Back to Journals » International Medical Case Reports Journal » Volume 9
Long-term follow-up of anatomical and functional macular changes after a single intravitreal implant of dexamethasone 0.7 mg for radiation macular edema secondary to proton beam therapy for choroidal melanoma
Authors Stringa F , Marzi F, Giannì L, Imparato M, Bianchi A, Bianchi PE
Received 28 July 2016
Accepted for publication 15 October 2016
Published 30 November 2016 Volume 2016:9 Pages 377—383
DOI https://doi.org/10.2147/IMCRJ.S118345
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Scott Fraser
Francesco Stringa, Federico Marzi, Laura Giannì, Manuela Imparato, Alessandro Bianchi, Paolo Emilio Bianchi
University of Pavia, Faculty of Medicine, University Eye Clinic, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy
Purpose: To describe the efficacy and safety of a single intravitreal implant of dexamethasone in a patient affected by radiation maculopathy due to proton beam radiotherapy for choroidal melanoma.
Patient and methods: Retrospective data of a 46-year-old woman treated with a single intravitreal injection of dexamethasone for radiation maculopathy due to proton beam radiotherapy were collected. The main outcome measures were best-corrected visual acuity and central retinal thickness. Intraocular pressure, anterior segment evaluation with slit lamp, macular changes depicted with spectral domain optical coherence tomography, retinal perfusion studied with fundus fluorescein angiography, and grade of macular edema using the Horgan classification were also evaluated during a 16-month follow-up.
Results: Macular edema occurred 25 months after radiation treatment in the left eye. The patient underwent a single intravitreal implant of dexamethasone. Preinjection visual acuity and central retinal thickness were 6/12 and 502 µm, respectively. After 8 months, visual acuity was 6/6 and remained stable until 16 months. Central retinal thickness was 269 µm at 16 months.
Conclusion: A single intravitreal implant of dexamethasone could effectively and stably improve visual acuity and central retinal thickness in some patients with radiation macular edema for 16 months after injection.
Keywords: retina, radiation maculopathy, macular edema, corticosteroid
Introduction
One of the most commonly used conservative treatments for choroidal melanoma is radiotherapy, having progressively replaced enucleation as the standard of care for the majority of tumors. The tumoricidal effect of radiation mainly results in damage to DNA, thereby reducing cell division.1
There are two main radiotherapeutic techniques for the treatment of uveal melanoma: brachytherapy suturing radioactive plaques to the sclera and external beam irradiation using charged particles, such as protons. The theoretical advantages of charged-particle irradiation are a high localized dose distribution and highly attractive depth-dose distribution patterns, but clinical experience has not completely substantiated these data.2
A predictable complication of proton beam therapy is radiation maculopathy (RM), which has been defined as a progressive occlusive retinal microangiopathy due to the iatrogenic damage toward endothelial cells and photoreceptors.3 RM represents the main cause of vision loss after charged-particle irradiation therapy, and its incidence appears to be dependent on radiation dose, tumor size, and distance from the fovea.4
The earliest and the most frequent clinical sign of RM is radiation macular edema (RME).5 Other funduscopic and angiographic findings typically include capillary non-perfusion, microaneurysms, telangiectasias, cotton-wool spots, hard exudates, perivascular sheathing, retinal and optic disc neovascularization.6
Fundus fluorescein angiography (FFA) is commonly used to evaluate macular perfusion in patients suffering from RM. It is also importantly used to distinguish focal from diffuse macular edema for prognostic and treatment purposes. Optical coherence tomography (OCT) is increasingly used to diagnose and monitor macular edema, allowing early detection of intraretinal cysts and photoreceptor loss.7
The treatment of RME is demanding. Laser photocoagulation,8,9 intravitreal injection of bevacizumab10,11 and ranibizumab,12 intravitreal injection of triamcinolone,13,14 and intravitreal dexamethasone implant have been described to treat RME.15 However, the best therapeutic approach is still uncertain.
The purpose of this article is to describe the anatomical changes in the macula with spectral domain (SD)-OCT and best-corrected visual acuity (BCVA) improvements in a patient affected by RME treated with a single intravitreal injection of 0.7 mg dexamethasone implant. Additionally, we aim to add further information in the current literature about RME and its effective treatments.
