Back to Journals » Cancer Management and Research » Volume 12
Long Noncoding RNA PRR34-AS1 Aggravates the Progression of Hepatocellular Carcinoma by Adsorbing microRNA-498 and Thereby Upregulating FOXO3
Authors Liu Z, Li Z, Xu B, Yao H, Qi S, Tai J
Received 19 May 2020
Accepted for publication 2 October 2020
Published 29 October 2020 Volume 2020:12 Pages 10749—10762
DOI https://doi.org/10.2147/CMAR.S263619
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Kenan Onel
This paper has been retracted.
Zhaoming Liu, 1 Zhen Li, 2 Binghui Xu, 1 Hao Yao, 1 Shuangyu Qi, 1 Jianxiong Tai 1
1Department of Hepatobiliary Surgery, Harrison International Peace Hospital, Hengshui, Hebei 053000, People’s Republic of China; 2Department of Interventional Therapy, Harrison International Peace Hospital, Hengshui, Hebei 053000, People’s Republic of China
Correspondence: Zhaoming Liu
Department of Hepatobiliary Surgery, Harrison International Peace Hospital, 180 Renmin East Road, Hengshui, Hebei 053000, People’s Republic of China
Email [email protected]
Purpose: Long noncoding RNAs are differentially expressed in hepatocellular carcinoma (HCC) and have been validated as essential regulators in HCC. However, there is limited knowledge regarding the detailed roles and mechanisms of most lncRNAs in HCC cells. In this study, the expression profiles of PRR34 antisense RNA 1 (PRR34-AS1) in HCC tissues and cell lines were determined. In addition, the detailed roles and underlying mechanisms of PRR34-AS1 in HCC cells were comprehensively elucidated.
Methods: Reverse transcription-quantitative polymerase chain reaction (PCR) was performed to measure PRR34-AS1 expression in HCC cells. Cell proliferation, apoptosis, and migration and invasion were evaluated in vitro using the cell counting kit-8 (CCK-8) assay, flow cytometric analysis, and transwell cell migration and invasion assays, respectively. In vivo tumor growth was determined using tumor xenograft experiments. The potential miRNA targets of PRR34-AS1 were predicted via bioinformatic analysis and further confirmed using the luciferase reporter assay, RNA immunoprecipitation assay, and reverse transcription-quantitative PCR.
Results: PRR34-AS1 was highly expressed in HCC tissues and cell lines, and its interference suppressed HCC cell proliferation, migration, and invasion but promoted cell apoptosis in vitro. In addition, loss of PRR34-AS1 decreased tumor growth in HCC cells in vivo. Mechanistically, PRR34-AS1 functions as a miR-498 sponge and subsequently increases forkhead box O3 (FOXO3) expression in HCC cells. Rescue experiments revealed that the suppressive effects triggered by PRR34-AS1 knockdown on the malignant characteristics of HCC cells could be abrogated by inhibiting miR-498 or restoring FOXO3 expression.
Conclusion: The depletion of PRR34-AS1 suppresses the oncogenicity of HCC cells by targeting the miR-498/FOXO3 axis. Therefore, the PRR34-AS1/miR-498/FOXO3 pathway may offer a basis for HCC treatment.
Keywords: PRR34 antisense RNA 1, forkhead box O3, ceRNA regulation model, polymerase chain reaction
Expression of Concern for this paper has been published
Introduction
Hepatocellular carcinoma (HCC) is the fifth-most common type of human cancer and the second-most common cause of cancer-related mortalities worldwide1 due to a lack of noticeable symptoms, difficulties in early detection, complex pathological mechanisms, and high death rates.2 In the last decade, the morbidity of HCC has significantly increased by approximately 3% per year in women and by approximately 4% per year in men.3 It has been estimated that there will be over 750,000 novel HCC cases and 55,000–85,000 deaths per year globally due to this fatal malignancy.4 Despite tremendous advancements in HCC diagnosis and therapy, the therapeutic effectiveness of HCC treatments remains unsatisfactory, with more than half of the patients suffering from recurrence and distant metastasis even after surgical excision.5,6 The overall 5-year survival rate of patients with HCC is approximately 5%, and the poor clinical outcomes are largely attributed to limited effective treatment options, delayed diagnosis, and the complex pathogenesis of HCC.7,8 Therefore, research into the mechanisms associated with hepatocarcinogenesis and cancer progression may contribute in identifying promising therapeutic targets as well as in developing new approaches for HCC management.
Long noncoding RNAs (lncRNAs) are short transcripts (over 200 nucleotides long) that lack protein-coding capacity.9 Several studies have revealed that lncRNAs contribute to diverse physiological and pathological processes via epigenetic, transcriptional, and post-transcriptional modulation.10 Accumulating evidence suggests that lncRNAs play key roles in carcinogenesis and cancer progression.11–13 The aberrant expression of lncRNAs is commonly observed in HCC cells. For instance, SNHG5,14 OIP5-AS1,15 and CASC1516 are upregulated in HCC cells, whereas RMRP,17 HAND2-AS1,18 and MIR22HG19 are expressed at low levels. The dysregulation of lncRNAs can have oncogenic or antioncogenic effects, and lncRNAs function in the regulation of numerous malignant characteristics.20,21
MicroRNAs (miRNAs) are a subgroup of noncoding RNA molecules of approximately 17–24 nucleotides. They can negatively regulate gene expression by base pairing with the 3′-untranslated regions (3′-UTRs) of their target mRNAs, resulting in transcript inhibition and/or mRNA degradation.22 To date, 474 miRNAs have been verified in the human genome and are estimated to regulate approximately 30% of protein-coding genes.23 They are critically implicated in the genesis and development of HCC because they exert essential activities, such as the regulation of cell growth, metastasis, tumor differentiation, and angiogenesis.24,25 Importantly, the proposed competing endogenous RNA (ceRNA) theory, which suggests that lncRNAs work as a miRNA sponge and prevent their binding to mRNAs, has received increasing attention.26 Therefore, it may be helpful to study the functions and mechanisms of lncRNAs in HCC cells to identify effective therapeutic targets.
