Back to Journals » Vascular Health and Risk Management » Volume 15
Long-term effects on survival after a 1-year multifactorial vascular risk factor intervention after stroke or TIA: secondary analysis of a randomized controlled trial, a 7-year follow-up study
Authors Hagberg G , Fure B, Sandset EC, Thommessen B, Ihle-Hansen H, Øksengård AR , Nygård S, Wyller TB, Ihle-Hansen H
Received 1 November 2018
Accepted for publication 11 January 2019
Published 7 February 2019 Volume 2019:15 Pages 11—18
DOI https://doi.org/10.2147/VHRM.S191873
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Harry Struijker-Boudier
Guri Hagberg,1,2 Brynjar Fure,3 Else Charlotte Sandset,4 Bente Thommessen,5 Håkon Ihle-Hansen,1,2 Anne Rita Øksengård,1 Ståle Nygård,6 Torgeir B Wyller,2,7 Hege Ihle-Hansen1,7
1Department of Internal Medicine, Bærum Hospital, Vestre Viken Hospital Trust, Drammen, Norway; 2Institute of Clinical Medicine, University of Oslo, Oslo, Norway; 3Department of Internal Medicine, Karlstad Central Hospital and Institute of Public Health, University of Tromsoe, Tromsoe, Norway; 4Department of Neurology, Oslo University Hospital, Oslo, Norway; 5Department of Neurology, Akershus University Hospital, Lørenskog, Norway; 6Department of Informatics, The Faculty of Mathematics and Natural Sciences, University of Oslo, Oslo, Norway; 7Department of Geriatric Medicine, Oslo University Hospital, Oslo, Norway
Background: Stroke and coronary heart disease share the same risk factors, and a multifactorial intervention after stroke may potentially result in the same reduction in cardiovascular mortality as seen after coronary events. We aimed to evaluate the effect on survival 7 years after a 1-year multifactorial risk factor intervention, and identify clinical predictors for long-term survival in a hospital-based cohort of patients with first-ever stroke or transient ischemic attack (TIA).
Materials and methods: We performed a secondary analysis of a randomized controlled trial including patients between February 2007 and July 2008 comparing an intensive risk factor intervention vs usual care the first year poststroke to prevent cognitive impairment. From February 2014 to July 2016, all patients were invited to a follow-up. For patients dying throughout the follow-up period, date of death was obtained from the medical record. Examination at baseline and 1-year follow-up included extensive assessment of vascular risk factors and cognitive assessments.
Results: A total of 195 patients were randomized. Mean (SD) age was 71.6 (12.5) years, 53.3% were male, mean (SD) body mass index (BMI) was 25.6 (4.1) kg/m². During follow-up, 35 patients in the intervention group and 41 in the control group died. Kaplan–Meier survival estimates show no significant difference in intention-to-treat (ITT) population or complete case (CC) population (log-rank P=0.29 vs log-rank P=0.07). In multivariable Cox proportional hazards regression analyses, lower age and higher BMI was independently associated with long-term survival, adjusted HR (95% CI) 1.08 (1.05–1.11) per year and 0.91 (0.85–0.97) per kg/m².
Conclusion: In this post hoc analysis, we found no significant effect on survival after 7 years of a multifactorial risk factor program given during the first year after first-ever stroke or TIA. Higher BMI was an independent predictor for long-term survival in this cohort.
Keywords: RCT, stroke, cardiovascular risk, risk factor management, secondary prevention
Introduction
Stroke remains one of the leading causes of disability and death worldwide,1 despite a decrease in stroke mortality in the last decades. The substantial long-term risk for recurrent stroke might justify prolonged management strategies for stroke survivors.2
After a coronary event, participation in a cardiac rehabilitation program reduces total and cardiovascular mortality, as well as hospital readmissions.3 Successful programs involve a multifactorial risk-reduction regimen including exercise. In contrast, stroke survivors have limited lifestyle support.4 Since stroke and coronary heart disease share many risk factors, a multifactorial intervention after stroke may potentially have beneficial effects on mortality.5
In the CAST study (Cognition After Stroke), 227 patients with first-ever stroke or transient ischemic attack (TIA) were randomized to a multifactorial intervention program aimed to preserve cognition. At 1 year poststroke, there were no statistically significant differences between the groups on cognition, but the intervention was associated with a reduction in anxiety and depression.6 More patients in the intervention group reached the targets for blood pressure and lipid values.7
Long-term follow-up of intervention studies poststroke are important. The aim of this follow-up and post hoc analysis was to evaluate the effect on survival after 7 years of the 1-year multifactorial risk factor intervention, and to identify clinical predictors for long-term survival in this hospital-based cohort of stroke survivors.
