Back to Journals » OncoTargets and Therapy » Volume 7
Long-term outcomes from dose-escalated image-guided intensity-modulated radiotherapy with androgen deprivation: encouraging results for intermediate- and high-risk prostate cancer
Authors Wilcox S, Aherne NJ, Benjamin LC, Wu B, de Campos Silva T, McLachlan CS, McKay MJ, Last AJ, Shakespeare TP
Received 31 March 2014
Accepted for publication 13 May 2014
Published 30 August 2014 Volume 2014:7 Pages 1519—1523
DOI https://doi.org/10.2147/OTT.S65238
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Shea W Wilcox,1,4 Noel J Aherne,2,4 Linus C Benjamin,1 Bosco Wu,1 Thomaz de Campos Silva,3 Craig S McLachlan,4 Michael J McKay,3,5 Andrew J Last,1 Thomas P Shakespeare1–4
1North Coast Cancer Institute, Port Macquarie, NSW, Australia; 2North Coast Cancer Institute, Coffs Harbour, NSW, Australia; 3North Coast Cancer Institute, Lismore, NSW, Australia; 4The University of New South Wales, Rural Clinical School, Sydney, NSW, Australia; 5The University of Sydney, Sydney, NSW, Australia
Purpose: Dose-escalated (DE) radiotherapy in the setting of localized prostate cancer has been shown to improve biochemical disease-free survival (bDFS) in several studies. In the same group of patients, androgen deprivation therapy (ADT) has been shown to confer a survival benefit when combined with radiotherapy doses of up to 70 Gy; however, there is currently little long-term data on patients who have received high-dose intensity-modulated radiotherapy (IMRT) with ADT. We report the long-term outcomes in a large cohort of patients treated with the combination of DE image-guided IMRT (IG-IMRT) and ADT.
Methods and materials: Patients with localized prostate cancer were identified from a centralized database across an integrated cancer center. All patients received DE IG-IMRT, combined with ADT, and had a minimum follow up of 12 months post-radiotherapy. All relapse and toxicity data were collected prospectively. Actuarial bDFS, metastasis-free survival, prostate cancer-specific survival, and multivariate analyses were calculated using the SPSS v20.0 statistical package.
Results: Seven hundred and eighty-two eligible patients were identified with a median follow up of 46 months. Overall, 4.3% of patients relapsed, 2.0% developed distant metastases, and 0.6% died from metastatic prostate cancer. At 5-years, bDFS was 88%, metastasis-free survival was 95%, and prostate cancer-specific survival was 98%. Five-year grade 2 genitourinary and gastrointestinal toxicity was 2.1% and 3.4%, respectively. No grade 3 or 4 late toxicities were reported. Pretreatment prostate specific antigen (P=0.001) and Gleason score (P=0.03) were significant in predicting biochemical failure on multivariate analysis.
Conclusion: There is a high probability of tumor control with DE IG-IMRT combined with androgen deprivation, and this is a technique with a low probability of significant late toxicity. Our long term results corroborate the safety and efficacy of treating with IG-IMRT to high doses and compares favorably with published series for the treatment of prostate cancer.
Keywords: dose-escalation, image-guided radiotherapy, treatment related toxicity, biochemical disease-free survival
Introduction
The utilization of dose-escalated (DE) radiotherapy for the primary treatment of clinically localized prostate cancer has become increasingly prevalent since the demonstration of improved outcomes with doses above 70 Gy.1,2 Intensity-modulated radiotherapy (IMRT) with image guidance has been widely accepted as a valuable technique of dose-escalation, with favorable long-term biochemical control and excellent mature toxicity profiles.3,4 The addition of androgen deprivation therapy (ADT) to conventional radiotherapy doses has also been shown to improve the outcomes of patients with localized prostate cancer.5–8 It is not surprising, then, that the RTOG 94-06 dose-escalation trial (a Phase I, II Dose Escalation Study using 3D-CRT for Adenocarcinoma of the Prostate), using 3D conformal radiotherapy and ADT, has demonstrated a trend towards increased biochemical disease-free survival (bDFS).9 Presently, there are few reports of long-term outcomes with ADT in combination with DE image-guided (IG)-IMRT. Variable use of ADT in some reports of DE IMRT make it difficult to accurately determine the clinical outcomes of this treatment combination, with increasing utilization requiring long-term mature follow up to determine the efficacy and safety of DE, particularly when combined with ADT.