A substantial number of lncRNAs are aberrantly expressed in HCC;27,28 however, knowledge regarding the detailed roles and mechanisms of most lncRNAs in HCC cells remains limited. In this study, we first measured PRR34 antisense RNA 1 (PRR34-AS1) expression in HCC cells and tissues and determined the roles of PRR34-AS1 in regulating the malignant characteristics of HCC cells. In addition, we comprehensively elucidated the mechanisms behind the oncogenic functions of PRR34-AS1 in HCC cells.
Materials and Methods
Tissue Sample and Cell Culture
HCC and adjacent normal tissues were collected from 65 patients in Harrison International Peace Hospital. All enrolled patients had not received radiotherapy, chemotherapy, or other anticancer treatments before the operation. All tissue specimens were immediately placed into liquid nitrogen and stored in liquid nitrogen until further use. This study was approved by the Ethics Committee of Harrison International Peace Hospital (2014#063) and performed in accordance with the Declaration of Helsinki. Written informed consent was obtained from all participants.
HCC cell lines, including Hep3B, HuH7, and BEL-7402, as well as transformed Human Liver Epithelial-3 cells (THLE-3) were purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). Two additional HCC cell lines (SNU-182 and SNU-398) were obtained from American Type Culture Collection (Manassas, VA, USA).
Hep3B cells were maintained in minimal essential medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) containing 10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.), 1% GlutaMAX, 1% nonessential amino acids, 1% sodium pyruvate 100 mM solution, and 1% penicillin/streptomycin (Gibco; Thermo Fisher Scientific, Inc.). HuH7 cells were cultured in Dulbecco’s Modified Eagle Medium (Gibco; Thermo Fisher Scientific, Inc.) with 10% FBS, 1% GlutaMAX, 1% nonessential amino acids, and 1% penicillin/streptomycin. RPMI-1640 (Gibco; Thermo Fisher Scientific, Inc.) containing 10% heat-inactivated FBS (Gibco; Thermo Fisher Scientific, Inc.) and 1% penicillin/streptomycin was used for the culturing of BEL-7402, SNU-182, and SNU-398 cells. Extra 1% GlutaMAX and 1% nonessential amino acids were added to the growth medium for SNU-182 cells. BEGM medium (Clonetics Corporation, Walkersville, MD) with 5 ng/mL EGF, 70 ng/mL Phosphoethanolamine and 10% FBS was used to culture THLE-3 cells. All cell lines were cultured at 37°C in a humidified atmosphere of 5% CO2.
Oligonucleotide, Plasmid, and Cell Transfection
miR-498 mimic and inhibitor were obtained from Ribobio (Guangzhou, China) and used to increase and decrease endogenous miR-498 expression, respectively. Negative control miRNA mimic (miR-NC) and negative control (NC) inhibitor were used as the controls for miR-498 mimic and miR-498 inhibitor, respectively. The corresponding sequences were as follows: miR-498 mimic, 5ʹ-CUUUUUGCGGGGGACCGAACUUU-3ʹ; miR-NC, 5ʹ-UUGUACUACACAAAAGUACUG-3ʹ; miR-498 inhibitor, 5ʹ- GAAAAACGCCCCCUGGCUUGAAA −3ʹ; and NC inhibitor 5ʹ-CAGUACUUUUGUGUAGUACAA-3ʹ.
Small interfering RNA (siRNA) targeting PRR34-AS1 (si-PRR34-AS1), siRNA scrambled control (si-NC), the forkhead box O3 (FOXO3) overexpressing plasmid pcDNA3.1/FOXO3, and empty pcDNA3.1 plasmid were all acquired from GenePharma Co., Ltd, (Shanghai, China). These oligonucleotides and plasmids were transfected into HCC cells using the Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA, USA). The si-PRR34-AS1 sequences were as follows: si-PRR34-AS1#1, 5ʹ-CTCTAATAATGGAAAAAAATTTA-3ʹ; si-PRR34-AS1#2, 5ʹ-ATCTATTTGACTTATAATAAATA-3ʹ; and si-PRR34-AS1#3, 5ʹ-TCGTTTTGTTTTGATTTATTTTA-3ʹ. The si-NC sequence was 5ʹ-CACGATAAGACAATGTATTT-3ʹ.
Cellular Nucleocytoplasmic Fractionation
Nuclear and cytoplasmic fractions were isolated using the Cytoplasmic and Nuclear RNA Purification Kit (Norgen Biotek, Thorold, Canada). The abundance of PRR34-AS1 in the nuclear and cytoplasmic fractions was evaluated via reverse transcription-quantitative polymerase chain reaction (RT-qPCR).
RT-qPCR
Total RNA was extracted from the cells using the TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.). The miRcute miRNA Isolation Kit (TIANGEN, Beijing, China) was used for miRNA extraction. RNA purity and quality were determined using the NanoDrop 2000c (Thermo Fisher Scientific, Inc.). Complementary DNA (cDNA) was synthesized from total RNA using the PrimeScript™ RT Reagent Kit (Takara Biotechnology Co., Ltd., Dalian, China), and qPCR was performed to detect the expression of PRR34-AS1 and FOXO3 using the SYBR® Premix Ex Taq™ (Takara Biotechnology Co., Ltd.). Glycerol-3-phosphate dehydrogenase (GAPDH) was used as the internal reference for PRR34-AS1 and FOXO3 expression.
