Back to Journals » Clinical Ophthalmology » Volume 13
Long-term changes of retinal pigment epithelium in the eyes with Vogt-Koyanagi-Harada disease observed by adaptive optics imaging
Authors Nakamura T, Hayashi A , Oiwake T
Received 29 December 2018
Accepted for publication 26 April 2019
Published 31 May 2019 Volume 2019:13 Pages 927—933
DOI https://doi.org/10.2147/OPTH.S199886
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Tomoko Nakamura, Atsushi Hayashi, Toshihiko Oiwake
Department of Ophthalmology, Graduate School of Medicine and Pharmaceutical Sciences, University of Toyama, Toyama, Japan
Purpose: To observe long-term changes of the retinal pigment epithelium (RPE) in the eyes of patients with Vogt-Koyanagi-Harada (VKH) disease using an adaptive optics (AO) fundus camera, and their correlation with the findings of spectral-domain optical coherence tomography (SD-OCT), fundus autofluorescence (AF) imaging.
Patients and methods: Three eyes of two patients with new-onset acute VKH disease were retrospectively studied. After the serous retinal detachment was resolved by high-dose corticosteroid treatment, the patients were examined with SD-OCT, blue-wavelength AF, near-infrared (NIR) AF, and an AO fundus camera. AO images of the macula were obtained using the rtx1TM, AO fundus camera. The area around the foveal center of the hyper-reflective lesion in AO imaging was measured manually. The time at which the serous retinal detachment resolved was set as the baseline, and AO and other images were obtained every 3 to 6 months from the baseline.
Results: In all three eyes, lesions with elevation or thickening of the RPE layer were observed with OCT imaging in the macula after the serous retinal detachment resolved. These lesions showed hyper-autofluorescence in NIR-AF image and hyper-reflective lesions with clear boundaries in AO image. The area of the hyper-reflective lesions of AO images in each eye showed an approximately 40% decrease at 6 months from baseline. However, the hyper-reflective lesion remained to some extent after 18 months in case 1 and 36 months in case 2.
Conclusions: By using OCT, fundus autofluorescence and AO images, it was possible to observe temporal changes of RPE layer in VKH eyes noninvasively. High-resolution AO images also allow us to observe for improvements in the elevation or thickening of the RPE layer quantitatively.
Keywords: adaptive optics, fundus autofluorescence, near-infrared imaging, Vogt-Koyanagi-Harada disease, retinal pigment epithelium
Introduction
Vogt-Koyanagi-Harada (VKH) disease is characterized by bilateral and diffuse choroiditis usually manifesting as serous retinal detachment in the acute stage, with the onset of granulomatous anterior segment inflammation soon thereafter.1 Patients with VKH disease are usually treated with high-dose systemic corticosteroids, either only orally or with initial intravenous administration followed by oral administration.
Several studies have used histopathologic examination to show the changes in the retinal pigment epithelium (RPE) that occur in VKH disease. It has been reported that in eyes with VKH disease, inflammatory cells infiltrate under the RPE layer and that RPE cells degenerate, proliferate, or drop out in cases with severe inflammation or long-term persistence or recurrence.2–5 Changes in the RPE in VKH have been observed with spectral-domain optical coherence tomography (SD-OCT) imaging as elevations or thickening of the RPE layer.6–8 These changes have been detected as mottled hyper-autofluorescent lesions in the near-infrared (NIR) light fundus autofluorescence (AF) images.6,8 However, there have been few reports of such RPE changes occurring over long-term follow-up. Additionally, these RPE changes have not been evaluated quantitatively over time. Adaptive optics (AO) fundus images are retinal imaging techniques that allow for the improvement of the lateral resolution of retinal images as compared to conventional imaging.9 The AO fundus camera allows not only observation of parafoveal cone photoreceptors, but also microvessel imaging and tracking of minute changes of cell groups, vessel sections, or lesions over time.10–13 The purpose of this study was to observe long-term RPE changes in the eyes of patients with VKH disease using SD-OCT, fundus autofluorescence, and an AO fundus camera.