This study reports on the 5-year clinical outcomes from one of the largest single-institution experiences with the combination of ADT and definitive DE IG-IMRT in patients with localized prostate cancer.
Materials and methods
Patient selection and staging
Following institutional ethics approval, the electronic medical records (Mosaiq; Elekta, Stockholm, Sweden) of an integrated cancer center (North Coast Cancer Institute, NSW, Australia) were searched to identify all patients with prostate cancer treated with definitive DE IG-IMRT and ADT, and with a minimum follow up of 12 months. Exclusion criteria included: patients who did not receive ADT, patients who were post-prostatectomy, patients who were node-positive, and patients with histology other than prostate adenocarcinoma. Staging computed tomography (CT) of the abdomen and pelvis, as well as bone scans were performed on all patients with Gleason 8–10, or with PSA >20 ng/mL. Patients were deemed low-risk if they had T2a disease or less, PSA <10 ng/mL, and Gleason 6 or less. High-risk patients had at least one of the following characteristics: T3 disease; PSA >20 ng/mL; or Gleason 8–10. All other patients were classified as being intermediate-risk.
All patients received ADT using leuprolide monotherapy with 3–6 months of neoadjuvant ADT, and high-risk patients received adjuvant ADT for a planned 2–3 years. All patients underwent transrectal ultrasound-guided insertion of gold fiducial markers and magnetic resonance imaging/CT fusion (unless contraindicated). Patients were planned and treated on an institutional bowel and bladder protocol, involving: low residue diet, the use of aperients, and pretreatment oral fluid regimen to achieve a comfortably full bladder and empty rectum. The planning CT scan (2 mm slices) was performed with the patient positioned supine and immobilized with ankle stocks. All clinical target volumes (CTVs) comprised prostate, the proximal 4–8 mm of seminal vesicles (SV), and any extracapsular extension. Patients with high-risk features had the distal SV: included in the CTV to either full dose (if SV magnetic resonance imaging was positive) or to 50 Gy equivalent via simultaneous integrated boost. All planning target volumes (PTVs) comprised CTV plus 5 mm uniform expansion. The total dose ranged from 73.8 Gy to 81 Gy in 1.8 to 2.0 Gy fractions. Image guidance was achieved by means of daily online kV portal images (matched to fiducial markers), with cone-beam CT on days 1–3, and weekly thereafter. Patients without fiducial markers (<1% of all patients) underwent daily cone-beam CT matching to soft tissue and bone. All patients were treated on Elekta Synergy linear accelerators. Biochemical failure was classified using the Phoenix definition (PSA nadir plus 2 ng/mL), and all patients with biochemical failure were restaged with CT and bone scans, with salvage androgen deprivation initiated when the PSA reached a level between 10 and 20 ng/mL. Metastatic failure was defined as the date of the first radiologically confirmed metastasis. All toxicity and relapse data was collected prospectively and recorded in the Mosaiq electronic medical record, and toxicity was scored using the common toxicity criteria (CTC) version 3 scoring system. The Kaplan-Meier method and Cox-regression multivariate analysis were used to calculate survival outcomes and predictive variables using the SPSS version 20 statistics package (IBM Corporation, Armonk, NY, USA). Variables included in the multivariate analysis included: age (using the median cut point of ≤71 versus >71 years), Gleason score (≤7 versus 8–10), PSA (using the median cut point of ≤11 ng/mL versus >11), and T-stage (T3–4 versus T1–2). All P-values were two tailed and considered statistically significant at a level <0.05.