The miRcute Plus miRNA First-Strand cDNA Kit (TIANGEN) was used to synthesize cDNA from miRNA. The cDNA was then subjected to PCR amplification to measure miR-498 expression using the miRcute Plus miRNA qPCR Kit (TIANGEN). The expression of miR-498 was normalized to that of U6 small nuclear RNA. Gene expression was analyzed using the 2−ΔΔCt method.
The primers were designed as follows: PRR34-AS1, 5′- CGATTTGGCGTTAACTTATTGA-3′ (forward) and 5′- ATCATTACAGAACATAATCAACAGGTA −3′ (reverse); FOXO3, 5′-ACTCATGCAGCGGAGCTCTAG-3′ (forward) and 5′-GTTCAGAGATGAAGGTCCGAACA-3′ (reverse); GAPDH, 5′-CGGAGTCAACGGATTTGGTCGTAT-3′ (forward) and 5′-AGCCTTCTCCATGGTGGTGAAGAC-3′ (reverse); miR-498, 5′- TCGGCAGGUUCAAGCCAGGGG −3′ (forward) and 5′- CACTCAACTGGTGTCGTGGA −3′ (reverse); and U6, 5′-GCTTCGGCAGCACATATACTAAAAT-3′ (forward) and 5′-CGCTTCACGAATTTGCGTGTCAT-3′ (reverse).
Cell Counting Kit-8 (CCK-8) Assay
Transfected cells were seeded into 96-well plates with five replicate wells. Each well contained a 100-μL cell suspension containing 2000 cells. Cell proliferation was monitored at 0, 24, 48, and 72 h after cell inoculation. Cells were incubated with 10 μL of the CCK-8 solution (Dojindo, Tokyo, Japan) at 37°C with 5% CO2 for 2 h. Finally, the absorbance was measured at 450 nm using a microplate reader.
Flow Cytometric Analysis
Transfected cells were detached by incubating them with ethylenediaminetetraacetic acid (EDTA)-free 0.25% trypsin and then rinsing them twice with ice-cooled phosphate buffer solution. Following centrifugation, the apoptosis of the transfected cells was detected using the Annexin V-fluorescein isothiocyanate (FITC) Apoptosis Detection Kit (Biolegend, San Diego, CA, USA). Briefly, transfected cells collected in a flow cytometer tube were made into a cell suspension and subsequently stained with 10 μL of annexin V-FITC and 5 μL of propidium iodide at room temperature for 20 min in the dark. The ratio of apoptotic cells was analyzed using a flow cytometer (FACScan; BD Biosciences, Franklin Lakes, NJ, USA).
Transwell Cell Migration and Invasion Assays
For cell migration, transfected cells were collected after 48 h of cultivation and suspended in FBS-free basal medium at a density of 5 × 105 cells/mL. The upper compartments of the transwell chambers (8-μm pore size; Corning Glass Works, Corning, N.Y., USA) were filled with 200 μL of the cell suspension, whereas 500 μL of the culture medium supplemented with 20% FBS were added into the basolateral compartments. After 24 h, the nonmigrated cells were removed with a cotton swab and the migrated cells were treated with 100% methanol and stained with 0.5% crystal violet. For cell invasion, the transwell chambers were coated with Matrigel (BD Biosciences, Franklin Lakes, NJ, USA), and the remaining procedures were similar to those of the migration assay. The migrated and invaded cells were imaged and counted using an IX31 inverted microscope (x200 magnification; Olympus Corporation, Tokyo, Japan).
Tumor Xenograft Experiments
The lentiviral vectors expressing short hairpin RNA (shRNA) sequences targeting PRR34-AS1 (sh-PRR34-AS1) and shRNA scrambled control (sh-NC) were prepared by GenePharma Co., Ltd. HuH7 cells were infected with the lentiviral vectors and incubated with puromycin to select the sh-PRR34-AS1 stably silenced HuH7 cells. The sequences of sh-PRR34-AS1 were 5ʹ-CCGGCTCTAATAATGGAAAAAAATTTACTCGAGTAAATTTTTTTCCATTATTAGAGTTTTTG-3ʹ and the sh-NC sequences were 5ʹ-CCGGCACGATAAGACAATGTATTTCTCGAGAAATACATTGTCTTATCGTGTTTTTG-3ʹ.
Male BALB/c nude mice (aged 4–6 weeks) were purchased from the Shanghai experimental animal center at the Chinese academy of sciences (Shanghai, China) and were randomly assigned into two groups: sh-PRR34-AS1 and sh-NC groups. The mice in the sh-PRR34-AS1 group were subcutaneously injected with approximately 1 × 107 HuH7 cells stably expressing sh-PRR34-AS1, whereas mice in the sh-NC group were inoculated with sh-NC stably transfected cells. After 1 week, tumor size was monitored every 4 days. All mice were euthanized at the end of the 31th day. Tumor xenografts were harvested, weighed, and preserved in liquid nitrogen. Tumor volume was calculated using the following formula: volume = 0.5 × (length × width2). All animal protocols were approved by the Institutional Animal Care and Use Committee of Harrison International Peace Hospital (2018#105), and performed in accordance with the NIH guidelines for the care and use of laboratory animals.
Bioinformatic Analysis
The expression profile of PRR34-AS1 in HCC cells and tissues and its relationship with overall survival were analyzed using the Gene Expression Profiling Interactive Analysis (GEPIA; http://gepia.cancer-pku.cn/), which includes the TCGA and GTEx databases. The potential miRNA targets of PRR34-AS1 were predicted using StarBase version 3.0 (http://starbase.sysu.edu.cn/). lncLocator (http://www.csbio.sjtu.edu.cn/bioinf/lncLocator/), a lncRNA subcellular localization predictor, was used to predict the location of PRR34-AS1.