Materials and methods
Trial design and randomization
CAST was a randomized, evaluator-blinded, controlled trial with two parallel groups. Patients with stroke (ischemic stroke or intracerebral hemorrhage) or TIA were randomized to intensive risk factor intervention in the outpatient clinic or standard care in the primary healthcare service. Patients in the intervention group were invited to follow-up at 3 and 6 months, for targeted and individualized multifactorial vascular risk factor management. Details of the intervention and the short-term results have previously been reported,7 with cognition as the primary endpoint, measured by the trail making test A (TMT-A)8 and the 10-word test from the Repeatable Battery for the Assessment of Neuropsychological Status.9 Anxiety and depression as secondary outcomes assessed with Hospital Anxiety and Depression Scale10 at 12 months and has previously been reported.6,7
Participants
All patients with a first-ever stroke or TIA, without known cognitive impairment, defined by a score <3.7 on the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE),11 admitted to the stroke unit of Baerum Hospital between February 2007 and July 2008, were invited to participate in the study. Patients with a life expectancy of more than 1 year and able to perform cognitive assessments were randomized following the acute phase (day 7–10). From February 2014 to July 2016, surviving participants were invited to a follow-up. For patients who died during follow-up, the date of death was obtained from the patients’ medical record and all-cause mortality was assessed through data from the Norwegian Causes of Death Register. Since this is a follow-up study, power calculation was based on the primary aim of the initial study.7
Assessments
At baseline, recorded vascular risk factors included treated hypertension prestroke, hyperlipidemia (total cholesterol >5.0 mmol/L, low-density lipoprotein [LDL]-cholesterol >3.0 mmol/L), diabetes mellitus (an established diagnosis or hemoglobin A1C [HbA1c] ≥7.0%), atrial fibrillation (AF), current smoking, and daily alcohol intake. Patients underwent neuroimaging with MRI or computerized tomography. Functional outcome was assessed with the modified Rankin scale (mRS).12 Neurological impairments were measured by the National Institute of Health Stroke Scale,13 and activities of daily living (ADL) were assessed using the Bartel ADL index14 at baseline and follow-up. Cognition was assessed with Mini Mental State Examination (MMSE),15 TMT-A, and 10-word tests at baseline, after 12 months, and at 7 years follow-up. The same team of physicians and study nurse were used during the study.
Fasting blood samples, electrocardiography, and blood pressure were collected at baseline and12 months follow-up. At the same time points, weight and height were measured and body mass index (BMI) calculated. Calculated BMI groups: 1: <18.5 kg/m2 underweight, 2: 18.5–24.9 kg/m2 normal weight, 3: 25.0–29.9 kg/m2 overweight, and 4: >30.0 kg/m2 obese. Self-reported smoking, alcohol use, physical activity, and current medication were recorded.
Intervention
Patients in the intervention group were invited to the outpatient clinic for a consultation with a study nurse and stroke physician at 3, 6, and 12 months. The intervention was based on the American Heart Association (AHA) 2006 recommendations regarding secondary stroke prevention16 and included medical treatment and promotion of a healthy lifestyle. Risk factor targets were blood pressure ≤140/90 mmHg, total cholesterol ≤5.0 mmol/L, LDL-cholesterol ≤3.0 mmol/L, HbA1c≤7.0%, homocysteine ≤15 µmol/L, and BMI ≤25 kg/m.2 The medical treatment was optimized, and patient education was given individually regarding stroke recurrence risk, prognosis, rehabilitation, and how to preserve brain health. Tailored advice regarding lifestyle changes included regular moderate physical activities and possibilities for strength and balance training and smoking cessation courses in groups if necessary. We recommended a diet rich in fruit, vegetables, and fish, low-fat dairy products, and less sugar in combination with encouragement to avoid excessive use of alcohol.
