Back to Journals » Journal of Asthma and Allergy » Volume 15
Long-Term Use of Maintenance Systemic Corticosteroids is Associated with Multiple Adverse Conditions in a Large, Real-World Cohort of US Adults with Severe Asthma
Authors Lugogo N, Chipps BE, Panettieri RA Jr , Trudo F, Ambrose CS
Received 3 June 2022
Accepted for publication 4 November 2022
Published 7 December 2022 Volume 2022:15 Pages 1753—1761
DOI https://doi.org/10.2147/JAA.S375005
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Luis Garcia-Marcos
Njira Lugogo,1 Bradley E Chipps,2 Reynold A Panettieri Jr,3 Frank Trudo,4 Christopher S Ambrose5
1University of Michigan, Ann Arbor, MI, USA; 2Capital Allergy & Respiratory Disease Center, Sacramento, CA, USA; 3Rutgers, The State University of New Jersey, New Brunswick, NJ, USA; 4AstraZeneca, Wilmington, DE, USA; 5AstraZeneca, Gaithersburg, MD, USA
Correspondence: Christopher S Ambrose, BioPharmaceuticals Medical, AstraZeneca, One MedImmune Way, Gaithersburg, MD, 20878, USA, Tel +1 301-398-4454, Email [email protected]
Abstract: There is growing recognition of the adverse consequences of maintenance systemic corticosteroid (mSCS) therapy in severe asthma (SA). The objective of this study was to describe the prevalence of potential adverse effects of long-term mSCS therapy in adults with specialist-confirmed SA in the United States (US). CHRONICLE is an ongoing, noninterventional, observational study of US adults with SA treated by allergists/immunologists and pulmonologists. Once enrolled, patients’ duration of mSCS therapy was reported by sites based on medical record review. For patients enrolled between February 2018 and February 2021, the prevalence of SCS-associated conditions was evaluated among those with no reported history of mSCS use, or mSCS use with < 2 years or ≥ 2 years cumulative duration. Prevalence and incidence estimates were adjusted for age and smoking history. Of 2793 patients enrolled, 311 and 231 had mSCS use for < 2 and ≥ 2 years, respectively. In adjusted analyses, adrenal insufficiency, pneumonia, type 2 diabetes, osteoporosis/osteopenia, congestive heart failure, coronary artery disease, hypertension, anxiety, and depression were statistically significantly associated with any mSCS use. By duration, mSCS use ≥ 2 years was associated with osteopenia/osteoporosis, coronary artery disease, adrenal insufficiency, and diabetes-related neuropathy; mSCS use < 2 years was associated with depression and osteopenia/osteoporosis, and diagnoses of depression, anxiety, and hypertension during the 12 months prior to enrollment. Overall, among patients with specialist-confirmed SA, mSCS use was associated with a high prevalence of multiple adverse conditions. Healthcare professionals should employ mSCS-sparing treatment strategies to avoid these negative consequences.
Keywords: duration, steroids, diabetes, pneumonia, osteoporosis
Introduction
Patients with severe and difficult-to-treat forms of asthma traditionally have been treated with maintenance systemic corticosteroid (mSCS) therapy.1 The Global Initiative for Asthma (GINA) global strategy document recommends mSCS as add-on therapy for patients with severe asthma (SA) uncontrolled by all other conventional therapies.2 However, GINA recommends that mSCS should only be considered after exclusion of all other contributory factors, and that patients be informed of potential side effects. In recent years, mSCS treatment for severe uncontrolled asthma has become less prevalent in the United States (US) with increased use of biologic (monoclonal antibody) therapies.3
There is a growing body of evidence from real-world studies describing the adverse consequences of mSCS therapy in asthma treatment. A matched cohort study utilizing medical record data from the United Kingdom found that patients treated with systemic corticosteroid (SCS) therapy had a significantly increased risk of multiple SCS-related complications including osteoporosis/osteoporotic fracture, pneumonia, cardio-/cerebrovascular diseases, cataract, sleep apnea, renal impairment, depression/anxiety, type 2 diabetes, and weight gain, compared with patients who were not treated with SCS.4 Use of mSCS was also found to be associated with increased burden and costs on the healthcare system in a retrospective study using administrative claims data in the US.5 Chung et al reported in a recent literature review that several observational studies suggest a direct dose-response relationship between mSCS use and various complications.6
However, studies reporting longer-term mSCS usage specifically in patients with SA are limited and inconsistent in their use of definitions of patient eligibility and mSCS treatment.1,6 In addition, there are limitations inherent in identifying patients with SA in studies relying on insurance claims or medical record databases. As a result, the aim of this analysis was to describe the prevalence of potential adverse effects of healthcare professional-confirmed mSCS use in a cohort of adults with specialist-confirmed SA, with a characterization of the risks associated with long-term treatment with mSCS.