RNA Immunoprecipitation (RIP) Assay
The EZ-Magna RIP™ RNA-Binding Protein Immunoprecipitation Kit (Millipore, Billerica, MA, USA) was used to perform the RIP assay. HCC cells were collected and incubated with RIP lysis buffer. The cell lysates were incubated overnight at 4°C with magnetic beads conjugated with anti-argonaute 2 (Ago2) or control IgG antibody (Millipore). After digestion with protease K, the immunoprecipitated RNA was extracted and subjected to RT-qPCR analysis to determine miR-498 and PRR34-AS1 enrichment.
Luciferase Reporter Assay
The fragments of PRR34-AS1 containing the miR-498 wild-type (wt) binding site was amplified using RT-qPCR and then inserted into pmirGLO dual-luciferase reporter vectors (Promega, Madison, WI, USA), generating the wt-PRR34-AS1 reporter plasmid. The GeneTailor™ Site-Directed Mutagenesis System (Invitrogen, Carlsbad, CA, USA) was used to perform binding site-directed mutagenesis and to produce the PRR34-AS1 mutant (mut) reporter plasmid mut-PRR34-AS1. The wt-FOXO3 and mut-FOXO3 reporter plasmids were constructed following the same experimental steps. Using the Lipofectamine 2000 reagent, wt or mut reporter plasmids alongside miR-498 mimic or miR-NC were introduced into HCC cells. After 48 h, the luciferase activity was determined using the Dual-Luciferase Assay Kit (Promega).
Western Blotting
Total protein was extracted from cultured cells using RIPA Lysis and Extraction Buffer (Invitrogen, Carlsbad, CA, USA). The concentration of total protein was detected using the BCA Protein Assay Kit (Beyotime; Shanghai, China). Equal amounts of protein were separated using 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred onto polyvinylidene fluoride membranes. After blocking with 5% skimmed milk for 2 h, the membranes were incubated overnight with specific primary antibodies targeting FOXO3 (cat. No. ab109629; Abcam, Cambridge, UK) or GAPDH (cat. No. ab181603; Abcam) and then probed with a horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G secondary antibody (cat. No. ab205718; Abcam). Finally, the Enhanced Chemiluminescence Detection System (Pierce; Thermo Fisher Scientific, Inc.) was used to develop protein signals.
Statistical Analysis
All results from three biological replicates were presented as mean ± standard deviation. The Student’s t-test was used to compare the data between the two groups, whereas the one-way analysis of variance followed by Tukey’s test was performed to test the differences among multiple groups. The chi-square test was used to determine the association between PRR34-AS1 expression and clinicopathological parameters in 65 HCC patients. The expression correlation between the two genes in the HCC tissues was determined using Pearson’s correlation coefficient. The overall survival rate was analyzed using the Kaplan–Meier method and tested with the Log rank test. P < 0.05 was considered to indicate statistical significance.
Results
PRR34-AS1 is Upregulated in HCC Cells, and Its Depletion Suppresses Cancer Progression
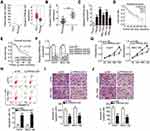
To determine the expression of PRR34-AS1 in HCC cells, GEPIA was used to analyze PRR34-AS1 expression in the TCGA and GTEx databases. The expression of PRR34-AS1 was upregulated in HCC tissues than in normal tissues (Figure 1A). To validate this observation, RT-qPCR was performed to detect PRR34-AS1 expression in 65 pairs of HCC and adjacent normal tissues. The data revealed that PRR34-AS1 was remarkably upregulated in HCC tissues than in adjacent normal tissues (Figure 1B). Additionally, the expression levels of PRR34-AS1 were measured in HCC cell lines (Hep3B, HuH7, BEL-7402, SNU-182, and SNU-398) and transformed Human Liver Epithelial-3 (THLE-3). Consistently, the expression level of PRR34-AS1 was higher in all five HCC cell lines than in THLE-3 (Figure 1C). Furthermore, data analysis obtained from TCGA and GTEx revealed that there was no obvious correlation between PRR34-AS1 expression and overall survival in patients with HCC (Figure 1D; P = 0.14), which is consistent with the results obtained from the Kaplan–Meier analyses of the 65 HCC patients (Figure 1E; P = 0.178).
Among the five HCC cell lines examined, HuH7 and SNU-182 relatively exhibited the highest PRR34-AS1 expression and were therefore selected for further experiments. PRR34-AS1 expression was silenced in HuH7 and SNU-182 cells via the transfection of si-PRR34-AS1. The silencing efficiency was verified using RT-qPCR. The results demonstrated the decreased expression of PRR34-AS1 in HuH7 and SNU-182 cells after the introduction of si-PRR34-AS1 (Figure 1F). si-PRR34-AS1#1 exhibited the highest efficiency in knocking down PRR34-AS1 expression and was therefore used in subsequent functional experiments. The CCK-8 assay and flow cytometric analysis were performed to assess HCC cell proliferation and apoptosis after PRR34-AS1 knockdown. We observed that loss of PRR34-AS1 clearly hindered the proliferation (Figure 1G) but induced the apoptosis (Figure 1H) of HuH7 and SNU-182 cells. Furthermore, both migratory (Figure 1I) and invasive (Figure 1J) abilities were apparently decreased in PRR34-AS1-silenced HuH7 and SNU-182 cells, as evidenced by the transwell cell migration and invasion assay results. Taken together, these findings suggest that PRR34-AS1 is upregulated in HCC cells and that it exhibits an oncogenic regulatory role in HCC malignancies.