Statistical analyses
Baseline characteristics are given in mean ± SD or as number and percentage as appropriate. Categorical variables were compared with Pearson’s chi-squared test and continuous variables with independent Student’s t-test. The long-term effect of the intervention upon survival was analyzed using Kaplan–Meier plots and Cox proportional hazard models adjusting for age and BMI. Predictors of survival were assessed using univariable and multivariable Cox proportional hazards regression analyses. The assumptions of proportional hazards were checked by visual inspection of log minus log plots. Variables with P<0.1 in univariable analyses were included in the multivariable analysis. Finally, as an exploratory analysis, we used Kaplan–Meier plots and log-rank test to study the effect of different BMI categories on survival. Analyses were performed using SPSS Statistic version 23.
Ethical approval and informed consent
Ethical approval for this study was obtained from the Regional Committee for Ethics in Medical Research and by the Data Protection Authorities (2013/1829). Written informed consent was obtained from all subjects or next of kind before inclusion in the study.
Results
Study population and intervention
Of the 227 patients included in 2007/2008, a total of 195 patients were randomized. Of the 13 patients who did not complete the intervention, eight patients discontinued and five died during the first year. Mean follow-up time was 7.3 years (0–9.9), with 76 deaths in total; 35 patients (36 %) in the intervention group and 41 (42 %) in the control group. Thus, the intention-to-treat (ITT) analysis comprised 98 patients in the intervention group and 97 in the control group, and the complete-case (CC) analysis comprised 85 patients in the intervention group and 97 in the control group. Flowchart of the study population is presented in Figure 1.
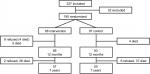  | Figure 1 Flowchart of the study population. |
At baseline there was no significant group differences regarding age, BMI, stroke subtype, risk factors, or MMSE scores. At 12 months, significantly more patients in the intervention group reached the treatment targets for systolic blood pressure and hyperlipidemia. Daily alcohol use and cigarette smoking were reduced in both the groups, but without any significant difference. No significant difference was seen regarding physical activity; mean 225±224 min/week in the intervention group and 192±201 min/week in the control group, P=0.30. At 12 months, mean BMI was significantly higher in the intervention group; mean 26.6±3.2 kg/m2 in the intervention group vs 25.7±4.5 kg/m2 in the control group, P=0.007. After 7 years, mean BMI was 26.8 (SD 3.8) kg/m2 in the intervention group and 26.7 (SD 4.0) kg/m2 in the control group, and ~70% of the survivors had a BMI >25 kg/m2. The baseline characteristics of the study population, including risk factor measurements after 1 year, are presented in Table 1.
Intervention and long-term effect on mortality
Kaplan–Meier survival curves for the ITT and the CC population, respectively, are presented in Figure 2. Log-rank P-values are 0.29 (ITT) and 0.07 (CC).
  | Figure 2 Kaplan–Meier survival curves for (A) the intention-to-treat and (B) complete case population. |
In multivariate Cox regression analysis, adjusted for age and BMI, we found no significant difference between the intervention and the control group, neither in ITT analysis (HR 0.92, 95% CI 0.56–1.52) nor in CC analysis (HR 0.71, 95% CI 0.41–1.23). Causes of death during follow-up are presented in Table 3.
  | Table 3 Causes of death during follow-up |
Predictors for long-term survival
All baseline variables shown in Table 1 were assessed using Cox proportional hazards regression analyses (Table 2). In the univariable models, higher age and higher mRS score were associated with higher mortality, whereas hyperlipidemia, higher BMI, and higher MMSE at discharge were associated with lower mortality. In the multivariable Cox regression model, including age, hyperlipidemia, AF, BMI, mRS at discharge, and MMSE at discharge, only younger age and higher BMI remained independent predictors for long-term survival over 7 years (HR 1.08, 95% CI 1.05–1.11, and HR 0.91, 95% CI 0.85–0.97, respectively). In a multivariable Cox regression model, without BMI, only age remained independent (HR 1.09, 95% CI 1.06–1.12).