Materials and Methods
Study Participants
The CHRONICLE study (NCT03373045) is an ongoing, noninterventional, observational study of US adults with SA treated by a diverse sample of US allergists/immunologists and pulmonologists. The design of the CHRONICLE study has been described in detail previously.7 Patients in the CHRONICLE study are required to have a physician-confirmed diagnosis of SA per European Respiratory Society/American Thoracic Society guidelines for at least 12 months prior to enrollment, with investigator confirmation that SA symptoms are not due to an alternative diagnosis.8 Patients must be adults (≥ 18 years) currently receiving care from subspecialist physicians at the participating site. In addition, patients must also meet one or more of the following criteria: 1) current use of FDA-approved monoclonal antibody therapy for SA; 2) use of systemic corticosteroids or other systemic immunosuppressants for ≥ 50% of the prior 12 months for SA; or 3) persistently uncontrolled asthma while receiving high-dosage inhaled corticosteroids (ICS) with additional controllers.
Patients were excluded if they were unable or unwilling to sign the written informed consent, not fluent in English or Spanish, unable to complete the study follow-up, or received an investigational therapy for asthma, allergy, atopic disease, or eosinophilic disease as part of a clinical trial during the 6 months prior to enrollment.7 Enrollment in clinical trials of investigational therapies after enrollment in CHRONICLE was allowed. Per protocol, sites approach all eligible patients for enrollment in CHRONICLE. Basic information was collected for all eligible patients including demographics, general medical history, and asthma-specific medical history. The CHRONICLE study was conducted in accordance with the principles of the Declaration of Helsinki (World Medical, 2013), the International Conference on Harmonisation guidelines for Good Clinical Practice, and applicable regulatory requirements.
Data Collection
At enrollment, sites reported comprehensive data on patient characteristics and asthma history, including asthma treatment, comorbidities, and relevant past medical events based on medical record review and knowledge of the patient’s medical history/status. Historical and current receipt of mSCS for asthma is explicitly collected on all enrolled patients; SCS dosage was reported by sites for the 12 months prior to enrollment and subsequently.
Statistical Analysis
In this analysis of the subgroup of CHRONICLE patients receiving mSCS, the unadjusted prevalence of potential SCS-associated conditions was evaluated among patients enrolled between February 2018 and February 2021. Patients with any mSCS use (defined as use of SCS for ≥ 30 consecutive days) were further categorized by cumulative duration of use more than or less than 2 years, the approximate median reported duration of use among those with mSCS use. Among conditions reported in at least 1% of patients, a ≥ 2-fold difference in prevalence between any 2 groups or a ≥ 10% absolute difference in prevalence between any 2 groups were identified as potential clinically relevant differences. In addition to overall prevalence of various conditions, annual rates of all-cause hospitalizations and pneumonia from 12 months prior to enrollment forward were calculated using this formula: annual rate = (total number of events x 365.25) / (total number of days in study for all participants).
Prevalence and incidence estimates were statistically adjusted for age and smoking history to account for those factors as potential confounders (ie, factors potentially associated with both the exposure and outcome). Because historical mSCS dosage was not available prior to 12 months before enrollment, long-term cumulative SCS dosage could not be evaluated as a factor. Odds ratios (ORs) with 95% confidence intervals (CIs) were used to assess the associations between mSCS duration and various comorbidities reported in at least 1% of patients. All analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC, USA). The CHRONICLE study protocol received central institutional review board (Advarra, Columbia, MD, USA) approval on November 3, 2017, and was registered on ClinicalTrials.gov on December 14, 2017 (NCT03373045).
