Back to Journals » Blood and Lymphatic Cancer: Targets and Therapy » Volume 12
Long-Term Survival with Ibrutinib Therapy in Elderly Patients with Newly Diagnosed Primary Central Nervous System Lymphoma
Authors Kuhlman JJ , Alhaj Moustafa M , Jiang L , Wang J , Gupta V , Tun HW
Received 1 February 2022
Accepted for publication 8 April 2022
Published 14 April 2022 Volume 2022:12 Pages 23—29
DOI https://doi.org/10.2147/BLCTT.S360442
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Wilson Gonsalves
Justin J Kuhlman,1 Muhamad Alhaj Moustafa,2 Liuyan Jiang,3 Jing Wang,1 Vivek Gupta,4 Han W Tun2
1Department of Internal Medicine, Mayo Clinic, Jacksonville, FL, USA; 2Division of Hematology and Oncology, Mayo Clinic, Jacksonville, FL, USA; 3Department of Pathology and Laboratory Medicine, Mayo Clinic, Jacksonville, FL, USA; 4Department of Radiology, Mayo Clinic, Jacksonville, FL, USA
Correspondence: Han W Tun, Division of Hematology and Oncology, Mayo Clinic, 4500 San Pablo Road S, Jacksonville, FL, 32224, USA, Tel +1 904-953-2000, Email [email protected]
Abstract: Primary central nervous system lymphoma (PCNSL) carries a dismal prognosis in elderly patients above 70 years of age with a median overall survival of 6 months. Novel therapeutic agents are urgently needed to improve survival outcomes in this age group. We describe the clinical presentation, diagnostic workup, and treatment outcome in two 80-year-old patients diagnosed with PCNSL who were treated with ibrutinib therapy. Both patients remain in complete remission following treatment with ibrutinib therapy. One patient is currently 4 years and the other is 2 years and 9 months from the time of initial diagnosis. We suggest that ibrutinib therapy has significant therapeutic activity against PCNSL in the newly diagnosed setting and should be evaluated in a clinical trial as part of front-line therapy, especially in elderly patients.
Keywords: newly diagnosed primary central nervous system lymphoma, ibrutinib, elderly adults, relapsed/refractory primary central nervous system lymphoma, clinical outcomes
Introduction
Primary central nervous system lymphoma (PCNSL) consists predominantly of non-germinal center diffuse large B cell lymphoma (NGC-DLBCL) confined to the brain, leptomeningeal compartment, eyes, cranial nerves, and spinal cord.1–3 Comprising less than 4% of non-Hodgkin lymphoma (NHL) cases and 4% of all primary brain tumors,4,5 PCNSL carries a predilection for older adults as the large majority of affected individuals are above 60 years of age with 37% of all cases found in those above 70 years old.6 According to an epidemiologic study evaluating over 25,000 PCNSL patients, the median overall survival (mOS) for combined age groups doubled from 12.5 months to 26 months over four decades, but the mOS for those above 70 years old remained dismal at 6 months.6 As such, elderly patients appear to be left behind with a poor prognosis. Novel agents with improved therapeutic efficacy and acceptable tolerability are therefore urgently needed to address this issue in the elderly, who are often unable to withstand standard chemotherapeutic agents or intensive consolidation treatments, such as autologous stem cell transplant or whole brain radiation. Some of these novel therapeutic agents include ibrutinib and immunomodulators, such as lenalidomide and pomalidomide, which have shown significant therapeutic activity in the relapsed/refractory setting of CNS lymphoma and vitreo-retinal lymphomas.7 We report two elderly patients with newly diagnosed PCNSL who have achieved long-term complete remission while on ibrutinib without any major toxicities.
