Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Long-Term Efficacy and Tolerability of a Medium G’ HA Filler with Tri-Hyal Technology on the Rejuvenation of the Mobile Facial Zone
Authors David M, Braccini F, Garcia P, Loreto F, Benadiba L, Gorj M, Grand-Vincent A, Rumyantseva Mathey E , Deutsch JJ, Ehlinger A, Cartier H, Nadra K, Fanian F
Received 20 March 2023
Accepted for publication 30 June 2023
Published 13 July 2023 Volume 2023:16 Pages 1795—1805
DOI https://doi.org/10.2147/CCID.S395353
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Michel David,1 Frederic Braccini,2 Philippe Garcia,3 Frederic Loreto,3 Laurent Benadiba,3 Mihai Gorj,3 Anne Grand-Vincent,3 Elena Rumyantseva Mathey,3 Jean-Jacques Deutsch,3 Agnès Ehlinger,4 Hugues Cartier,5 Karim Nadra,6 Ferial Fanian6
1Private Clinic, Metz, France; 2Private Clinic, Nice, France; 3Private Clinic, Paris, France; 4Private Clinic, Thionville, France; 5Private Clinic, Arras, France; 6Laboratories Fill-Med, Paris, France
Correspondence: Ferial Fanian, Tel +33673761820, Email [email protected]
Purpose: Injectable hyaluronic acid-based fillers are commonly used for the correction of skin contour irregularities and to smooth skin depressions formed by volume loss during the aging process. These fillers are particularly efficient to restore perioral skin depressions/wrinkles or to correct topographical anomalies. The European directives require a continuous evaluation of the performance of these medical devices, particularly for CE marked products.
Methods: An 18-month prospective randomized single-blind study for the efficacy and safety of ART FILLER Universal (AFU) was performed on the lips, the nasolabial folds, and the marionettes lines. The evaluations were performed on 153 subjects enrolled in this study. The efficacy, the longevity, and the safety were evaluated for the injected areas via area specific clinical scoring after a single injection with the filler and with no re-touch.
Results: We showed here that filler injection induced potent improvements of volume restoration after a single injection on all the treated areas. These beneficial properties of the filler were significant 3 weeks after injection and during the whole study period. Moreover, injections of the filler were well tolerated by the subjects. The recorded adverse events are routinely seen with HA fillers for face volume corrections, and most of these local reactions resolved within 14 days.
Conclusion: AFU was well tolerated and showed a continuous efficacy for at least 18 months, in exploratory analyses.
Keywords: hyaluronic acid, dermal fillers, facial injections, anti-aging, skin rejuvenation, perioral area
Introduction
The perioral region is significantly impacted by facial aging and commonly targeted by facial rejuvenation strategies.1 In addition to lips thinning, the most significant signs of midface aging are the appearance of deepening nasolabial folds and marionette lines. The nasolabial folds are defined by facial wrinkles that extend from the edge of the nose to the mouth’s outer corners. The marionette lines run vertically from the mouth corners to the chin. These skin structures appear due a loss of dermal collagen synthesis, the subcutaneous fat shift, and bone resorption.2
Hyaluronic acid (hyaluronan or HA) is one of the most frequently used dermal fillers allowing the expansion of soft tissues, the filling of wrinkles, and the treatment of skin irregularities.3 Once injected, hyaluronic acid shows a significant effect in the maintenance of tissue augmentation for up to nine months.4 This longevity property is permitted by its chemical cross-linking to chemical agent such as the 1,4-butanediol diglycidyl ether.
ART FILLER Universal (AFU) has a specific combination of different crosslinked hyaluronic acid of non-animal origin, slowly absorbable over time and containing 0.3% by weight of lidocaine hydrochloride for its anesthetic properties. The concentration of lidocaine within this filler is used by many similar fillers and has no influence on tolerability and efficacy and showed significant benefits in terms of reducing the pain sensation during injections.5,6 Regarding its chemical properties, AFU has no major innovative characteristics compared to other existing HA-based products.
The European directives require manufacturers of injectable fillers to provide the evaluation of the safety and performance of their products according to the different anatomical zones. On the other hand, the medical devices with CE marks need a continuous evaluation of their performance, their safety, and their clinical benefits. In this context, we carried out, in post-CE marking, a prospective and non-comparative study to document AFU properties regarding the adequate aesthetic correction of lips, the nasolabial folds, and the marionette lines as well as their immediate and long-term tolerance. The main objective of this study was to measure the aesthetic improvement of the injected areas from baseline to 21 days post injection after a single injection of AFU. The secondary objectives were to assess the long-term effectiveness of the volume improvement on the different injected zones over 540 days (18 months), as well as to assess the safety and tolerance of AFU.