Kaplan–Meier survival curves of calculated BMI groups are presented in Figure 3.
  | Figure 3 Kaplan–Meier survival curves of calculated body mass index groups. |
At baseline, seven patients had BMI <18.5 kg/m2, 74 between 18.5 and 24.9 kg/m2, 72 between 25 and 29.9 kg/m2, and 24 patients ≥30 kg/m2.
Discussion
Although mortality was lower with a maximum difference of ~20% at 7 years, we found no significant effect on survival after 7 years of a multifactorial risk factor program given during the first year after first-ever stroke or TIA. Lower age and higher BMI were independent predictors for 7 years survival in this cohort.
Intervention and long-term effect on mortality
This is a post hoc analysis and clearly underpowered to detect differences in clinical outcomes. Our findings must be interpreted with caution, but the trend toward better survival in the intervention group is similar to the results of a trial of 70 patients randomized to lifestyle intervention followed for 3 years.17 Moreover, a recent systematic review and meta-analysis indicated that lifestyle intervention following stroke is significantly associated with lower blood pressure,18 an important contributor to the general decline in stroke mortality. In our study, significantly more patients in the intervention group reached the treatment targets for systolic blood pressure and hyperlipidemia at 12 months. The intervention in this study included advice regarding diet and encouragement to preform regular moderate physical activity, but no significant differences were observed between the groups at 12 months. Physical activity is associated with reduced cardiovascular mortality,19 but more specific training programs and strict dietary advices are probably needed to see any difference, as shown in previous studies.20,21 Our study differs from previous trials in that the study population was older, we included all types of stroke, and the follow-up was longer.
The cardiac model of rehabilitation, with pharmacological and lifestyle interventions, has widespread availability, and studies show effect regardless of age.22 Small studies have indicated that stroke patients can benefit in a similar manner as patients with heart diseases, both in the postacute period and later in the chronic phase.23–25 A large ongoing trial with multidomain approach after stroke or TIA is the intensified secondary prevention intending a reduction of recurrent events in TIA and minor stroke patients (INSPiRE-TMS),26 with an estimated completion date of December 2018.
Predictors for long-term survival
Lower age and higher BMI were independent predictors for long-term survival in this cohort. Taking BMI out of the multivariate Cox regression model, since patients in the intervention group had higher BMI at 12 months, did not change these results. There is an increasing evidence suggesting an inverse relationship between BMI and total poststroke mortality.27,28 However, there is no prospective data on the effect of weight loss as part of secondary prevention on survival after stroke in adults.29 The negative association between low weight and mortality may have several explanations. Most importantly, undernourished patients tend to suffer from more stroke complication including infections and gastrointestinal bleedings during their hospital stay.30 Furthermore, weight loss after stroke may be preceded by poor nutritional status in the early phase, especially due to swallowing problems.30,31 In the general population, low BMI is an independent risk factor of total mortality in the elderly,32 and poor outcome in underweight patients could be a contributing factor to our results. At present, AHA guidelines for secondary prevention after stroke or TIA recommend screening for obesity with measurement of BMI, but the usefulness of weight loss among patients with a recent TIA or stroke is uncertain.33 Our findings support these recommendations, in contrast to guidelines from 2006 which recommended weight loss in those with BMI >25 kg/m2.
The impact of different risks factors of stroke differ according to both age and etiology.34 Looking at the causes of death, fewer patients in the intervention group died of vascular disease. However, due to small numbers, these results are difficult to interpret. With increasing age conventional vascular risk factors lose their predictive value for stroke, as functional status and cognition being more important.35 In line with this, and with mean age 71.6 years and all stroke types included at baseline, our findings seem reasonable.
Strengths and limitations
This is a post hoc analysis of a study originally designed and powered to test whether a multifactorial vascular risk factor intervention could prevent cognitive impairment, underpowered to study survival. Furthermore, the inclusion of both TIA, ischemic and hemorrhagic stroke, with possible different attributable risks by different cardiovascular risk factors, in combination with the limited sample size, makes it difficult to find any group differences. Only patients with life expectancy more than 1 year were included in the study, so our findings might not be applied to most of the stroke patients. In addition, our findings might be biased by comorbidities and age-related complications due to high age at baseline and long-time follow-up. However, the strength of our trial is the randomized controlled design in a representative sample of stroke survivors, with long-term follow-up by the same team of physicians and study nurse, which may produce data of high quality. Long-term follow-up of RCTs after stroke is missing, so our study adds important clinical knowledge.