Case #1
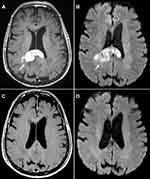
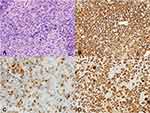
An 80-year-old female with hypertension, chronic kidney disease (CKD), and distant history of right-sided breast cancer in remission presented to her local ophthalmologist with painless vision loss in the right eye over the previous 2–3 weeks. Ophthalmological examination revealed papilledema and vitreous cells. Magnetic resonance imaging (MRI) of the brain showed a well-defined splenial mass in the corpus callosum measuring approximately 2.5 cm in diameter and involving the posterior right central cerebrum, external capsule, cingulate gyrus, and contiguous cingulate sulcus leptomeninges (Figure 1A and B). Full body computed tomography (CT) scan showed no evidence of malignancy. Cerebral spinal fluid (CSF) studies and vitrectomy of the right eye were conducted without definitive pathologic diagnosis. A stereotactic biopsy of the right corpus callosal lesion was performed and revealed a diffuse proliferation of large neoplastic lymphocytes (Figure 2A). Immunohistochemistry (IHC) demonstrated that the lymphoma cells were positive for CD20, BCL2 (90%), MYC (80%), PAX5, and MUM1; negative for CD10, BCL6, and CD30 (Figure 2B-E). The Ki-67 proliferation index was 100% (Figure 2F). Fluorescence in situ hybridization (FISH) showed 3 or more copies of MYC (at 8q.24) in 70% of nuclei without translocation, consistent with a diagnosis of “double expressor” DLBCL.1 Due to stage 3 CKD with an estimated creatinine clearance (Cr Cl) between 50–60 mL/min, the patient was initiated on renally dosed high-dose methotrexate (HD-MTX) at 1.75 g/m2 (50% dose reduction of regular dose 3.5 g/m2 as calculated by: 100 – Cr Cl = % dose reduction) alongside rituximab and temozolomide. Following her first dose of MTX, the patient experienced worsening kidney function with Cr Cl decreasing to 40–50 mL/min, and the patient was switched to ibrutinib 560 mg daily in combination with rituximab 375 mg/m2 weekly for 8 weeks. She received oral dexamethasone 4 mg twice daily with taper for 2 weeks around the time of diagnosis and IV dexamethasone 20 mg weekly with each rituximab treatment. One month after switching to ibrutinib with continued rituximab/steroidal therapy, the patient underwent repeat MRI of the brain, which demonstrated a partial response to therapy with interval decrease in splenial mass size and associated mass effect. Four months later while being maintained on ibrutinib alone, a repeat MRI revealed complete remission without any evidence of residual or recurrent lymphoma (Figure 1C and D). She is currently 4 years out from her original diagnosis and remains in complete remission while on ibrutinib. She enjoys an ECOG performance status of 1 with retained cognitive function.
Case #2
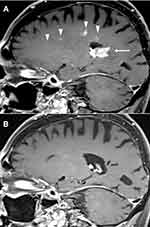
An 80-year-old male with coronary artery disease, stage 2 CKD, and distant history of lung cancer in remission presented to his primary care physician with a one-month history of rapidly progressive cognitive impairment along with left leg and right upper extremity weakness. He was ataxic with multiple near falls. MRI of the brain demonstrated a large enhancing lesion involving the splenium of the corpus callosum and extending into the occipital horns of both lateral ventricles, the left centrum ovale, and the left frontal deep white matter (Figure 3A). Stereotactic needle biopsy confirmed a pathologic diagnosis of NGC-DLBCL. IHC revealed lymphoma cells, which stained positive for CD20, BCL2, and MUM1 with a proliferation index of 70% by Ki-67 (Figure 4A-D); negative for CD10, BCL6, MYC, and CD30. A full body positron emission tomography (PET) was negative for malignancy outside of the CNS. Due to an estimated Cr Cl between 60–70 mL/min, the patient was initiated on renally dosed HD-MTX at 2.45 g/m2 (30% dose reduction from 3.5 g/m2), rituximab, and ibrutinib. His kidney function immediately deteriorated with a Cr Cl between 35–40 mL/min. He was therefore switched to ibrutinib 560 mg daily in combination with rituximab 375 mg/m2 weekly for 8 weeks. He received oral dexamethasone 4 mg twice daily with taper for 2 weeks around the time of diagnosis and IV dexamethasone 20 mg weekly with each rituximab treatment. He underwent an MRI of brain less than 3 months following his original diagnosis while on ibrutinib therapy alone, which demonstrated significant interval improvement and complete resolution of the left splenial mass with residual T2 signaling in the periventricular white matter. A repeat MRI of the brain 7 months thereafter confirmed a complete response with clear resolution of all pre-existing lesions (Figure 3B). He is currently 2 years and 9 months out from his original diagnosis and remains on ibrutinib while in complete remission. He enjoys an ECOG performance status of 0 with excellent cognitive function.