Materials and Methods
Population
Before their inclusion, all of the subjects submitted to this study signed a written informed consent. The study strictly followed the requirements of the Declaration of Helsinki and is GCP adapted. The subjects were pre-selected between day −120 and day −7 by the company SYRES or the investigators involved in the study according to pre-defined inclusion criteria. Subjects were males or females >19 years old, a Fitzpatrick Phototype I to IV, having a score of ≥0 and ≤2 on the lip volume fullness scale from Medicis, and a grade ≥2 and ≤4 for the folds scale from Bazin. The selected subjects were informed about the study process (Figure 1A). The per-protocol population evaluated in this study was defined by individuals who completed the follow-up visit at days 21, 90, 180, 270, 360, 450, and 540 and a global aesthetic score was determined. The safety population comprises all individuals registered in the study and for whom at least one injection of the evaluated product has been executed.
Test Products and Procedure of Injection
AFU is a crosslinked, hyaluronic acid of non-animal origin, slowly absorbable over time, colorless, sterile, non-pyrogenic, physiologically transparent, viscoelastic gel containing 0.3% by weight of lidocaine hydrochloride for its anesthetic properties. It contains 25 mg of 3 types of HA: very long chain with molecular weight of around 3 million Dalton, long chain HA with the molecular weight of 1 million Dalton, and free HA with the molecular weight of 1 million Dalton. The product contains no LMW HA. This gel comes in a pre-filled 1.2 mL graduated disposable syringe with two 27G1/2 × 13 mm needles. The volume of product injected was adapted to the corrected area, but a maximum of 1.2 mL each side was authorized to be injected for the nasolabial folds, 1.2 mL each side for the marionette lines, and 1.0 mL for the lips (Figure 1B). The injection technique used for the filler was intradermal injection with a 27G1/2 (13 mm) TSK needle or a 25G/55 mm cannula (SoftFil). The processes of injection, massaging, and evaluation of the correction were performed depending on the injected areas and the habits of physicians. For injection by cannula, a local anesthetic could be injected at the entry of the needle. No regional anesthesia through nerve blocks was authorized for this study for all zones.
Evaluation Criteria
The primary criterion of the study was the Global Aesthetic Clinical Score (GACS)7 that was evaluated by the investigator in the presence of the subject at each visit and for all the face studied area. This GACS scoring contains a 7-grade scale (0 to 3 points with a 0.5 point interval). No sagging or volume loss was scored 0, and extreme sagging or extreme volume loss was scored 3. According to photographic scale, the Medicis Lips Fullness (MLFS) and the Bazin scale for the nasolabial folds and the marionette lines were used in order to confirm the aesthetic improvement observations. These different scales have been previously validated and published.8,9
Ethical Approval
This study protocol was submitted by FILORGA Laboratories to the Ile de France V CPP (St Antoine hospital, 284 rue du Faubourg Saint Antoine, 75,012 Paris, France) (People Protection Committee). The set-up and initiation of this study was done in the investigative centers after the favorable decision of the CPP was obtained. The favorable decision was obtained on April 2, 2019.
Data Collection and Analysis
The execution of this study and the training of physicians involved in the use of the devices studied was provided by Laboratoires FILL-MED. The evolution of the global aesthetic score between the basal value and the value of the selected day was calculated for each treated zone on the PP population. Mean and median variations in scores between the initial injection day and the follow-up visits were evaluated. A statistical Wilcoxon test was used to compare the significance of the score evolutions. The evolution of the global aesthetic 7-grade scale (score 0 to 3) between the baseline value (D0) and the other time points was calculated for each treated zone on the PP population. The percentage of success was also evaluated by the ratio of satisfactory responses for each visit.
The collected data can be shared by request to the corresponding author.