Results
A total of 2793 patients enrolled in CHRONICLE from February 2018 to February 2021 (Table 1); 2251 patients had no reported history of any mSCS use, and 542 patients had reported mSCS use, with 311 and 231 patients with cumulative mSCS use of < 2 and ≥ 2 years, respectively. The mean age of all enrolled patients was 54 years (range: 18–94). Most patients were female (69%) and reported White race (75%). Patient demographic and baseline characteristics were generally similar among those with no reported use of mSCS and those who use mSCS (Table 1).
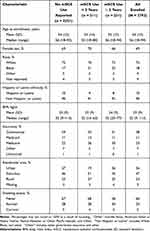 |
Table 1 Demographic and Baseline Characteristics of Enrolled Patientsa |
Among all enrolled patients with mSCS use, the median (interquartile range [IQR]) duration of mSCS use was 584 (191–1264) days (Table 2). The median (IQR) duration of SCS use for the < 2 and ≥ 2 years of mSCS groups was 260 (90–456) and 1433 (992–2251) days, respectively. The median (IQR) prednisone equivalent daily dose was lower among those also receiving biologics, at 20.0 (10.0, 40.0) mg, compared with 24.5 (10.0, 40.0) mg among those not receiving biologics.
 |
Table 2 Characteristics of mSCS Use in Enrolled Patients |
Unadjusted Analysis
Hypertension, osteopenia/osteoporosis (assessed by either clinical diagnosis or by T-score), cataract, adrenal insufficiency, coronary artery disease, history of cardiac procedures (including coronary artery bypass grafting, stent, and angioplasty), stroke, and myocardial infarction were more prevalent in patients with any mSCS use than in patients with no mSCS use (Figure 1).
Annual rates of all-cause hospitalization and pneumonia were higher in patients with any mSCS use than in patients who reported no mSCS use (Table 3). Patients who had a cumulative mSCS use of < 2 years had the highest annual rates of all-cause hospitalization and pneumonia.
 |
Table 3 Incidence of Select Events from 12 Months Prior to Enrollment Forward |
Adjusted Analyses
Compared with patients who reported no mSCS use, patients with any mSCS use were statistically significantly more likely to have adrenal insufficiency (OR: 3.46; 95% CI 1.81–6.61), pneumonia ever and since the 12 months prior to enrollment ([OR: 2.07; 95% CI 1.52–2.81] and [OR: 2.65; 95% CI 1.64–4.29]), or type 2 diabetes (OR: 1.41; 95% CI 1.09–1.83), respectively) (Figure 2).
Additionally, mSCS use was associated with osteoporosis/osteopenia and specific cardiovascular and neuropsychiatric comorbidities. Adjusted ORs for associations with osteoporosis/osteopenia by T-score, by any clinical diagnosis, and by clinical diagnosis since the 12 months before enrollment ranged from 2.41 to 3.01, all of which were statistically significant. Statistically significant associations were found between select cardiovascular comorbidities and any mSCS use including congestive heart failure (OR: 2.28), coronary artery disease (OR: 1.58), and hypertension (OR: 1.59). Active diagnoses of anxiety (OR: 1.64) and depression (OR: 1.34; 95% CI 1.06–1.69), as well as a new-onset depression in the 12 months prior to enrollment (OR: 2.14) were statistically significantly associated with any mSCS use.
Analyzed by duration of mSCS use, short-term (< 2 years) and long-term (≥ 2 years) mSCS use were associated with significant increases in morbidity (Figure 3). With longer duration of mSCS use ≥ 2 years, stronger associations were observed with osteopenia/osteoporosis by clinical diagnosis, coronary artery disease, adrenal insufficiency, and neuropathy related to diabetes. Stronger associations were observed for mSCS use duration < 2 years with depression, osteopenia/osteoporosis by T-score, as well as depression, anxiety, and hypertension diagnosed in the 12 months prior to enrollment.