Materials and methods
The patient provided written informed consent to publish this case report. Retrospective data of a patient who underwent intravitreal injection of 0.7 mg dexamethasone “drug delivery system” (DDS; Ozurdex®; Allergan, Irvine, CA, USA) for RME due to proton beam therapy for choroidal melanoma in the left eye were collected, as well as tumor data and radiation characteristics. The implant is made of biodegradable polymer (Novadur™; Allergan), which permits dual-phase pharmacokinetics, first delivering a burst of dexamethasone to rapidly reach therapeutic concentration, followed by a lower sustained release.16
The patient underwent a complete ocular examination before the injection, including BCVA, slit-lamp examination, funduscopy, and intraocular pressure (IOP) measurement with applanation tonometry. FFA, SD-OCT, and color fundus photography were performed with a Spectralis HRA+OCT (Heidelberg Engineering, Heidelberg, Germany) and 3D-OCT 2000 SD-OCT (Topcon Corp, Tokyo, Japan). Retinal thickness was automatically calculated using the Spectralis HRA+OCT mapping software.
The diagnosis of RME was made on the basis of anamnesis, funduscopy, FFA, OCT and classified using the Early Treatment Diabetic Retinopathy Study (ETDRS) classification for diabetic macular edema. Therefore, the term “clinically significant RME” indicated any of the following characteristics: thickening of the retina at or within 500 µm of the center of the macula; hard exudates at or within 500 µm of the center of the macula, if associated with thickening of the adjacent retina; a single or multiple zones of retina thickening 1 disk area or larger, within 1 disk diameter from the center of the macula.17
Retinal perfusion was evaluated with FFA, whereas OCT findings were assessed using the OCT-based grading system for RME, proposed by Horgan et al. This is a 5-point classification: grade 1, includes extrafoveal noncystoid macular edema; grade 2, extrafoveal cystoid edema; grade 3, foveolar noncystoid edema; grade 4, mild-to-moderate foveolar cystoid edema; and grade 5, severe cystoid foveolar edema.18
Before treatment administration, informed consent about the off-label nature of dexamethasone 0.7 mg implant in RME was obtained. The injection was performed in the vitreous cavity through pars plana at 5-mm inferonasally from limbus.
Scheduled visits occurred 15 days after the injection, at 2 months, every 2 months until 8 months, and then every 4 months until a total follow-up of 16 months. During each visit, BVCA, IOP measurement, slit-lamp examination, funduscopy, SD-OCT, and color fundus photography were performed.
Results
A 46-year-old woman with vision loss due to RME in consequence to proton beam therapy for choroidal melanoma was treated with intravitreal dexamethasone 0.7 mg implant in the left eye at the University Eye Clinic, IRCCS Policlinico San Matteo, Pavia, Italy.
At the time of the proton beam treatment, the tumor width was 4.4 mm, the length was 6.9 mm, and the thickness was 2.32 mm. It was located inferiorly to the macula and the distances of its most posterior edge from the fovea and the optic disc were 6.21 and 5.05 mm, respectively. The tumor was graded T1, according to the 7th edition of the American Joint Committee on Cancer/International Union Against Cancer Classification System for uveal melanoma. The proton beam treatment had been performed at the Centre Lacassagne (Nice, France), and the total radiation dose was 60 cobalt gray equivalent, which had been delivered in four consecutive days. Also, according to the treatment planning model, the percentage of macula receiving 50%/90% of the maximal dose was 35/0. Further information on the actual proton beam treatment performed at Centre Lacassagne was unfortunately unavailable.
RME occurred 25 months after proton beam therapy, and the intravitreal injection of the implant was performed 20 days after the detection of RME.
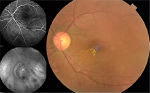
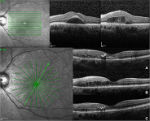
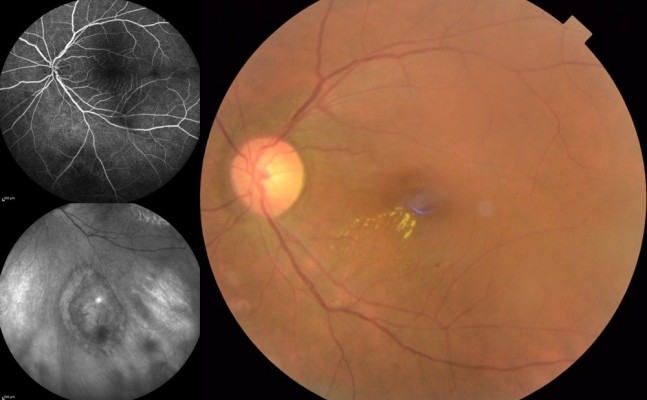
At the preinjection assessment, no systemic risk factors for the development of ME, such as diabetes mellitus and hypertension, were reported. Slit-lamp examination showed no signs of radiation cataract, radiation papillopathy, retinal neovascularization, or any other pathologies that might have affected visual acuity. BCVA was 6/12 and IOP 12 mmHg. Fundus exploration showed retinal exudates, microaneurysms, and narrowing of retinal vessels with focal irregularities. No signs of retinal ischemia were observed on FFA images, but an area of hypofluorescence in the foveal region was evident (Figure 1). According to OCT-based grading scale proposed by Horgan et al, RME was classified to grade 2 and SD-OCT images showed foveal neuroepithelium detachment, intraretinal cysts, and hard exudates (Figure 2). Retinal thickness map calculated by the Spectralis HRA+OCT mapping software shows a clinically significant RME and a central foveal thickness (CFT) of 500 µm (Figure 3).