Results
Population and Procedure
For this prospective and non-comparative study, a total of 153 subjects were enrolled (9 males and 144 females) with a mean age of 51.7 years (27–77 years old). Within this intention-to-treat population, 69% had no history of aesthetic treatment, 26% had been previously submitted to an HA injection, and the remaining 5% experienced other type of aesthetic interventions. The subject’s weight was recorded before injection and during the study to follow their body mass index (BMI). The average BMI was 24.1 and remained stable during the study. Seven individuals have withdrawn from the study before the final evaluation visit (D540). The subjects were injected into a maximum of 3 different areas including the lips, the nasolabial folds, and the marionette lines (Figure 1B). The efficacy of the procedure was assessed at 3 weeks post injection and the persistence of the correction was evaluated at 6, 9, 12, 15, and 18 months (Figure 1A). Among the enrolled population, 50 subjects were injected in the lips, 130 in the nasolabial folds, and 64 in the marionette lines. All of the patients injected in the marionette line were injected also on the nasolabial fold. The cross injection between nasolabial fold and lip volume was possible but not obligatory. No touch up re-injections were allowed during the study. The mean injection volume was 1.2 mL for the lips and 2.4 mL for the nasolabial fold as well as for the marionette lines.
Evolution of the GACS Score and the Success Rate
The primary objective of the study was to objectively quantify the improvement, according to a Global Aesthetic Clinical Scoring (GACS), of lip volume and nasolabial folds alone or with the marionette line, after a single injection of the filler. The evolution of the GACS between the basal value and the value on D21 was calculated for each treated zone. A reduction of at least one grade in the scale (−0.5 point) was defined as a satisfactory correction (except for the lips where an increase of 1 grade (+0.5 point) was defined as a satisfactory correction). As shown in Figure 2, AFU injection induced a significant GACS modification for all areas after 3 weeks. A favorable score evolution of 0.7 for the lips, 1.1 for the nasolabial folds, and 1.2 for the marionette lines were detected 21 days post injection. These significant evolutions were preserved during all the study period until day 540 (Figure 2A–2C). Furthermore, the success percentage 18 months post injection, represented by the subjects for whom the initial GACS score was positively modified from 1 grade (=0.5 point on the GACS scale), was of 32% for the lips, 74% for the nasolabial folds, and 82% for the marionette lines (Figure 3A–3C).
Evaluation of the Efficacy Duration of the Fillers with Area Specific Scales
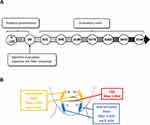
To confirm the persistence of the observed corrections, area specific scales were used to assess the evolution of each injected area. The Medicis Lips Fullness scale (MLFS) was significantly increased by 77% at day 21, and this augmentation was kept significant until day 540 (+38%) (Figure 4A). The Bazin score for the nasolabial folds severity decreased significantly by 47% at day 21 and remained significant during all the investigation period (−22% at D540) (Figure 4B). Similarly, the Bazin score for the marionette lines severity was reduced by −50% at day 21 and was kept significantly lower until day 540 by at least −27% (Figure 4C).
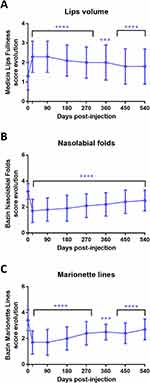 |
Figure 4 Evolution of the Medicis Lips Fullness score (A), the Bazin Nasolabial Fold score (B), and the Bazin Marionette Lines score (C). Mean ± sd, ****p < 0.0001, ***p < 0.001, compared to D0. |
The Aesthetic Benefits of the Filler are Confirmed by the GAIS Score and the Rosenberg Self-Esteem Score
The subjects' and investigators' satisfaction were assessed by the 7-grade Global Aesthetic Improvement Scale (GAIS) score (+3 for very much improved, −3 for very much worse). As shown in Figure 5, the GAIS score assessed by the investigators was closely related to the score recorded by the subjects themselves. Of note, a global improvement for all the treated areas was still observable at D540. Interestingly, despite the lower percentage of success between the injected areas at D540 (Figure 3A), the lip injections showed the best satisfaction rate according to the GAIS score (+1 at D540). The benefit of the filler injections and their aesthetic outcomes showed also a positive psychological impact as demonstrated by the evolution of the Rosenberg self-esteem score based on the results from the questionnaire of self-esteem on the whole population of the study. We found that AFU injections induced a significant improvement on the subject’s self-esteem as early as 3 weeks post injection which remained stable until 18 months (Figure 6).
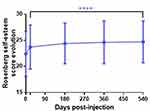 |
Figure 6 Evolution of the Rosenberg self-esteem score based on the results from the questionnaire of self-esteem on the whole population of the study. Mean ± sd, ****p < 0.0001, compared to D0. |
Safety Assessment
The aspect and the sensitivity of the treated areas during and after injections were evaluated.