PRR34-AS1 Functions by Adsorbing miR-498 in HCC Cells
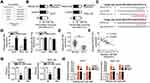
To explore the detailed mechanisms by which PRR34-AS1 regulates the oncogenicity of HCC, lncLocator analysis and cellular nucleocytoplasmic fractionation were performed to determine its localization in HCC cells. PRR34-AS1 was predicted to be located in the cytoplasm (Figure 2A).This prediction was reconfirmed using the cellular nucleocytoplasmic fractionation assay (Figure 2B). Extensive evidence has shown that cytoplasmic lncRNAs can act as miRNA sponges, functionally liberating miRNA-targeted mRNAs. Bioinformatic analysis was conducted to identify potential miRNAs with complementary base pairing to PRR34-AS1. Two miRNAs, miR-498 and miR-3614-5p, were predicted to be sequestered by PRR34-AS1 (Figure 2C). After PRR34-AS1 knockdown in HuH7 and SNU-182 cells, the expression levels of miR-498 were found to be increased, whereas those of miR-3614-5p were unaffected (Figure 2D). Therefore, miR-498 was selected for further experiments.
miR-498 expression was detected in the 65 pairs of HCC and adjacent normal tissues. RT-qPCR analysis showed that miR-498 was evidently downregulated in HCC tissues than in adjacent normal tissues (Figure 2E). Furthermore, the expressions of PRR34-AS1 and miR-498 were inversely correlated in the 65 HCC tissues (Figure 2F; r = −0.7021, P < 0.0001), as demonstrated using Pearson’s correlation coefficient. Luciferase reporter assays were conducted to analyze the binding interaction between miR-498 and PRR34-AS1 in HCC cells. The wild-type and mutant binding sites between miR-498 and PRR34-AS1 were presented in Figure 2C. As illustrated in Figure 2G, the luciferase activity of the wt-PRR34-AS1 reporter vector but not that of the mut-PRR34-AS1 reporter vector was reduced by miR-498 upregulation in HuH7 and SNU-182 cells, indicating that miR-498 directly binds to PRR34-AS1. Furthermore, the RIP assay results demonstrated that PRR34-AS1 and miR-498 were statistically enriched in anti-Ago2 pellets (Figure 2H). Taken together, these data suggest that PRR34-AS1 functions as an miR-498 sponge in HCC cells.
miR-498 is an Antioncogenic miRNA in HCC Cells
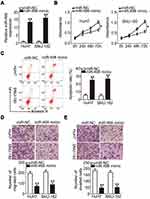
To identify the roles of PRR34-AS1 in HCC cells, HuH7 and SNU-182 cells were transfected with miR-498 mimic to generate miR-498-overexpressing cells (Figure 3A). Cell proliferation was detected via the CCK-8 assay, and the results showed that transfection with miR-498 mimic resulted in a substantial decrease in cell proliferation (Figure 3B). Additionally, compared with the miR-NC group, ectopic miR-498 expression strikingly promoted HuH7 and SNU-182 cell apoptosis (Figure 3C). Furthermore, miR-498-overexpressing HuH7 and SNU-182 cells presented impaired migratory (Figure 3D) and invasive (Figure 3E) abilities compared with miR-NC-transfected cells. These results collectively demonstrate that miR-498 plays cancer-inhibiting roles during HCC progression.
FOXO3 is Directly Targeted by miR-498 Under the Control of PRR34-AS1 in HCC Cells
A previous study has identified FOXO3 (Figure 4A) as a direct target of miR-498 in HCC cells. To confirm this observation, wt-FOXO3 and mut-FOXO3 reporter plasmids were constructed and transfected into HuH7 and SNU-182 cells in the presence of miR-498 mimic or miR-NC. The luciferase reporter assay results showed that the luciferase activity of wt-FOXO3 was initially decreased by miR-498 overexpression, whereas that of mut-FOXO3 was minimally affected in HuH7 and SNU-182 cells (Figure 4B). In addition, transfection with miR-498 mimic led to significantly reduced FOXO3 expression at both the mRNA (Figure 4C) and protein (Figure 4D) levels in HuH7 and SNU-182 cells. Furthermore, the expression of FOXO3 mRNA was dramatically upregulated in HCC tissues than in adjacent normal tissues (Figure 4E). Notably, the abundance of FOXO3 mRNA in the 65 HCC tissue specimens was inversely related to the level of miR-498 (Figure 4F; r = −0.6675, P < 0.0001). The above results demonstrate FOXO3 as a direct target of miR-498 in HCC cells.
To investigate the manner in which PRR34-AS1 regulates FOXO3 levels in HCC cells, the impacts of PRR34-AS1 silencing on FOXO3 mRNA and protein expression in HuH7 and SNU-182 cells were evaluated. The results showed that FOXO3 mRNA (Figure 4G) and protein (Figure 4H) levels were apparently decreased by PRR34-AS1 depletion. Additionally, PRR34-AS1 expression presented a positive correlation with FOXO3 mRNA in the 65 HCC tissues (Figure 4I; r = 0.6265, P < 0.0001). To determine whether PRR34-AS1 regulates FOXO3 expression by adsorbing miR-498, miR-498 inhibitor or NC inhibitor was transfected into PRR34-AS1-depleted HuH7 and SNU-182 cells. RT-qPCR and Western blotting were used to determine the expression of FOXO3. The results confirmed that loss of PRR34-AS1 obviously decreased FOXO3 mRNA (Figure 4J) and protein (Figure 4K) expressions in HuH7 and SNU-182 cells. Significantly, this influence was abolished by the addition of the miR-498 inhibitor. Collectively, these results demonstrate that PRR34-AS1 acts as a molecular sponge for miR-498 in HCC cells, thereby increasing the expression of its downstream target gene FOXO3.