Discussion
We report two elderly patients with newly diagnosed PCNSL who have been on treatment with ibrutinib therapy before achieving long-term complete remission. Both patients could not tolerate HD-MTX based induction therapy due to worsening kidney function with decreased Cr Cl and were treated with rituximab and ibrutinib for 8 weeks followed by maintenance ibrutinib alone. It is worth noting, however, that both patients received a single dose of renally dosed HD-MTX before transitioning to ibrutinib, steroids, and rituximab, and only thereafter, underwent repeat imaging to assess treatment response. Consequently, it is difficult to strictly assess ibrutinib’s influence in the initial treatment response of both cases due to comitant use of rituximab with steroids and a single cycle of HD-MTX. However, the complete response witnessed on later imaging while only receiving ibrutinib alone suggests significant therapeutic efficacy from ibrutinib therapy. Case #1 especially highlights ibrutinib’s role in obtaining a complete response as initial imaging only demonstrated a partial response to previous therapies, and it was only after continuing ibrutinib monotherapy when the patient attained a complete response. This patient’s response is particularly striking as she harbored an aggressive B cell lymphoma with double expressor phenotype and a proliferation index of 100%. Case #2 represents a case of NGC-DLBCL without evidence of double hit lymphoma or double expressor phenotype. Because no definitive biomarkers have been identified in predicting treatment response to ibrutinib, negative MYC expression in this patient’s case did not carry any predictive value in determining response to ibrutinib therapy.8 Nonetheless, the patient responded quickly to treatment and experienced near complete remission on initial imaging following single dose HD-MTX, steroids, rituximab, and ibrutinib. Months later while on ibrutinib monotherapy, repeat MRI confirmed complete resolution of all pre-existing lesions. Since then, both patients in the current series have maintained long-term complete remission, far surpassing the 6 month mOS previously reported in those above 70 years of age.6 Despite the limitation of only having two patients in the current series, the long-term complete remission seen in both cases suggests that ibrutinib has significant therapeutic activity against PCNSL in the newly diagnosed setting. This finding is especially significant for older adults who are often unable to withstand standard chemotherapeutic agents due to a poor functional status or significant comorbidities (eg CKD).
Ibrutinib is an inhibitor of Bruton’s tyrosine kinase (BTK), an important signaling molecule in B cell receptor and Toll-like receptor signaling pathways which are constitutively active in PCNSL.9–12 It can cross the blood brain barrier quite well with a median CNS penetration of 43.4%, 35.1%, and 28.7% at dose levels of 560 mg, 700 mg, and 840 mg, respectively.11 Ibrutinib has been tested in two clinical trials as monotherapy for relapsed/refractory PCNSL, secondary CNS lymphoma, and primary vitreo-retinal lymphoma (Table 1).8,10 Although response rates to ibrutinib were high in both trials, progression free survival was rather short with only a few long-term responders. It appears that ibrutinib is more efficacious against ocular relapsed disease than brain/spinal cord relapsed disease.8 Rapid response to ibrutinib in the newly diagnosed setting of PCNSL has only been reported in a clinical trial whereby ibrutinib monotherapy (560–840 mg daily for two weeks) was used as a pre-treatment before initiation of a combination therapy (TEDDi-R; temozolomide, etoposide, Doxil, dexamethasone, ibrutinib, and rituximab).11 The overall response rate to the short-lived ibrutinib monotherapy was 83% with all responders achieving partial response. Besides BTK inhibition with ibrutinib, other therapeutic options for older patients with PCNSL who often cannot tolerate intensive chemoimmunotherapy include immunomodulators (ie, lenalidomide and pomalidomide) as well as phosphatidylinositol 3-kinase (PI3K) inhibitors, such as idelalisib, which has shown therapeutic activity in systemic B cell lymphomas.13 Although it remains to be seen whether or not PI3K inhibitors are effective in PCNSL treatment, they are currently being investigated in early phase clinical trials in the relapsed/refractory setting (ClinicalTrials.gov identifier: NCT03581942).