Discussion
This observational cohort study demonstrated an increased prevalence of multiple adverse effects of mSCS in adults with specialist-confirmed SA. Compared with patients who reported no mSCS use, patients with any mSCS use were more likely to have hypertension, pneumonia, osteopenia/osteoporosis (clinical diagnosis or by T-score), cataract, adrenal insufficiency, coronary artery disease, and history of cardiac procedures, stroke, and myocardial infarction. These results are consistent with other real-world studies describing the adverse consequences of mSCS therapy in asthma treatment in much larger cohorts,4,5 but which lacked specialist confirmation of SA and mSCS use.
Our findings of increased risk of some adverse effects with duration of mSCS receipt of 2 years or longer (adrenal insufficiency, osteopenia by clinical diagnosis, pneumonia, coronary artery disease) complement findings from other studies of mSCS effects. Dalal et al reported in a retrospective analysis that the likelihood of SCS users to develop any adverse consequences associated with mSCS increased with SCS dose exposure, suggesting a direct dose-response relationship between mSCS use and adverse consequences. Patients with low (< 5 mg/day), medium (≥ 5–10 mg/day), and high (> 10 mg/day) SCS exposure had a 2.50-fold, 2.95-fold, and 3.32-fold risk, respectively, of developing SCS-related complications compared with patients with no SCS exposure.5 Compared with other studies, the median daily SCS dose among mSCS users was high, at 20 mg/day prednisone equivalent dose. In a systematic literature review of 23 real-world studies encompassing Europe, Asia, and North America, Bleecker et al reported that average daily SCS doses for patients with severe disease ranged from 4.0 mg to 21.4 mg.1 The high daily mSCS dose in the current study is consistent with the fact that the cohort represents the most difficult-to-treat asthma patients managed by US subspecialists.
Differences in statistically significant comorbidities between patients receiving < 2 years of mSCS or ≥ 2 years of mSCS versus no prior mSCS use could be due to multiple factors. Anxiety, depression, and hypertension diagnosed in the 12 months prior to enrollment were significant in the adjusted analysis of patients with < 2 years mSCS use versus no prior use, but not significant in the analysis of patients with ≥ 2 years of mSCS use. Mood changes and onset or worsening of psychiatric diseases are known side effects of mSCS use.9 HCPs may choose to discontinue mSCS treatment in patients experiencing psychiatric comorbidities, in line with current treatment guidelines.10 Similarly, onset of hypertension in patients beginning treatment with mSCS may lead HCPs to consider another treatment option to reduce patient morbidity.10 Coronary artery disease was significant in the adjusted analysis of patients with ≥ 2 years mSCS use versus no prior use, but not significant in the analysis of patients with < 2 years of mSCS use. Previous studies of patients with asthma have observed an increasing prevalence of adverse outcomes with increasing duration of SCS treatment.6 In aggregate, the results of this and prior studies emphasize that patients’ asthma treatment should be regularly assessed to minimize SCS use and exposure.
Limitations
A limitation of this analysis is that outcomes were assessed only by duration of use as data were not available to describe mSCS dosage before 12 months prior to enrollment. However, one advantage of this approach is that it aligns with the information most readily available to treating physicians based on patient-reported medical history, as data on historical mSCS dosage may not be available or accurately reported. Data were also not available to describe the interventions that patients may have received to treat the adverse consequences of mSCS use. This analysis does not describe the standard of care for children and adolescents with SA or patients not treated by a specialist. Use of mSCS is reported by the site based on medical record review and knowledge of the patient’s medical history, but it was not confirmed by pharmacy record review. As a result, there may be recall bias and missing data from medical records. Although the OR analyses were adjusted for age and smoking status, residual confounding due to other unmeasured factors is still possible.
Conclusions
This study highlights the negative consequences of mSCS therapy in a cohort of patients with specialist-confirmed SA and provides novel insights regarding the effects of long-term specialist-reported mSCS use for SA. There is a clear increased risk of serious medical comorbidities when exposed to mSCS compared with those not exposed, consistent with prior research. Further research is needed to prospectively quantify the risks associated with longer-term exposure to intermittent bursts of SCS. Providers should employ mSCS-sparing treatment strategies, such as biologic treatments, and maximize pharmacologic adherence to avoid the negative consequences of SCS therapy for SA.