miR-498 Downregulation and FOXO3 Upregulation Both Abrogate the Inhibitory Actions of PRR34-AS1 Downregulation in HCC Cells
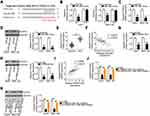
To determine whether the biological activities of PRR34-AS1 in HCC cells are mediated by the regulation of the miR-498/FOXO3 axis, HuH7 and SNU-182 cells previously transfected with si-PRR34-AS1 were treated with miR-498 inhibitor or NC inhibitor. The RT-qPCR results verified the transfection efficiency of the miR-498 inhibitor (Figure 5A). si-PRR34-AS1 transfection obviously suppressed HuH7 and SNU-182 cell proliferation (Figure 5B) but promoted their apoptosis (Figure 5C); the effects were alleviated by miR-498 inhibitor treatment. Additionally, the migration (Figure 5D) and invasion (Figure 5E) of HuH7 and SNU-182 cells impaired by PRR34-AS1 deficiency were partially restored by the addition of the miR-498 inhibitor.
Rescue experiments were performed in HuH7 and SNU-182 cells to test whether FOXO3 was required for the PRR34-AS1-mediated regulation of the malignant features in HCC. First, the overexpression efficiency of pcDNA3.1/FOXO3 was evaluated by measuring the changes in FOXO3 protein expression in HuH7 and SNU-182 cells after the transfection of pcDNA3.1/FOXO3 or pcDNA3.1. The protein level of FOXO3 was significantly increased in pcDNA3.1/FOXO3-transfected HuH7 and SNU-182 cells (Figure 5F). pcDNA3.1/FOXO3 or pcDNA3.1, in combination with si-PRR34-AS1, was transfected into HuH7 and SNU-182 cells, and cell proliferation, apoptosis, migration, and invasion were analyzed. Restoring FOXO3 expression partly relieved the impacts of PRR34-AS1 silencing on the proliferation (Figure 5G), apoptosis (Figure 5H), migration (Figure 5I), and invasion (Figure 5J) of HuH7 and SNU-182 cells. Therefore, the actions of PRR34-AS1 in HCC cells were performed by regulating the output of the miR-498/FOXO3 axis.
Loss of PRR34-AS1 Decreases Tumor Growth in HCC Cells in vivo
To address the hepatocarcinogenesis role of PRR34-AS1 in vivo, xenograft models were established by injecting HuH7 cells stably expressing sh-PRR34-AS1 or sh-NC into nude mice. Tumor volumes (Figure 6A and B) and weights (Figure 6C) were markedly decreased in the subcutaneous tumor xenografts derived from sh-PRR34-AS1 stably transfected HuH7 cells. Tumor xenografts were harvested and subjected to RT-qPCR analysis to determine the expression levels of PRR34-AS1 and miR-498. The data revealed that PRR34-AS1 was downregulated in the subcutaneous tumors from the sh-PRR34-AS1 group (Figure 6D), whereas the levels of miR-498 presented the opposite trend (Figure 6E). Additionally, Western blotting analysis revealed that FOXO3 protein expression was remarkably decreased in PRR34-AS1-deficient tumor xenografts (Figure 6F). Therefore, the interference of PRR34-AS1inhibits the tumor growth of HCC due to altered miR-498/FOXO3 expression.
Discussion
Recent studies have discovered that lncRNAs are differentially expressed in HCC, and they have been validated as crucial regulators in HCC cells.16,29,30 lncRNAs perform important roles in tumor differentiation, growth, metastasis, epithelial–mesenchymal transition, and radiochemotherapy resistance as well as other aspects by regulating gene expression and cancer-related signaling pathways.31 Therefore, further studies on tumor-associated lncRNAs in HCC are warranted for the identification of potential therapeutic targets. In the current study, the expression and detailed roles of PRR34-AS1 in HCC were determined. In addition, a series of mechanistic studies were performed to elucidate the interactions of PRR34-AS1 with miRNAs and mRNAs in relation to its regulation of HCC oncogenicity.
PRR34-AS1 has been shown to promote the protection of propofol pretreatment against ischemia/reperfusion injury;32 however, the expression and functions of PRR34-AS1 in HCC cells remain poorly understood. In the present study, TCGA and GTEx databases and clinical specimens were used to evaluate the expression of PRR34-AS1 in HCC cells. The expression of PRR34-AS1 in HCC tissues was higher than that in normal tissues. Following PRR34-AS1silencing in vitro, we assessed the changes in HCC cell proliferation, apoptosis, migration, and invasion. Our results displayed that PRR34-AS1 interference inhibited HCC cell proliferation, migration, and invasion and promoted cell apoptosis. Tumor xenograft experiments confirmed that loss of PRR34-AS1 reduced tumor growth in HCC cells in vivo.
The above findings led us to investigate the precise mechanisms by which PRR34-AS1 regulates the aggressiveness of HCC cells. The ceRNA regulation theory has recently been proposed to describe the working mechanism of cytoplasmic lncRNAs.33 lncRNAs harbor miRNA-response elements and can compete with other genes to bind to miRNAs, thereby decreasing the miRNA-mediated suppression of miRNA targets.26 Accordingly, the localization of PRR34-AS1 was first predicted using lncLocator, a lncRNA subcellular localization predictor. In addition, cellular nucleocytoplasmic fractionation was performed to isolate the nuclear and cytoplasmic fractions of HCC cells. Both fractions were subjected to RT-qPCR analysis to determine the distribution of PRR34-AS1. PRR34-AS1 was found to be mostly distributed in the cytoplasm of HCC cells, implying its potential ability to function as a molecular sponge for miRNAs.