Patients and methods
Patients
This was a single-center, retrospective study that was conducted in accordance with the Institutional Guidelines of the University of Toyama and was approved by the Institutional Review Board. The procedures conformed to the tenets of the World Medical Association’s Declaration of Helsinki. All subjects provided their written informed consent before participation in the study. Three eyes of two consecutive patients with new-onset acute VKH disease who visited Toyama University Hospital were studied retrospectively. Only patients with elevation or thickening of the RPE layer in OCT image after resolution of serous retinal detachment were included in this study. The diagnosis of VKH was based on established international criteria.14 Both patients underwent comprehensive eye examinations, including measurement of decimal best-corrected visual acuity, fundus examination, SD-OCT, scanning laser ophthalmoscopy (SLO) blue-light (BL) and NIR-AF imaging, and AO fundus camera.
Imaging modalities
OCT images were obtained with an SD-OCT (RS-3000 Advance; Nidek, Aichi, Japan).
After the serous retinal detachment was resolved in OCT image, BL-AF and NIR-AF images (all with Heidelberg spectralis HRA; Heidelberg Engineering, Heidelberg, Germany), and AO images were taken. BL-AF images were acquired with an excitation filter of 486 nm and a barrier filter of 500 nm. NIR-AF images were acquired with an excitation filter of 786 nm and a barrier filter of 830 nm. The field of view was set to 30°×30°and 512×512 pixel resolution centered on the fovea. AO images were obtained at the macula with an rtx1TM AO fundus camera (Imagine Eyes, Orsay, France). Each AO image was obtained a 4°×4° retinal area over an acquisition time of 4 seconds. Multiple images were recorded at the foveal center and at 2° superior, inferior, temporal, and nasal from the foveal center. We focused the AO camera at the depth of the reflection of cone mosaics. The time at which the serous retinal detachment resolved was set as the baseline, and AO and other images were obtained every 3 to 6 months from the baseline. Case 1 was observed for 18 months, and case 2 was observed for 36 months from the baseline.
AO image processing and analysis
The images were processed with software tools supplied by the manufacturer (CK V.1.3 and AOdetect V.0.1). These images were registered using a cross-correlation method and averaged to produce a final image with an enhanced signal-to-noise ratio.15 Montage images were created using image editing software (i2k Retina; DualAlign LLC, Clifton Park, NY). The area of hyper-reflective lesions observed with AO imaging was measured, and the change during the follow-up period was investigated. The area of hyper-reflective lesions within the range of 1.1×2.2 mm around the foveal center was manually measured using the ImageJ software (National Institutes of Health, Bethesda, MD). The scales of the macular images were calculated using Littmann’s method.16
Results
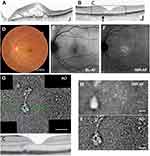
Case 1 was a 33-year-old man. Before treatment, the left eye showed serous retinal detachment with massive subretinal and intraretinal fluid (Figure 1A). At the initial visit, his best-corrected decimal visual acuity was 0.6 in the right eye and 0.7 in the left eye. Two courses of steroid pulse therapy followed by 70 mg daily dose of oral prednisolone were performed. The serous retinal detachment completely resolved within 4 weeks and best-corrected decimal visual acuity improved 1.0 in both eyes. The dose of prednisolone was gradually tapered over 15 months. In the OCT images when the serous retinal detachment disappeared, lesions involving elevation of the RPE layer were observed at the nasal side of the fovea (Figure 1B). Slight hyper-fluorescence in the BL-AF image and hyper-autofluorescence in NIR-AF image were observed at the nasal side of the fovea. (Figure 1E and F). Figure 1G is a panoramic image of AO around the fovea. The lesions corresponding to elevation of the RPE layer in the OCT images showed highly reflective lesions in AO image (Figure 1G and C). In the AO image, the boundary of the highly reflective lesion was clear (Figure 1H and I).