Conclusion
We found no significant effect on survival after 7 years of a multifactorial risk factor program given during the first year after first-ever stroke or TIA. Higher BMI is an independent predictor for long-term survival in this cohort.
Data sharing statement
The data sets are available from the first and corresponding author on reasonable request.
Acknowledgments
We appreciate the contribution of all our participants. We also thank our dedicated study nurse and the Department of Geriatric, Stroke and Rehabilitation Medicine and Department of Medical Research, Bærum Hospital, Vestre Viken Hospital Trust for their support.
Author contributions
BF, BT, ARØ, HeIH, TBW, and GH researched literature, conceived the study, and contributed to the statistical analysis plan. HeIH, BF, BT, and GH were involved in protocol development and gaining ethical approval. HåIH, HeIH, and GH were involved in patient recruitment. HåIH, HeIH, ECS, GH, and SN were involved in data analysis. GH wrote the first draft of the manuscript. All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Lackland DT, Roccella EJ, Deutsch AF. Factors influencing the decline in stroke mortality: a statement from the American Heart Association/American Stroke Association. Stroke. 2014;45(1):315–353. | ||
Edwards JD, Kapral MK, Fang J, Swartz RH. Long-term morbidity and mortality in patients without early complications after stroke or transient ischemic attack. CMAJ. 2017;189(29):E954–E961. | ||
Piepoli MF, Corrà U, Benzer W, et al. Secondary prevention through cardiac rehabilitation: from knowledge to implementation. A position paper from the cardiac rehabilitation section of the European association of cardiovascular prevention and rehabilitation. Eur J Cardiovasc Prev Rehabil. 2010;17(1):1–17. | ||
Rudd AG, Lowe D, Hoffman A, Irwin P, Pearson M. Secondary prevention for stroke in the United Kingdom: results from the National sentinel audit of stroke. Age Ageing. 2004;33(3):280–286. | ||
Winstein CJ, Stein J, Arena R. Guidelines for adult stroke rehabilitation and recovery: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2016;47(6):e98–e169. | ||
Ihle-Hansen H, Thommessen B, Fagerland MW, et al. Effect on anxiety and depression of a multifactorial risk factor intervention program after stroke and TIA: a randomized controlled trial. Aging Ment Health. 2014;18(5):540–546. | ||
Ihle-Hansen H, Thommessen B, Fagerland MW, et al. Multifactorial vascular risk factor intervention to prevent cognitive impairment after stroke and TIA: a 12-month randomized controlled trial. Int J Stroke. 2014;9(7):932–938. | ||
Rm R. Validity of the TRAIL making test as an indicator of organic brain damage. Percept Mot Skills. 1958;8:271–276. | ||
Randolph C, Tierney MC, Mohr E, Chase TN. The repeatable battery for the assessment of neuropsychological status (RBANS): preliminary clinical validity. J Clin Exp Neuropsychol. 1998;20(3):310–319. | ||
Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370. | ||
Jorm AF, Scott R, Cullen JS, Mackinnon AJ. Performance of the informant questionnaire on cognitive decline in the elderly (IQCODE) as a screening test for dementia. Psychol Med. 1991;21(3):785–790. | ||
Wilson JT, Hareendran A, Hendry A, Potter J, Bone I, Muir KW. Reliability of the Modified Rankin Scale across multiple raters: benefits of a structured interview. Stroke. 2005;36(4):777–781. | ||
Goldstein LB, Bertels C, Davis JN. Interrater reliability of the NIH Stroke Scale. Arch Neurol. 1989;46(6):660–662. | ||
Sulter G, Steen C, De Keyser J. Use of the Barthel index and modified Rankin scale in acute stroke trials. Stroke. 1999;30(8):1538–1541. | ||
Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. | ||