Using bioinformatics analysis, miR-498 and miR-3614-5p were predicted to be sequestered by PRR34-AS1.miR-498 and miR-3614-5p expression in response to PRR34-AS1 silencing in HCC cells were determined using RT-qPCR. miR-498 was identified as the only one candidate whose expression was strikingly increased in HCC cells after PRR34-AS1 depletion. Moreover, miR-498 was downregulated in HCC tissues, and an inverse correlation was identified between the expression levels of miR-498 and PRR34-AS1 in HCC tissues. Furthermore, luciferase activity and RIP assays verified the direct binding relationship between miR-498 and PRR34-AS1 in HCC cells. After validating FOXO3 as a direct target of miR-498, we further explored the regulatory relationship among PRR34-AS1, miR-498, and FOXO3 in HCC cells. Our results demonstrated that PRR34-AS1 positively regulates FOXO3 expression in HCC cells by acting as an miR-498 sponge. Taken together, a ceRNA regulatory network involving PRR34-AS1, miR-498, and FOXO3 was identified in HCC cells.
miR-498 is expressed at low levels in many types of human cancers, including HCC.34 The results of functional experiments illustrate the tumor-suppressing functions of miR-498 in HCC cells, which are consistent with those of a previous study.34 FOXO3, a direct target of miR-498 in HCC cells, is a member of the forkhead box class O transcription factor family.35 Studies have revealed the high expression of FOXO3 in HCC cells and have confirmed its pro-oncogenic actions during HCC genesis and progression.36–38 In the present study, rescue experiments were conducted, and the results showed that the suppressive effects triggered by PRR34-AS1 knockdown on the malignant features of HCC cells could be abrogated by inhibiting miR-498 or restoring FOXO3. Taken together, it can be inferred that PRR34-AS1 acts as an miR-498 sponge to increase the expression of FOXO3 in HCC cells, thereby exhibiting cancer-promoting actions during cancer progression.
In this study, we did not overexpress PRR34-AS1 expression, and determine the effects of PRR34-AS1 upregulation on the malignant phenotypes of HCC cells. Additionally, we did not explore whether knockdown of PRR34-AS1 may regulate important signal pathways in HCC. They were limitations of our study, and we will resolve them in the near future.
Conclusion
In summary, PRR34-AS1 is significantly highly expressed in HCC tissues and cell lines. PRR34-AS1 promotes the oncogenicity of HCC cells by adsorbing miR-498 and subsequently increasing the expression of FOXO3. Therefore, the PRR34-AS1/miR-498/FOXO3 pathway may offer a basis for HCC treatment.
Disclosure
The authors declare that they have no competing interests.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34.
2. Hartke J, Johnson M, Ghabril M. The diagnosis and treatment of hepatocellular carcinoma. Semin Diagn Pathol. 2017;34(2):153–159. doi:10.1053/j.semdp.2016.12.011
3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30.
4. Kulik L, El-Serag HB. Epidemiology and management of hepatocellular carcinoma. Gastroenterology. 2019;156(2):477–491 e471. doi:10.1053/j.gastro.2018.08.065
5. Portolani N, Coniglio A, Ghidoni S, et al. Early and late recurrence after liver resection for hepatocellular carcinoma: prognostic and therapeutic implications. Ann Surg. 2006;243(2):229–235. doi:10.1097/01.sla.0000197706.21803.a1
6. Ye LY, Chen W, Bai XL, et al. Hypoxia-induced epithelial-to-mesenchymal transition in hepatocellular carcinoma induces an immunosuppressive tumor microenvironment to promote metastasis. Cancer Res. 2016;76(4):818–830. doi:10.1158/0008-5472.CAN-15-0977
7. Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391(10127):1301–1314. doi:10.1016/S0140-6736(18)30010-2
8. Said A, Ghufran A. Epidemic of non-alcoholic fatty liver disease and hepatocellular carcinoma. World J Clin Oncol. 2017;8(6):429–436. doi:10.5306/wjco.v8.i6.429
9. St Laurent G, Wahlestedt C, Kapranov P. The landscape of long noncoding RNA classification. Trends Genet. 2015;31(5):239–251. doi:10.1016/j.tig.2015.03.007
10. Melissari MT, Grote P. Roles for long non-coding RNAs in physiology and disease. Pflugers Arch. 2016;468(6):945–958. doi:10.1007/s00424-016-1804-y
11. Huarte M. The emerging role of lncRNAs in cancer. Nat Med. 2015;21(11):1253–1261.
12. Liz J, Esteller M. lncRNAs and microRNAs with a role in cancer development. Biochim Biophys Acta. 2016;1859(1):169–176. doi:10.1016/j.bbagrm.2015.06.015
13. Wu Y, Shao A, Wang L, et al. The role of lncRNAs in the distant metastasis of breast cancer. Front Oncol. 2019;9:407. doi:10.3389/fonc.2019.00407
14. Hu PA, Miao YY, Yu S, Guo N. Long non-coding RNA SNHG5 promotes human hepatocellular carcinoma progression by regulating miR-363-3p/RNF38 axis. Eur Rev Med Pharmacol Sci. 2020;24(7):3592–3604.