Seventy-nine subjects had no adverse event after the injections while a total of 74 patients (48% of the total population injected) reported 187 adverse events during the whole study (including all areas). Among them, N=57 (30%) were not related to the injections, N=110 (59%) were possibly related to injection, N=17 (9%) probably, and N=3 (2%) doubtfully. These adverse events concerned mainly edema (28%), hematoma (20%), erythema (8%), irregularities at palpation (14%), or pain (14%). As shown in Figure 7, these adverse events were classified from slight to severe. Twenty-seven events were recorded after lip injections, 34 events after nasolabial fold injections, 18 events after marionette line injections, and 5 events not applicable to the zones. Forty-five adverse events had missing data regarding the injection zone. However, no severe event has been reported. Overall, most of these detected events are routinely seen when using HA filling procedures for face volume corrections, and most of these local reactions were resolved within 14 days after injection.
 |
Figure 7 Summary of the adverse events related to injections over the whole study and categorized in slight, moderate, and important events in %. |
Discussion
Injection of HA-based fillers for wrinkle correction becomes a common aesthetic procedure in modern cosmetic practice. The choice of this filler type for safe interventions is based on its characteristics including the comfort of injection, long-lasting, resistance to deformation after injection,10 biocompatibility, reversibility,11 and a low degree of immunogenicity.12
In this study, we report the immediate and the long-term clinical results of AFU injection in the lips, the nasolabial folds, and the marionette lines. Our observations revealed a satisfactory result on all the investigated area (a significant improvement of at least 1 grade observed for more than 80% of the subjects, from 86% for the lips to 96% for the marionette lines). The beneficial effects were persistent and remained significant 18 months post injection. Many similar studies assessing HA fillers efficacy on these perioral areas were limited in their duration or with touch-up possibilities,rendering difficult the assessments of longevity.13–15 Therefore, thanks to the 18-month follow-up protocol, the observations performed in this study can be used as suitable data to determine and compare the longevity of HA-based filler injections. These observations confirmed the long-term efficiency of this medium G’ HA-based filler (120–220 Pa) in the perioral zone but when we compare the results with the previously published article on another HA filler with the same technology (Tri-Hyal Technology) and on the same area (nasolabial fold, lip volume, and marionette line), we could observe that the success rate is deeply dependent to the G’.16 For each evaluated area, a higher success rate was obtained at day 21 (main criteria time point) for the product with higher G’ (ART FILLER® Lips, G’=180–280 Pa, data published in the company brochure) versus AFU (G’=120–220 Pa, data published in the company brochure) except the lip volume. For lip volume, the success rate of AFU at day 21 is 72.73% while for AFL is 76.47%. However, this parameter is 32% at 18 months for AFU while it is 33% for the lips. The success rate is 74% for nasolabial fold injected by AFU compared to 98.4% for AF LIPS and 82% vs 94.4% for the marionette lines regarding AFU and AF Lips respectively.16
The satisfactory results obtained on the per-protocol population were consistent with results from the intention-to-treat population. When taken together, the treated areas showed an average improvement of the global score that remained observed for 90% of the subjects after 3 months, 81.5% after 6 months, 70% after 12 months, and 57% after 18 months (the best longevity was observed for the marionette lines, then the nasolabial folds). Whereas at least 88% of the subjects were satisfied with the global aesthetic improvement 21 days after injection, they were still at least 63% to be satisfied after 12 months and 49% after 18 months. Interestingly, the efficiency observed in our study for the nasolabial folds was better than recently published observations by Cheon et al using two different HA-based fillers (DIVAVIVA medium and Restylane Perlane).15 52 weeks after injection, 74% of subjects perceived an improvement in the GAIS scale after AFU injection; and only 37.8% with DIVAVIVA and 34.1% with Restylane. This difference could be explained by the maximal injected dose. Here, a maximal dose of 1.2 mL was tolerated whereas 1.0 mL only was tolerated in Cheon et al's study.
The injections by AFU were well tolerated by the subjects and induced mainly some minor side effects. Most reported reactions were slight pain during injection, and erythema or edema or pain just after injection. Most of them had disappeared after 3 weeks. These reported side effects were those typically expected following HA filler injection,17,18 confirming the safety of the tested filler for the treatment of the selected areas in this study.
In conclusion, this study confirmed the performance and safety of the injectable filler AFU. When compared to previous publications assessing the efficacy of HA-based fillers in lips, marionette lines, or nasolabial folds, our study confirms the non-inferiority and the safety of this filler up to 18 months.
Disclosure
The hyaluronic acid-based filler under investigation in this study is a product developed and commercialized by FILLMED Laboratories. Dr Ferial Fanian and Dr Karim Nadra are both employees of FILLMED Laboratories. Dr Frederic Loreto reports personal fees from Filorga, during the conduct of the study. None of the other authors declared any potential conflicts of interest with respect to the research, authorship, and publication of this article.