Results
Outcomes
Between January 2005 and March 2011, 782 patients with localized prostate cancer were treated with DE IG-IMRT and ADT, with a median follow up of 46 months. The characteristics of patients are summarized in Table 1. Median age was 71 years (range 48–85), median PSA was 11 ng/mL (range 0.6–180), and Gleason scores were 6.6% for Gleason 5–6, 56.3% for Gleason 7, and 37.1% for Gleason 8–10. The median IMRT dose delivered was 78 Gy (range 73.8–81).
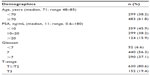  | Table 1 Patient demographics |
Overall, 34 of 782 (4.3%) patients suffered a biochemical relapse. Distant metastases developed in 16 (2.0%) patients, with five (0.6%) patients dying as a result of metastatic prostate cancer. At 5 years, the overall bDFS was 88%, metastasis-free survival was 95%, and prostate cancer-specific survival was 98% (Figures 1–3, respectively).
  | Figure 1 Biochemical disease-free survival. |
  | Figure 2 Metastasis-free survival. |
  | Figure 3 Prostate cancer-specific survival. |
Toxicity
Treatment was well tolerated, with late genitourinary (GU) or gastrointestinal (GI) toxicity uncommon. At 5 years, 2.1% of patients experienced grade 2 GU symptoms, with no grade 3 or 4 toxicities reported (Table 2). Similarly, 5-year GI toxicity was low, with 3.4% of patients developing grade 2 GI toxicity, and no late grade 3 or 4 toxicity reported. Rates of grade 1 and 2 erectile dysfunction were 56.9% and 9.1%, respectively, with no grade 3 erectile dysfunction reported.
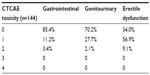  | Table 2 Late (5-year) toxicity outcomes |
Multivariate analysis
On Cox multivariate-regression analysis, only the initial PSA (P=0.001) and the Gleason score (P=0.03) were significant for predicting biochemical failure. A Gleason score >7 was also found to significantly predict for metastatic failure (P=0.01), with no variable significantly predictive of prostate cancer-specific mortality. Additional covariates included in the analysis and found to be nonsignificant were; age (P=0.33), T-stage (P=0.96), and radiation dose (P=0.27).
Discussion
The optimum treatment of clinically localized prostate cancer remains controversial, with three efficacious treatments available: surgery; brachytherapy; and external beam radiotherapy.10–12 Most published comparisons do not incorporate modern external-beam radiotherapy techniques utilizing DE IMRT with daily online image-guidance (IG) and few of these series routinely combine radiotherapy with ADT. To our knowledge, the present series is the largest reported series combining DE IG-IMRT and ADT for patients with localized prostate cancer. This single-institution experience showed a high 5-year bDFS of 88%, with very low levels of late toxicity.
It seems likely that both IG and DE contribute to accurate delivery of a tumoricidal dose whilst minimizing toxicity. This technique has been shown by other authors to accurately deliver high-dose radiotherapy to the clinical target volume with sparing of normal tissues.13 Randomized studies of DE show clear advantages in terms of bDFS,1,2 with respectable toxicity profiles when using conformal techniques.14 Series omitting daily online IG would be expected to show inferior outcomes compared to the current study, considering the known interfraction target motion.15 Despite careful adherence to dose constraints, without image guidance, there will inevitably be variability in dose delivery to the target volume and to the organs at risk.