BCVA was 6/6.4 fifteen days after the injection; it improved to 6/7.5 at 2 months and to 6/6 at 6 months, then it remained stable for the remaining 10 months. CFT decreased from 500 to 419 µm after 15 days and to 213 µm after 2 months, then it progressively increased to 285 µm at 8 months, leveling-off at 269 µm by the end of the follow-up (Figure 3). SD-OCT images acquired at each visit showed reduction of the foveal detachment 15 days after the injection and a progressive reduction of intraretinal cysts. After the foveal detachment restoration, no disruption nor discontinuity of the ellipsoid zone was evident (Figure 2).
There were no complications related to the implant injection and no signs of cataract progression were visible. An increase of 25 mmHg in the IOP was reported 2 months after the injection, but it decreased to 15 mmHg after 1-month therapy with a topical beta-blocker and remained stable until the end of the follow-up.
Discussion
Recently, it has been hypothesized that the pathophysiology of radiation retinopathy could be comparable to that of diabetic retinopathy. As a matter of fact, they both are occlusive retinal microangiopathies in which retinal inflammation pathways play a key role in the development of the pathology. Furthermore, the liberation of high levels of vascular endothelial growth factor (VEGF), due to retinal ischemia and macrophages activation, has been reported.19 Therefore, not surprisingly, the administration of intravitreal corticosteroids and antiangiogenic agents have shown positive effects in terms of reducing macular edema and stabilizing or improving visual function. Uncontrolled studies have demonstrated promising results in patients with RME treated with continuous intravitreal injections of anti-VEGF drugs for a mean follow-up of 38 and 54 months.20,21 Nevertheless, a minor proportion of patients treated with antiangiogenic drugs achieved higher and stable visual acuities. Moreover, recurrent RME was also present and further injections were required, on average, from every 4 to 8 weeks.20,22
It has been assumed that poor visual outcome after injection could be related to undetected macular ischemia or late-onset treatment.23
Increased VEGF levels in eyes with RM may represent only one aspect of the entire pathogenesis. Corticosteroids are well-known anti-inflammatory agents that act against several processes involved in the pathogenesis of macular edema.24 They control capillary permeability by stabilizing endothelial cell tight junctions,25 block leukocyte migration,26 and prevent the production of proinflammatory cytokines and VEGF.27,28
Similar to anti-VEGF therapies, intravitreal injections of triamcinolone have been proved to effectively reduce RME on OCT analysis. However, multiple injections were required and recurrent RME appeared after 3 and 6 months.13,14
The 0.7 mg dexamethasone DDS, which has been approved by the Food and Drug Administration for the treatment of macular edema in retinal vein occlusion and non-infectious uveitis, has been developed for sustained drug release up to 3–6 months. Safety concerns include cataract formation and IOP elevation that is most often transitory and responsive to medical management.16 Previous evidence has described intravitreal injections of dexamethasone implant as “rescue treatments” in eyes affected by chronic RME unresponsive to anti-VEGF treatment (bevacizumab) and triamcinolone acetonide.29,30 Data of naïve RME treated with dexamethasone implant are still poor, but good efficacy and safety results have been published, resizing the concerns due to the raise in IOP and cataract development. However, the beneficial effects reported lasted up to 5 months.15
Despite the fact that a recent study has shown similar outcomes concerning CFT and BCVA after anti-VEGF or corticosteroid intravitreal treatment for RME,31 we opted for the 0.7 mg dexamethasone DDS (Ozurdex). Our choice was guided by its good safety profile, broad multistep action on retinal inflammation, and high anti-inflammatory activity, six times greater than triamcinolone.32
To the best of our knowledge, this case describes the highest results in terms of improvement and stability in CFT and BCVA obtained after a single injection of 0.7 mg dexamethasone DDS for RME due to proton beam therapy in a 16-month follow-up, one of the longest available in literature. We believe that this result could be related to the absence of retinal ischemia on FFA images,16 the integrity of the photoreceptors and ellipsoid zone layers on OCT scans, and a prompt treatment after RME detection.31 As we expected, according to the dexamethasone DDS pharmacokinetics, there was a partial recurrence of RME 8 months after injection. Nevertheless, after this episode, not only BVCA remained stable at 6/6 but also CFT progressively decreased by the end of the follow-up (Figure 2). This phenomenon could be correlated with the retinal fluid clearance acted by Müller cells, whose water absorption activity has proven to be partially stimulated by corticosteroids.33 Therefore, the duration of corticosteroids effect on RME absorption may be related to the Müller cells integrity.