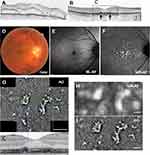
Case 2 was a 43-year-old man. As in case 1, massive serous retinal detachment and inner retinal fluid were observed before treatment (Figure 2A). At the initial visit, his best-corrected decimal visual acuity was 0.6 in both eyes. The steroid pulse was performed one course, and then 50 mg daily dose of oral prednisolone was administered. The serous retinal detachment completely resolved within 6 weeks and best-corrected decimal visual acuity improved 1.0 in both eyes. At the time when the serous retinal detachment resolved, lesions involving a thickened RPE layer were observed in both eyes in the OCT image. Figure 2B shows an OCT image of right eye obtained at 18 months from baseline. Elevation of the RPE layer was observed around the fovea. In the BL-AF, NIR-AF, and AO panoramic images acquired at 18 months from the baseline (Figure 2E, F and G), similar to those of case 1, slight hyper-fluorescence in the BL-AF image and multiple hyper-autofluorescence in NIR-AF image were observed. The lesions corresponding to elevation or thickening of the RPE layer in the OCT images showed highly reflective lesions in AO image (Figure 2G and C). In the AO image, the boundary of the highly reflective lesion was clearer than NIR-AF image (Figure 2H and I).
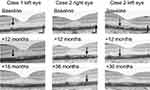
The time course changes near the fovea as determined by OCT are shown in Figure 3. Lesions involving elevation or thickening of the RPE layer were observed, but by the end of the follow-up period, some of them had disappeared.
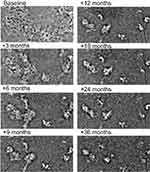
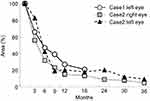
Figure 4 shows changes in hyper-reflective lesions at the fovea of the AO image in the left eye of case 2. The area of each hyper-reflective lesion in the AO image gradually decreased during the follow-up. The area of the hyper-reflective lesions was measured using ImageJ software and changes of the area in each eye are shown in Figure 5. The area of the hyper-reflective lesions in each eye showed an approximately 40% decrease at 6 months from baseline. However, the hyper-reflective lesion remained to some extent after 18 months in case 1 and 36 months in case 2.
Discussion
Our study had two findings. First, in the eyes with VKH that display an elevation or thickening of the RPE layer on OCT images, hyper-autofluorescence in NIR-AF image and hyper-reflective areas in AO image are observed. In addition, the hyper-reflective areas in the AO images correspond to the location of the elevation or thickening of the RPE layer in OCT images. Second, the areas of hyper-reflective area in AO images gradually decreased in size, but some remained.
Several studies have reported changes in the RPE layer as shown by SD-OCT in eyes with VKH disease. Koizumi and colleagues have reported cases showing multifocal thickening of the RPE layer.6 Zhou et al reported that RPE hyperplasia was seen in 28 of 58 eyes in VKH cases over 12 months.7 In addition, Vasconcelos-Santos et al reported RPE thickening on OCT in a case of chronic VKH exhibiting a sunset-glow fundus.8 In a reported case of sympathetic ophthalmia, SD-OCT revealed a hyper-reflective lesion at the level of the RPE.17 These reports showed changes in the RPE layer as seen in our cases. Changes in retinal pigment epithelium in VKH disease have been examined histologically. Inomata and Rao observed the five enucleated eyes of patients with VKH disease and detected changes in RPE histologically. They reported that lymphocytes and epithelial cells infiltrate under the RPE layer in the acute phase, resulting in elevation of the RPE layer. After the inflammation of the choroid subsided, lymphocytes and epithelial cells in the nodules disappeared, and the quiescent nodules consisted only of proliferated RPE cells.2,3 The changes of RPE layer observed by SD-OCT are considered to be these inflammatory changes in VKH disease.
Our cases showed slight hyper-autofluorescence in the BL-AF images and hyper-autofluorescence region in the macula in the NIR-AF images.