15. Shi C, Yang Q, Pan S, et al. LncRNA OIP5-AS1 promotes cell proliferation and migration and induces angiogenesis via regulating miR-3163/VEGFA in hepatocellular carcinoma. Cancer Biol Ther. 2020;21:604–614. doi:10.1080/15384047.2020.1738908
16. Wang C, Zi H, Wang Y, Li B, Ge Z, Ren X. LncRNA CASC15 promotes tumour progression through SOX4/Wnt/beta-catenin signalling pathway in hepatocellular carcinoma. Artif Cells Nanomed Biotechnol. 2020;48(1):763–769. doi:10.1080/21691401.2019.1576713
17. Shao C, Liu G, Zhang X, Li A, Guo X. Long noncoding RNA RMRP suppresses the tumorigenesis of hepatocellular carcinoma through targeting microRNA-766. Onco Targets Ther. 2020;13:3013–3024. doi:10.2147/OTT.S243736
18. Bi HQ, Li ZH, Zhang H. Long noncoding RNA HAND2-AS1 reduced the viability of hepatocellular carcinoma via targeting microRNA-300/SOCS5 axis. Hepatobiliary Pancreat Dis Int. 2020. doi:10.1016/j.hbpd.2020.02.011
19. Gao L, Xiong DD, He RQ, et al. MIR22HG as a tumor suppressive lncRNA in HCC: a comprehensive analysis integrating RT-qPCR, mRNA-seq, and microarrays. Onco Targets Ther. 2019;12:9827–9848. doi:10.2147/OTT.S227541
20. Liu WG, Xu Q. Long non-coding RNA XIST promotes hepatocellular carcinoma progression by sponging miR-200b-3p. Eur Rev Med Pharmacol Sci. 2019;23(22):9857–9862.
21. Tian X, Wu Y, Yang Y, et al. Long noncoding RNA LINC00662 promotes M2 macrophage polarization and hepatocellular carcinoma progression via activating Wnt/β-catenin signaling. Mol Oncol. 2020;14(2):462–483. doi:10.1002/1878-0261.12606
22. Harries LW. Long non-coding RNAs and human disease. Biochem Soc Trans. 2012;40(4):902–906. doi:10.1042/BST20120020
23. Lajos R, Braicu C, Jurj A, et al. A miRNAs profile evolution of triple negative breast cancer cells in the presence of a possible adjuvant therapy and senescence inducer. J BUON. 2018;23(3):692–705.
24. Rui T, Xu S, Feng S, Zhang X, Huang H, Ling Q. The mir-767-105 cluster: a crucial factor related to the poor prognosis of hepatocellular carcinoma. Biomark Res. 2020;8:7. doi:10.1186/s40364-020-0186-7
25. Yu D, Zhu L, Tu H, Wu L, Yang H, Xu C. miR-4698-Trim59 axis plays a suppressive role in hepatocellular carcinoma. Front Biosci. 2020;25:1120–1131. doi:10.2741/4849
26. Abdollahzadeh R, Daraei A, Mansoori Y, Sepahvand M, Amoli MM, Tavakkoly-Bazzaz J. Competing endogenous RNA (ceRNA) cross talk and language in ceRNA regulatory networks: a new look at hallmarks of breast cancer. J Cell Physiol. 2019;234(7):10080–10100. doi:10.1002/jcp.27941
27. Lin Y, Jian Z, Jin H, et al. Long non-coding RNA DLGAP1-AS1 facilitates tumorigenesis and epithelial-mesenchymal transition in hepatocellular carcinoma via the feedback loop of miR-26a/b-5p/IL-6/JAK2/STAT3 and Wnt/beta-catenin pathway. Cell Death Dis. 2020;11(1):34. doi:10.1038/s41419-019-2188-7
28. Wu S, Chen S, Lin N, Yang J. Long non-coding RNA SUMO1P3 promotes hepatocellular carcinoma progression through activating Wnt/beta-catenin signalling pathway by targeting miR-320a. J Cell Mol Med. 2020;24(5):3108–3116. doi:10.1111/jcmm.14977
29. Gong X, Zhu Z. Long noncoding RNA HOTAIR contributes to progression in hepatocellular carcinoma by sponging miR-217-5p. Cancer Biother Radiopharm. 2020. doi:10.1089/cbr.2019.3070
30. Fan L, Huang X, Chen J, et al. Long non-coding RNA MALAT1 contributes to sorafenib resistance by targeting miR-140-5p/Aurora-A signaling in hepatocellular carcinoma. Mol Cancer Ther. 2020;19:1197–1209. doi:10.1158/1535-7163.MCT-19-0203
31. Huang Z, Zhou JK, Peng Y, He W, Huang C. The role of long noncoding RNAs in hepatocellular carcinoma. Mol Cancer. 2020;19(1):77.
32. Fang H, Zhang FX, Li HF, et al. PRR34-AS1 overexpression promotes protection of propofol pretreatment against ischemia/reperfusion injury in a mouse model after total knee arthroplasty via blockade of the JAK1-dependent JAK-STAT signaling pathway. J Cell Physiol. 2020;235(3):2545–2556. doi:10.1002/jcp.29158
33. Ye Y, Shen A, Liu A. Long non-coding RNA H19 and cancer: a competing endogenous RNA. Bull Cancer. 2019;106(12):1152–1159. doi:10.1016/j.bulcan.2019.08.011
34. Li W, Jiang H. Up-regulation of miR-498 inhibits cell proliferation, invasion and migration of hepatocellular carcinoma by targeting FOXO3. Clin Res Hepatol Gastroenterol. 2020;44(1):29–37. doi:10.1016/j.clinre.2019.04.007
35. Huang H, Tindall DJ. Dynamic FoxO transcription factors. J Cell Sci. 2007;120(Pt 15):2479–2487. doi:10.1242/jcs.001222
36. Song SS, Ying JF, Zhang YN, et al. High expression of FOXO3 is associated with poor prognosis in patients with hepatocellular carcinoma. Oncol Lett. 2020;19(4):3181–3188.
37. Zhao W, Dai Y, Dai T, et al. TRIP6 promotes cell proliferation in hepatocellular carcinoma via suppression of FOXO3a. Biochem Biophys Res Commun. 2017;494(3–4):594–601. doi:10.1016/j.bbrc.2017.10.117
38. Yang Z, Liu S, Zhu M, et al. PS341 inhibits hepatocellular and colorectal cancer cells through the FOXO3/CTNNB1 signaling pathway. Sci Rep. 2016;6:22090.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.