Finally, a raise in IOP occurred 2 months after injection, but it was transient and managed with eye drops; no signs of progression of cataract were detected.
Conclusion
We conclude that 0.7 mg dexamethasone DDS could be chosen as a first-line treatment for RME in some patients who have previously undergone proton beam therapy for choroidal melanoma. Furthermore, our report shows that very good and stable results in terms of visual acuity and intraretinal edema reduction could be achieved after a single intravitreal injection in a patient with the integrity of the foveal ellipsoid zone on SD-OCT and no signs of macular ischemia on FFA.
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Anderson C, Fleming P, Wilkinson A, Singh AD. Principles of radiation therapy. In: Singh AD, Damato PE, Pe’er J, Murphree AL, Perry JD, editors. Clinical Ophthalmic Oncology. 1st ed. Philadelphia, PA: Saunders-Elsevier; 2007:40–44. | ||
Gragoudas ES, Goitein M, Verhey, Munzenreider J, Urie M, Suit H, Koehler A. Proton beam irradiation of uveal melanomas. results of 5 1/2-year study. Arch Ophthalmol. 1982;100(6):928–934. | ||
Archer DB, Amoaku WM, Gardiner TA. Radiation retinopathy. Clinical, histopathological, ultrastructural and experimental correlations. Eye (Lond). 1991;5(Pt 2):239–251. | ||
Guyer DR, Mukai S, Egan KM, Seddon JM, Walsh SM, Gragoudas ES. Radiation maculopathy after proton beam irradiation for choroidal melanoma. Ophthalmology. 1992;99(8):1278–1285. | ||
Mukai S, Guyer DR, Gragoudas S. Radiation retinopathy. In: Albert DM, Jakobiaec FA, editors. Principles and Practice in Ophthalmology – Vol 2. Philadelphia, PA: WB Saunders; 1994:1038–1041. | ||
Gragoudas S, Li W, Lane AM, Munzenrider J, Egan KM. Risk for radiation maculopathy and papillopathy after intraocular irradiation. Ophthalmology. 1999;106(8):1571–1577. | ||
Levitz LM. The use of optical coherence tomography to determine the severity of radiation retinopathy. Ophthalmic Surg Lasers Imaging. 2005;36(5):410–411. | ||
Kinyoun JL, Zamber RW, Lawrence BS, Barlow WE, Arnold AM. Photocoagulation treatment for clinically significant radiation macular oedema. Br J Ophthalmol. 1995;79(2):144–149. | ||
Hykin PG, Shields C, Shields JA, Arevalo JF. The efficacy of focal laser therapy in radiation-induced macular edema. Ophthalmology. 1998;105(8):1425–1429. | ||
Gupta A, Muecke GS. Treatment of radiation maculopathy with intravitreal injection of bevacizumab (Avastin). Retina. 2008;28(7):964–968. | ||
Finger PT. Radiation retinopathy is treatable with anti-vascular endothelial growth factor bevacizumab (Avastin). Int J Radiat Oncol Biol Phys. 2008;70(4):974–977. | ||
Finger PT. Intravitreous ranimizumab (Lucentis) for radiation maculopathy. Arch Ophthalmol. 2010;128(2):249–252. | ||
Sutter FK, Gillies MC. Intravitreal triamcinolone for radiation-induced macular edema. Arch Ophthalmol. 2003;121(10):1491–1493. | ||
Shields CL, Demirici H, Dai V, et al. Intravitreal triamncinolone acetonide for radiation maculopathy after plaque radiotherapy for choroidal melanoma. Retina. 2005;25(7):868–874. | ||
Baillif S, Maschi C, Gastaud P, Caujolle JP. Intravitreal dexamethasone 0.7-mg implant for radiation macular edema after proton beam therapy for choroidal melanoma. Retina. 2013;33(9):1784–1790. | ||
London NJ, Chiang A, Haller JA. The dexamethasone drug delivery system: indications and evidence. Adv Ther. 2011;28(5):351–366. | ||