 |
Table 1 Therapeutic Activity of Ibrutinib Monotherapy in Relapsed/Refractory PCNSL, SCNSL, and PVRL in Clinical Trials |
It is worth noting that ibrutinib has been associated with aspergillosis in relapsed/refractory PCNSL.8,10,11,14 The incidence for aspergillosis appears to be increased when ibrutinib is combined with other steroids and/or chemotherapeutic agents. Mechanistically, BTK plays an important role in the innate immune response, and when BTK signaling is inhibited within immune cells (ie, phagocytes), there appears to be an increased susceptibility to aspergillosis.11,14 Despite not receiving anti-fungal prophylaxis while on long-term ibrutinib, the patients in the current series have not experienced any signs of aspergillosis or infectious complications. Prophylaxis was deemed unnecessary since long term combination therapy and/or extended use of steroids was avoided in both patients. For individuals receiving ibrutinib therapy as part of combination therapy, anti-fungal prophylaxis should be considered in order to mitigate the risk of aspergillosis.15
Conclusion
In conclusion, our series describes what appears to be the first report of two elderly patients with newly diagnosed PCNSL who have achieved long-term complete remission while on ibrutinib therapy. Although both patients received concomitant steroids and one cycle of HD-MTX chemotherapy during initial treatment, it appears that ibrutinib has significant therapeutic activity against PCNSL in the newly diagnosed setting. We suggest that ibrutinib therapy should be brought to the frontline and evaluated in clinical trials for newly diagnosed PCNSL as a monotherapy or in combination with other therapeutic agents, especially in elderly patients who often cannot withstand standard chemotherapeutic regimens.
Ethics and Consent
Written informed consent was obtained from both patients in the current series for the publication of this manuscript and accompanying images. Institutional approval was not required for publication.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Disclosure
The authors declare that there are no conflicts of interest regarding the publication of this manuscript.
References
1. Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375–2390.
2. Grommes C, DeAngelis LM. Primary CNS lymphoma. J Clin Oncol. 2017;35(21):2410.
3. Ferreri AJ. How I treat primary CNS lymphoma. Blood J Am Soc Hematol. 2011;118(3):510–522.
4. Chukwueke UN, Nayak L. Central nervous system lymphoma. Hematol Oncol Clin. 2019;33(4):597–611.
5. Villano J, Koshy M, Shaikh H, Dolecek T, McCarthy B. Age, gender, and racial differences in incidence and survival in primary CNS lymphoma. Br J Cancer. 2011;105(9):1414–1418.
6. Mendez JS, Ostrom QT, Gittleman H, et al. The elderly left behind—changes in survival trends of primary central nervous system lymphoma over the past 4 decades. Neuro-Oncology. 2018;20(5):687–694.
7. Grommes C, Nayak L, Tun HW, Batchelor TT. Introduction of novel agents in the treatment of primary CNS lymphoma. Neuro-Oncology. 2019;21(3):306–313.
8. Soussain C, Choquet S, Blonski M, et al. Ibrutinib monotherapy for relapse or refractory primary CNS lymphoma and primary vitreoretinal lymphoma: final analysis of the Phase II ‘proof-of-concept’iLOC study by the Lymphoma study association (LYSA) and the French oculo-cerebral lymphoma (LOC) network. Eur J Cancer. 2019;117:121–130.
9. Chapuy B, Roemer MG, Stewart C, et al. Targetable genetic features of primary testicular and primary central nervous system lymphomas. Blood J Am Soc Hematol. 2016;127(7):869–881.
10. Grommes C, Pastore A, Palaskas N, et al. Ibrutinib unmasks critical role of Bruton tyrosine kinase in primary CNS lymphoma. Cancer Discov. 2017;7(9):1018–1029.
11. Lionakis MS, Dunleavy K, Roschewski M, et al. Inhibition of B cell receptor signaling by ibrutinib in primary CNS lymphoma. Cancer Cell. 2017;31(6):833–843. e835.
12. Wilson WH, Young RM, Schmitz R, et al. Targeting B cell receptor signaling with ibrutinib in diffuse large B cell lymphoma. Nat Med. 2015;21(8):922–926.
13. Aksu S, Ayyildiz O, Etgul S, et al. Idelalisib at the Crossroads of B-Cell Lymphoproliferative Disorders. UHOD-Uluslararasi Hematoloji-Onkoloji Dergisi. 2016;26(1):43.
14. Grommes C, Younes A. Ibrutinib in PCNSL: the curious cases of clinical responses and aspergillosis. Cancer Cell. 2017;31(6):731–733.
15. Simard J, Melani C, Lakhotia R, et al. Preliminary results of a response-adapted study of ibrutinib and isavuconazole with temozolomide, etoposide, liposomal doxorubicin, dexamethasone, rituximab (TEDDI-R) for secondary CNS lymphoma. Blood. 2020;136:24–25.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.