Vasconcelos-Santos et al reported that in the case of chronic VKH disease, the autofluorescence signal increases or decreases in BL-AF, and they hypothesized that the abnormally high hyperautofluorescence observed in the area of RPE proliferation might be a result of the presence of fluorophores either within the RPE cells or the outer segments of photoreceptors.8 Koizumi et al also compared BL-AF and NIR-AF images, and demonstrated that NIR-AF showed similar patterns to BL-AF, but the patterns were more evident in the NIR-AF images.6 Their results related to autofluorescence images are consistent with those of our case, and hyperautofluorescence areas in NIR-AF images were observed as hyper-reflective areas in the AO images. Infrared imaging is known to be particularly advantageous for identifying subretinal structures, and melanin is considered to be the most likely candidate for increased NIR reflectance and NIR fluorescence.18–20 Similarly, NIR is used in AO cameras. We speculate that the hyper-reflective regions in AO images correspond to the areas of RPE changes, such as thickening of the RPE layer or proliferated RPE cells. Because the elevation or thickening of the RPE layer observed in the OCT image was consistent with the hyper-reflective region in the AO image, the origin of the NIR reflection is considered to be melanin; RPE cells contain high levels of melanin,21 and the proliferation of RPE cells has been histologically reported to occur in VKH eyes. Similar image was shown in the report by Mrejen et al. They observed a soft drusen with an AO fundus camera, and the area where the RPE layer was raised by a sub-RPE drusen displayed as a highly reflective lesion in the AO image.22
In this study, the high-resolution image obtained by the AO camera enabled us to quantitatively evaluate the area considered to correspond with these types of changes in the RPE layer. Further, Gocho et al used an AO camera to observe the eyes with geographic atrophy, and they reported on the better resolution observed along the borders and presence of hyporeflective clumps than SLO images. They also measured the spot sizes associated with geographic atrophy in the AO images and showed their progression.12 As shown in Figure 5, the area of the highly reflective lesions in each eye observed using the AO camera was gradually decreased. In this study, we set the depth of focus of the AO camera to the level where the cone mosaic was visible. Since the bright spot of the cone mosaic is known to be obtained approximately in the ellipsoid and interdigitation zone,23 the AO image shown in this study was taken at the approximate depth of the ellipsoid zone and the interdigitation zones. The gradual decrease in the hyper-reflective lesion in the AO image was thought to be due to a reduction in the elevation of the RPE layer. As seen in the OCT image (Figure 3), the elevation or thickening of the RPE layer was partially disappeared during the follow-up period. According to the previous histological studies, the RPE layer was plausibly elevated by the infiltration of inflammatory cells. But when the inflammation was resolved, the inflammatory cells decreased in the sub-RPE space and the lesion where the RPE layer was elevated diminished as well.2,3 Considering of the time courses changes of OCT and histological reports, we speculate that the hyper-reflective lesions observed in the AO image were caused by the accumulation of the inflammatory cells under the RPE and the time courses of these lesions.
There are some limitations to our study, including the small number of cases and the retrospective nature of the study. In addition, this AO camera cannot observe the RPE cell mosaic itself. This study only speculates on changes that could have occurred in the RPE layer by comparing the AO findings with OCT and fundus autofluorescent images. However, using high-resolution AO imaging, we observed and quantitatively analyzed the areas that were thought to correspond with RPE changes in the eyes of VKH patients for the first time.
In conclusion, by using OCT, fundus autofluorescence, and AO images, it was possible to observe temporal changes in the RPE layer of VKH eyes noninvasively. High-resolution AO images also allow us to observe for improvements in the elevation or thickening of the RPE layer quantitatively, which in turn is useful in monitoring the improvement of the disease.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Moorthy RS, Inomata H, Rao NA. Vogt-Koyanagi-Harada syndrome. Surv Ophthalmol. 1995;39(4):265–292.
2. Inomata H, Rao NA. Depigmented atrophic lesions in sunset glow fundi of Vogt-Koyanagi-Harada disease. Am J Ophthalmol. 2001;131(5):607–614.
3. Rao NA. Pathology of Vogt-Koyanagi-Harada disease. Int Ophthalmol. 2007;27(2–3):81–85. doi:10.1007/s10792-006-9029-2
4. Inomata H, Minei M, Taniguchi Y, Nishimura F. Choroidal neovascularization in long-standing case of Vogt-Koyanagi-Harada disease. Jpn J Ophthalmol. 1983;27(1):9–26.