Data Sharing Statement
CHRONICLE is an ongoing study; individual de-identified participant data cannot be shared until the study concludes. The full study protocol is available upon request of the corresponding author. Individuals who were or were not involved in the study may submit publication proposals to the study’s Publication Steering Committee by contacting the corresponding author.
Acknowledgments
Medical writing support was provided by Anne Kangethe, PhD, and Casey Demko, MS, of Oxford PharmaGenesis Inc., Newtown, PA, USA, which was in accordance with Good Publication Practice (GPP3) guidelines, and funded by AstraZeneca, Wilmington, DE, USA. Interim findings of this analysis were presented as a poster presentation at the American Thoracic Society 2020 International Conference. The poster’s abstract was published in the conference abstract supplement to the American Journal of Respiratory and Critical Care Medicine (DOI: 10.1164/ajrccm-conference.2020.201.1_MeetingAbstracts.A6474).
Funding
This work was supported by and the CHRONICLE study is funded by AstraZeneca.
Disclosure
Njira Lugogo reports having received consulting fees for advisory board participation from Amgen, AstraZeneca, Genentech, GSK, Novartis, Regeneron, Sanofi, and Teva; honoraria for non-speakers’ bureau presentations from GSK and AstraZeneca; and travel support from AstraZeneca; her institution received research support from Amgen, AstraZeneca, Avillion, Gossamer Bio, Genentech, GSK, Regeneron, Sanofi, and Teva. Bradley E. Chipps reports personal fees from AstraZeneca, GlaxoSmithKline, Regeneron, Sanofi Genzyme, and Genentech for speaker’s bureau and advisor for consult, outside the submitted work. Reynold A. Panettieri Jr has advisory board membership in and has grant support from AstraZeneca, Sanofi, Genentech, Regeneron, Novartis, and also reports grants for honorarium from AstraZeneca, RIFM, Genentech, Merck & Co., and TEVA; grants from Equllium, Novartis, MedImmune, Origo, ACTIV-1, Janssen, and Vault Health, outside the submitted work. Frank Trudo and Christopher S. Ambrose are employees and shareholders of AstraZeneca. The authors report no other conflicts of interest in this work.
References
1. Bleecker ER, Menzies-Gow AN, Price DB, et al. Systematic literature review of systemic corticosteroid use for asthma management. Am J Respir Crit Care Med. 2020;201(3):276–293. doi:10.1164/rccm.201904-0903SO
2. Global Initiative for Asthma (GINA). Pocket guide for asthma management and prevention; 2020. Available from: https://ginasthma.org/wp-content/uploads/2020/04/Main-pocket-guide_2020_04_03-final-wms.pdf.
3. Moore WC, Panettieri RA, Trevor J, et al. Biologic and maintenance systemic corticosteroid therapy among US subspecialist-treated patients with severe asthma. Ann Allergy Asthma Immunol. 2020;125(3):294–303.e291.
4. Price DB, Trudo F, Voorham J, et al. Adverse outcomes from initiation of systemic corticosteroids for asthma: long-term observational study. J Asthma Allergy. 2018;11:193–204.
5. Dalal AA, Duh MS, Gozalo L, et al. Dose-response relationship between long-term systemic corticosteroid use and related complications in patients with severe asthma. J Manag Care Spec Pharm. 2016;22(7):833–847.
6. Chung LP, Upham JW, Bardin PG, Hew M. Rational oral corticosteroid use in adult severe asthma: a narrative review. Respirology. 2020;25(2):161–172.
7. Ambrose CS, Chipps BE, Moore WC, et al. The CHRONICLE study of US adults with subspecialist-treated severe asthma: objectives, design, and initial results. Pragmat Obs Res. 2020;11:77–90.
8. Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43(2):343–373.
9. Price D, Castro M, Bourdin A, Fucile S, Altman P. Short-course systemic corticosteroids in asthma: striking the balance between efficacy and safety. Eur Respir Rev. 2020;29(155):190151.
10. Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention; 2021. Available from: https://ginasthma.org/archived-reports/.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