Early Treatment of Diabetic Retinopathy Study Group. Photocoagulation for macular edema. Early treatment of diabetic retinopathy study report number 1. Arch Ophthalmol. 1982;103(12):1798–1806. | ||
Horgan N, Shields CL, Mashayeki A, Shields JA. Classification and treatment of radiation maculopathy. Curr Opin Ophthalmol. 2010;21(3):233–238. | ||
Irvine AR, Wood IS. Radiation retinopathy as an experimental model for ischemic proliferative retinopathy and rubeosis iridis. Am J Opthalmol. 1987;103(6):790–797. | ||
Finger PT, Chin KJ, Semenova AE. Intravitreal anti-VEGF therapy for macular radiation retinopathy: a 10-year study. Eur J Ophthalmol. 2016;26(1):60–66. | ||
Shah NV, Houston SK, Markoe AM, Feuer W, Murray TG. Early SD-OCT diagnosis followed by prompt treatment of radiation maculopathy using intravitreal bevacizumab maintains functional visual acuity. Clin Ophthalmol. 2012;6:1739–1748. | ||
Finger PT, Mukkamala SK. Intravitreal anti-VEGF bevacizumab (Avastin) for external beam related radiation retinopathy. Eur J Ophthalmol. 2011;21(4):446–551. | ||
Mashayeki A, Rojanaporn D, Al-Dahmash S, Shields CL, Shields JA. Monthly intravitreal bevacizumab for macular edema after iodine-125 plaque radiotherapy of uveal melanoma. Eur J Ophthalmol. 2014;24(2):228–234. | ||
Leopold IH. Nonsteroidal and steroidal anti-inflammatory agents. In: Sears ML, Tarkkanen A, editors. Surgical Pharmacology of the Eye. New York, NY: Raven Press; 1985:83–133. | ||
Antonetti DA, Wolpert EB, DeMaio L, Harhaj NS, Scaduto RC Jr. Hydrocortisone decreases retinal endothelial cell water and solute flux coincident with increased content and decreased phosphorylation of occludin. J Neurochem. 2002;80(4):667–677. | ||
Mizuno S, Nishiwaki A, Morita H, Miyake T, Ogura Y. Effects of periocular administration of triamcinolone acetonide on leukocyte-endothelium interactions in the ischemic retina. Invest Opthalmol Vis Sci. 2007;48(6):2831–2836. | ||
Nehmè A, Edelman J. Dexamethasone inhibits high glucose-, TNF-alpha- and IL-1 beta-induced secretion of inflammatory and angiogenic mediators from retinal microvascular pericytes. Invest Ophthalmol Vis Sci. 2008;49(5):2030–2038. | ||
Edelman JL, Lutz D, Castro MR. Corticosteroids inhibit VEGF-induced vascular leakage in a rabbit model of blood-retinal and blood-aqueous barrier breakdown. Exp Eye Res. 2005;80(2):249–258. | ||
Russo A, Avitabile T, Uva M, et al. Radiation macular edema after Ru-106 plaque brachytherapy for choroidal melanoma resolved by an intravitreal dexamethasone 0.7-mg implant. Case Rep Ophthalmol. 2012;3(1):71–76. | ||
Tarman L, Langmann G, Mayer C, Weger M, Haas A, Wackernagel W. Ozurdex(®) reduces the retinal thickness in radiation maculopathy refractory to bevacizumab. Acta Opthalmol. 2014;92(8):e694–e696. | ||
Seibel I, Hager A, Riechardt AI, Davids AM, Böker A, Joussen AM. Antiangiogenic or corticosteroids treatment in patients with radiation maculopathy after proton beam therapy for uveal melanoma. Am J Ophthalmol. 2016;168:31–39. | ||
Goldfien A. Adrenocorticosteroids and adrenocortical antagonists. In: Katzung BG, editor. Basic and Clinical Pharmacology. 6th ed. London, UK: Prentice Hall International; 1995:592–607. | ||
Reichenbach A, Wurn A, Pannicke T, Iandiev I, Wiedemann P, Bringmann A. Müller cells as players in retinal degeneration and edema. Graefe Arch Clin Exp Opthalmol. 2007;245(5):627–636. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.