5. Yamaki K, Gocho K, Sakuragi S. Pathogenesis of Vogt-Koyanagi-Harada disease. Int Ophthalmol Clin. 2002;42(1):13–23.
6. Koizumi H, Maruyama K, Kinoshita S. Blue light and near-infrared fundus autofluorescence in acute Vogt-Koyanagi-Harada disease. Br J Ophthalmol. 2010;94(11):1499–1505. doi:10.1136/bjo.2009.164665
7. Zhou M, Jiang C, Gu R, Sun Z, Huynh N, Chang Q. Correlation between retinal changes and visual function in late-stage Vogt-Koyanagi-Harada disease: an optical coherence tomography study. J Ophthalmol. 2015;2015:916485. doi:10.1155/2015/916485
8. Vasconcelos-Santos DV, Sohn EH, Sadda S, Rao NA. Retinal pigment epithelial changes in chronic Vogt-Koyanagi-Harada disease: fundus autofluorescence and spectral domain-optical coherence tomography findings. Retina. 2010;30(1):33–41. doi:10.1097/IAE.0b013e3181c5970d
9. Liang J, Williams DR, Miller DT. Supernormal vision and high-resolution retinal imaging through adaptive optics. J Opt Soc Am A Opt Image Sci Vis. 1997;14(11):2884–2892.
10. Carroll J, Kay DB, Scoles D, Dubra A, Lombardo M. Adaptive optics retinal imaging–clinical opportunities and challenges. Curr Eye Res. 2013;38(7):709–721. doi:10.3109/02713683.2013.784792
11. Paques M, Meimon S, Rossant F, et al. Adaptive optics ophthalmoscopy: application to age-related macular degeneration and vascular diseases. Prog Retin Eye Res. 2018;66:1–16. doi:10.1016/j.preteyeres.2018.07.001
12. Gocho K, Sarda V, Falah S, et al. Adaptive optics imaging of geographic atrophy. Invest Ophthalmol Vis Sci. 2013;54(5):3673–3680. doi:10.1167/iovs.12-10672
13. Loganadane P, Delbosc B, Saleh M. short-term progression of diabetic hard exudates monitored with high-resolution camera. Ophthalmic Res. 2019;61(1):3–9. doi:10.1159/000493858
14. Read RW, Holland GN, Rao NA, et al. Revised diagnostic criteria for Vogt-Koyanagi-Harada disease: report of an international committee on nomenclature. Am J Ophthalmol. 2001;131(5):647–652.
15. Zitova B, Flusser J. Image registration methods: a survey. Image Vision Comput. 2003;21(11):977–1000. doi:10.1016/S0262-8856(03)00137-9
16. Bennett AG, Rudnicka AR, Edgar DF. Improvements on Littmann’s method of determining the size of retinal features by fundus photography. Graefes Arch Clin Exp Ophthalmol. 1994;232(6):361–367.
17. Muakkassa NW, Witkin AJ. Spectral-domain optical coherence tomography of sympathetic ophthalmia with Dalen-Fuchs nodules. Ophthalmic Surg Lasers Imaging Retina. 2014;45(6):610–612. doi:10.3928/23258160-20141008-01
18. Elsner AE, Burns SA, Weiter JJ, Delori FC. Infrared imaging of sub-retinal structures in the human ocular fundus. Vision Res. 1996;36(1):191–205.
19. Keilhauer CN, Delori FC. Near-infrared autofluorescence imaging of the fundus: visualization of ocular melanin. Invest Ophthalmol Vis Sci. 2006;47(8):3556–3564. doi:10.1167/iovs.06-0122
20. Weinberger AW, Lappas A, Kirschkamp T, et al. Fundus near infrared fluorescence correlates with fundus near infrared reflectance. Invest Ophthalmol Vis Sci. 2006;47(7):3098–3108. doi:10.1167/iovs.05-1104
21. Lapierre-Landry M, Carroll J, Skala MC. Imaging retinal melanin: a review of current technologies. J Biol Eng. 2018;12:29. doi:10.1186/s13036-018-0124-5
22. Mrejen S, Sato T, Curcio CA, Spaide RF. Assessing the cone photoreceptor mosaic in eyes with pseudodrusen and soft Drusen in vivo using adaptive optics imaging. Ophthalmology. 2014;121(2):545–551. doi:10.1016/j.ophtha.2013.09.026
23. Zhang Y, Cense B, Rha J, et al. High-speed volumetric imaging of cone photoreceptors with adaptive optics spectral-domain optical coherence tomography. Opt Express. 2006;14(10):4380–4394. doi:10.1364/OE.14.004380
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.