Smith SC, Allen J, Blair SN, Sn B, et al. AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update: endorsed by the National Heart, lung, and Blood Institute. Circulation. 2006;113(19):2363–2372. | ||
Kono Y, Yamada S, Yamaguchi J, et al. Secondary prevention of new vascular events with lifestyle intervention in patients with noncardioembolic mild ischemic stroke: a single-center randomized controlled trial. Cerebrovasc Dis. 2013;36(2):88–97. | ||
Ia D, Sm VS, Ee VW, et al. Lifestyle interventions to prevent cardiovascular events after stroke and transient ischemic attack: systematic review and meta-analysis. Stroke. 2017;48(1):174–179. | ||
Vatten LJ, Nilsen TI, Holmen J. Combined effect of blood pressure and physical activity on cardiovascular mortality. J Hypertens. 2006;24(10):1939–1946. | ||
Ngandu T, Lehtisalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (finger): a randomised controlled trial. Lancet. 2015;385(9984):2255–2263. | ||
Teuschl Y, Matz K, Firlinger B, et al. Preventive effects of multiple domain interventions on lifestyle and risk factor changes in stroke survivors: evidence from a two-year randomized trial. Int J Stroke. 2017;12(9):976–984. | ||
Rodrigues P, Santos M, Sousa MJ, et al. Cardiac rehabilitation after an acute coronary syndrome: the impact in elderly patients. Cardiology. 2015;131(3):177–185. | ||
Kirk H, Kersten P, Crawford P, Keens A, Ashburn A, Conway J. The cardiac model of rehabilitation for reducing cardiovascular risk factors post transient ischaemic attack and stroke: a randomized controlled trial. Clin Rehabil. 2014;28(4):339–349. | ||
Lennon O, Carey A, Gaffney N, Stephenson J, Blake C. A pilot randomized controlled trial to evaluate the benefit of the cardiac rehabilitation paradigm for the non-acute ischaemic stroke population. Clin Rehabil. 2008;22(2):125–133. | ||
Tang A, Marzolini S, Oh P, McIlroy WE, Brooks D. Feasibility and effects of adapted cardiac rehabilitation after stroke: a prospective trial. BMC Neurol. 2010;10(1):40. | ||
Leistner S, Michelson G, Laumeier I, et al. Intensified secondary prevention intending a reduction of recurrent events in TIA and minor stroke patients (INSPiRE-TMS): a protocol for a randomised controlled trial. BMC Neurol. 2013;13(1):11. | ||
Scherbakov N, Dirnagl U, Doehner W. Body weight after stroke: lessons from the obesity paradox. Stroke. 2011;42(12):3646–3650. | ||
Doehner W, Schenkel J, Anker SD, Springer J, Audebert HJ. Overweight and obesity are associated with improved survival, functional outcome, and stroke recurrence after acute stroke or transient ischaemic attack: observations from the TEMPiS trial. Eur Heart J. 2013;34(4):268–277. | ||
Curioni C, André C, Veras R. Group.Weight reduction for primary prevention of stroke in adults with overweight or obesity. Cochrane Database Syst Rev. 2006;25(3):Cd006062. | ||
FOOD Trial Collaboration. Poor nutritional status on admission predicts poor outcomes after stroke: observational data from the food trial. Stroke. 2003;34(6):1450–1456. | ||
Kim Y, Kim CK, Jung S, Ko SB, Lee SH, Yoon BW. Prognostic importance of weight change on short-term functional outcome in acute ischemic stroke. Int J Stroke. 2015;10(Suppl A1000):62–68. | ||
Gulsvik AK, Thelle DS, Mowé M, Wyller TB. Increased mortality in the slim elderly: a 42 years follow-up study in a general population. Eur J Epidemiol. 2009;24(11):683–690. | ||
Kernan WN, Ovbiagele B, Black HR. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(7):2160–2236. | ||
O’Donnell MJ, Chin SL, Rangarajan S, et al. Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): a case-control study. Lancet. 2016;388(10046):761–775. | ||
Sabayan B, Gussekloo J, de Ruijter W, Westendorp RGJ, de Craen AJM. Framingham stroke risk score and cognitive impairment for predicting first-time stroke in the oldest old. Stroke. 2013;44(7):1866–1871. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