The relative contributions of DE with robust IG and ADT to the excellent outcomes in this series cannot be quantified, and there is no existing randomized study addressing this question. However, in conventional-dose external-beam radiotherapy, the use of ADT has been shown to produce consistent advantages in terms of bDFS and, in some studies, overall survival outcomes.5–8 The mechanism for this advantage is not entirely certain; however, there is some evidence that androgen deprivation impacts favorably on both local (prostate) disease as well as distant micrometastatic disease.16 This underlies the rationale for the use of ADT even in the setting of DE IG-IMRT, and due to the potential benefits shown in at least one analysis there have been calls for randomized studies evaluating this.9
The present study is not the only study to demonstrate excellent outcomes when using DE IG-IMRT. Eade et al17 demonstrated very high rates of bDFS and low rates of treatment-related toxicity in a series of low-risk patients. In that series, IG-IMRT demonstrated superior bDFS and lower late GI and GU toxicity compared to low dose rate (LDR) brachytherapy. Similarly, Takeda et al18 recently published encouraging efficacy and toxicity results of 141 patients treated with DE IG-IMRT at 5-years follow-up. The study by Tomita et al19 showed similar results to the present study with regards to low toxicity and encouraging bDFS. They reported on a cohort of 241 patients treated with helical tomotherapy, with a median follow-up of 35 months, with the majority of patients receiving ADT. The rates of late grade 3 GI and GU toxicities were 0.8% and 1.2%, respectively, with a 3-year overall bDFS of 99.4%. The use of DE with ADT, as reported by Zapatero et al,20 has previously demonstrated good results, even in the absence of daily online IG and IMRT.
The present study is a retrospective analysis of patients from a single institution and is no substitute for a randomized controlled trial investigating DE IG-IMRT and ADT, however, the widespread implementation of these treatments requires stringent reporting of toxicity and efficacy. The duration of ADT required for the apparent improvement in survival outcomes with radiotherapy is still to be determined, with benefits on tumor control ideally balanced against possible side effects from hormonal therapy; randomized controlled trials to investigate this are needed. In addition, all patients in our study received DE IG-IMRT, with encouraging biochemical control, however, the necessity of DE for all risk groups is unknown, and further investigation into risk factors influencing this treatment decision is required. Longer-term follow-up of our cohort is also necessary as, although we demonstrate excellent 5-year results, it is well known that treatment failure continues to develop with time, and it is possible that toxicity rates could increase in the future. Similar studies with longer-term follow-up are therefore required.
Conclusion
We conclude that there is a high probability of tumor control with DE IG-IMRT combined with ADT, a technique with a low probability of significant late toxicity at 5 years. The long-term disease control and toxicity outcomes of this large cohort treated exclusively in one integrated cancer center corroborate the safety and efficacy of ADT and modern IMRT treating to high doses. Ongoing follow-up is needed to monitor tumor control and rates of late toxicity, as are randomized studies to investigate the optimal duration of ADT and patient selection for DE radiotherapy.
Disclosure
The authors report no conflicts of interest in this work.
References
Peeters ST, Heemsbergen WD, Koper PC, et al. Dose-response in radiotherapy for localized prostate cancer: results of the Dutch multicenter randomized phase III trial comparing 68 Gy of radiotherapy with 78 Gy. J Clin Oncol. 2006;24(13):1990–1996. | |
Kuban DA, Tucker SL, Dong L, et al. Long-term results of the MD Anderson randomized dose-escalation trial for prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70:67–74. | |
Zelefsky MJ, Chan H, Hunt M, Yamada Y, Shippy AM, Amols H. Long-term outcome of high dose intensity modulated radiation therapy for patients with clinically localized prostate cancer. J Urol. 2006;176:1415–1419. | |