References
1. Morera Serna E, Serna Benbassat M, Terre Falcon R, Murillo Martin J. Anatomy and aging of the perioral region. Facial Plast Surg. 2021;37(2):176–193. doi:10.1055/s-0041-1725104
2. Hu X, Xue Z, Qi H, Chen B. Comparative study of autologous fat vs hyaluronic acid in correction of the nasolabial folds. J Cosmet Dermatol. 2017;16(4):e1–e8. doi:10.1111/jocd.12333
3. Gilbert E, Hui A, Waldorf HA. The basic science of dermal fillers: past and present Part I: background and mechanisms of action. J Drugs Dermatol. 2012;11(9):1059–1068.
4. Narins RS, Bowman PH. Injectable skin fillers. Clin Plast Surg. 2005;32(2):151–162. doi:10.1016/j.cps.2004.12.002
5. Brandt F, Bank D, Cross SL, Weiss R. A lidocaine-containing formulation of large-gel particle hyaluronic acid alleviates pain. Dermatol Surg. 2010;36 Suppl 3:1876–1885. doi:10.1111/j.1524-4725.2010.01777.x
6. Lupo MP, Swetman G, Waller W. The effect of lidocaine when mixed with large gel particle hyaluronic acid filler tolerability and longevity: a six-month trial. J Drugs Dermatol. 2010;9(9):1097–1100.
7. Kopera D, Palatin M, Bartsch R, et al. An open-label uncontrolled, multicenter study for the evaluation of the efficacy and safety of the dermal filler Princess VOLUME in the treatment of nasolabial folds. Biomed Res Int. 2015;2015:195328. doi:10.1155/2015/195328
8. Bazin RD. Skin aging atlas; 2007:1.
9. Carruthers A, Carruthers J, Hardas B, et al. A validated grading scale for marionette lines. Dermatol Surg. 2008;34(2):S167–172. doi:10.1111/j.1524-4725.2008.34366.x
10. Kablik J, Monheit GD, Yu L, Chang G, Gershkovich J. Comparative physical properties of hyaluronic acid dermal fillers. Dermatol Surg. 2009;35(1):302–312. doi:10.1111/j.1524-4725.2008.01046.x
11. Brandt FS, Cazzaniga A, Brandt F. Hyaluronic acid gel fillers in the management of facial aging. Clin Interv Aging. 2008;3(1):153–159. doi:10.2147/CIA.S2135
12. Bentkover SH. The biology of facial fillers. Facial Plast Surg. 2009;25(2):73–85. doi:10.1055/s-0029-1220646
13. Noh TK, Moon HR, Yu JS, et al. Effects of highly concentrated hyaluronic acid filler on nasolabial fold correction: a 24-month extension study. J Dermatolog Treat. 2016;27(6):510–514. doi:10.3109/09546634.2016.1170759
14. Narins RS, Brandt FS, Dayan SH, Hornfeldt CS. Persistence of nasolabial fold correction with a hyaluronic acid dermal filler with retreatment: results of an 18-month extension study. Dermatol Surg. 2011;37(5):644–650. doi:10.1111/j.1524-4725.2010.01863.x
15. Cheon HI, Kim JH, Kim BJ, Lee YW. Efficacy and safety of a new hyaluronic acid filler for nasolabial folds: a 52-week, multicenter, randomized, evaluator/subject-blind, split-face study. J Cosmet Dermatol. 2021;20(5):1467–1473. doi:10.1111/jocd.13773
16. Ehlinger-David A, Gorj M, Braccini F, et al. A prospective multicenter clinical trial evaluating the efficacy and safety of a hyaluronic acid-based filler with Tri-Hyal technology in the treatment of lips and the perioral area. J Cosmet Dermatol. 2023;22(2):464–472. doi:10.1111/jocd.15169
17. Goodman GJ, Liew S, Callan P, Hart S. Facial aesthetic injections in clinical practice: pretreatment and posttreatment consensus recommendations to minimise adverse outcomes. Australas J Dermatol. 2020;61(3):217–225. doi:10.1111/ajd.13273
18. Ortiz AE, Ahluwalia J, Song SS, Avram MM. Analysis of U.S. food and drug administration data on soft-tissue filler complications. Dermatol Surg. 2020;46(7):958–961. doi:10.1097/DSS.0000000000002208
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.