reduce dynamic hyperinflation during exercise in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1996;153(3):967–975. | ||
Kanner RE, Anthonisen NR, Connett JE; Lung Health Study Research Group. Lower respiratory illnesses promote FEV(1) decline in current smokers but not ex-smokers with mild chronic obstructive pulmonary disease: results from the lung health study. Am J Respir Crit Care Med. 2001;164(3):358–364. | ||
Menezes AMB, Montes de Oca M, Pérez-Padilla R, et al. Increased risk of exacerbation and hospitalization in subjects with an overlap phenotype: COPD-asthma. Chest. 2014;145(2):297–304. | ||
López-Campos JL, Peces-Barba G, Soler-Cataluña JJ, et al. Chronic obstructive pulmonary disease history assessment in Spain: a multidimensional chronic obstructive pulmonary disease evaluation. study methods and organization. Arch Bronconeumol. 2012;48(12):453–459. |
Supplementary materials
  | Figure S1 Correlation between (A) IC and (B) IC/TLC ratio with post-bronchodilator FEV1. |
  | Figure S2 Longitudinal change of annual (A) FEV1 and (B) IC (mL) in COPD patients during 3-year follow-up according to history of exacerbation. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.