Spratt DE, Pei X, Yamada J, Kollmeier MA, Cox B, Zelefsky MJ. Long-term survival and toxicity in patients treated with high-dose intensity modulated radiation therapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2013;85(3):686–692. | |
Pilepich MV, Winter K, Lawton CA, et al. Androgen suppression adjuvant to definitive radiotherapy in prostate carcinoma – long-term results of phase III RTOG 85-31. Int J Radiat Oncol Biol Phys. 2005;61(5):1285–1290. | |
Bolla M, Collette L, Blank L, et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomised trial. Lancet. 2002;360(9327):103–106. | |
Hanks GE, Pajak TF, Porter A, et al. Phase III trial of long-term adjuvant androgen deprivation after neoadjuvant hormonal cytoreduction and radiotherapy in locally advanced carcinoma of the prostate: the Radiation Therapy Oncology Group Protocol 92-02. J Clin Oncol. 2003;21(21):3972–3978. | |
Crook J, Ludgate C, Malone S, et al. Final report of multicenter Canadian Phase III randomized trial of 3 versus 8 months of neoadjuvant androgen deprivation therapy before conventional-dose radiotherapy for clinically localized prostate cancer. Int J Radiat Oncol Biol Phys. 2009;73(2):327–333. | |
Valicenti RK, Bae K, Michalski J, et al. Does hormone therapy reduce disease recurrence in prostate cancer patients receiving dose-escalated radiation therapy? An analysis of Radiation Therapy Oncology Group 94-06. Int J Radiat Oncol Biol Phys. 2011;79(5):1323–1329. | |
Zelefsky MJ, Eastham JA, Cronin AM, et al. Metastasis after radical prostatectomy or external beam radiotherapy for patients with clinically localized prostate cancer: a comparison of clinical cohorts adjusted for case mix. J Clin Oncol. 2010;28(9):1508–1513. | |
Zwahlen DR, Andrianopoulos N, Matheson B, Duchesne GM, Millar JL. High-dose-rate brachytherapy in combination with conformal external beam radiotherapy in the treatment of prostate cancer. Brachytherapy. 2010;9(1):27–35. | |
Azelie C, Gauthier M, Mirjolet C, et al. Exclusive image guided IMRT vs radical prostatectomy followed by postoperative IMRT for localized prostate cancer: a matched-pair analysis based on risk groups. Radiat Oncol. 2012;7:158. | |
Eade TN, Guo L, Forde E, et al. Image-guided dose-escalated intensity-modulated radiation therapy for prostate cancer: treating to doses beyond 78 Gy. BJU Int. 2012;109(11):1655–1660. | |
Dearnaley DP, Khoo VS, Norman AR, et al. Comparison of radiation side-effects of conformal and conventional radiotherapy in prostate cancer: a randomised trial. http://www.ncbi.nlm.nih.gov/pubmed/9929018. Lancet. 1999;353(9149):267–272. | |
Ahern NJ, Herden J, Wood M, et al. Daily fiducial based tracking of seminal vesicle motion in image guided dose escalated IMRT: Are we kidding ourselves regarding seminal vesicle coverage? [abstract]. Int J Radiat Oncol Biol Phys. 2009;75(3)(Suppl):S297. | |
Soloway MS, Sharifi R, Wajsman Z, McLeod D, Wood DP Jr, Puras-Baez A. Randomized prospective study comparing radical prostatectomy alone versus radical prostatectomy preceded by androgen blockade in clinical stage B2 (T2bNxM0) prostate cancer. The Lupron Depot Neoadjuvant Prostate Cancer Study Group. J Urol. 1995;154:424–428. | |
Eade TN, Horwitz EM, Ruth K, et al. A comparison of acute and chronic toxicity for men with low-risk prostate cancer treated with intensity-modulated radiation therapy or (125)I permanent implant. Int J Radiat Oncol Biol Phys. 2008;71(2):338–345. | |
Takeda K, Takai Y, Narazaki K, et al. Treatment outcome of high-dose image-guided intensity-modulated radiotherapy using intra-prostate fiducial markers for localized prostate cancer at a single institute in Japan. Radiat Oncol. 2012;7:105. | |
Tomita N, Soga N, Ogura Y, et al. Preliminary results of intensity-modulated radiation therapy with helical tomotherapy for prostate cancer. J Cancer Res Clin Oncol. 2012;138:1931–1936. | |
Zapatero A, García-Vicente F, Martín de Vidales C, et al. Long-term results after high-dose radiotherapy and adjuvant hormones in prostate cancer: how curable is high-risk disease? Int J Radiat Oncol Biol Phys. 2011;81(5):1279–1285. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
