Back to Journals » OncoTargets and Therapy » Volume 12
Long noncoding RNA LINC00515 promotes cell proliferation and inhibits apoptosis by sponging miR-16 and activating PRMT5 expression in human glioma
Received 13 December 2018
Accepted for publication 1 March 2019
Published 8 April 2019 Volume 2019:12 Pages 2595—2604
DOI https://doi.org/10.2147/OTT.S198087
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Carlos E Vigil
Zhangyi Wu, Yihai Lin
Department of Neurosurgery, Zhejiang Tongde Hospital, Hangzhou, Zhejiang Province, People’s Republic of China
Introduction: In recent years, an increasing amount of literature has demonstrated the functional role of long non-coding RNA (lncRNA) in human diseases. LINC00515 is a newly defined lncRNA and is reported to act as an oncogene in multiple myeloma. However, the function of LINC00515 in glioma is still uncertain.
Materials and methods: We examined the expression levels of LINC00515 in human glioma tissues and cell lines using real-time PCR analysis. In addition, we confirmed the distribution of LINC00515 in glioma cells and suppressed LINC00515 expression with siRNAs. CCK-8, colony formation assay and apoptosis analysis were used to study the function of LINC00515 in glioma progression. Then, we used bioinformatics prediction and subsequent experiments to reveal the underlying molecular mechanism.
Results: We found that LINC00515 was up-regulated in glioma tissues and cell lines. LINC00515 was mainly located in the cytoplasm in glioma cells. Knockdown of LINC00515 led to decreased proliferation and increased apoptosis of glioma cells. Mechanistically, our data indicated that there was a LINC00515/miR-16/PRMT5 regulatory axis in glioma. LINC00515 could activate PRMT5 expression and promote glioma progression by acting as a sponge of miR-16.
Conclusion: LINC00515 expression is elevated in human glioma and promotes growth and inhibits apoptosis of glioma cells. The regulatory cascade LINC00515/miR-16/PRMT5 plays a critical role in glioma progression.
Keywords: LINC00515, miR-16, PRMT5, glioma
Introduction
Brain tumors account for about 2% in worldwide human cancer incidence rate and glioma, a subtype of primary brain tumor, can cause significant morbidity and have very poor prognosis.1 Glioma commonly occurs in the central nervous system and is reported to represent 75% of malignant primary brain tumors in adults.2 Glioma can be divided into four grades (I, II, III, IV) in accordance with World Health Organization (WHO) grade criteria.3 Survival rates of glioma after diagnosis are significantly associated with their grades. Despite development in cancer therapeutic strategies, surgery, radiation and chemotherapy still make up the greatest proportion of glioma treatments which lead to remaining high mortality and poor prognosis in malignant glioma.4 The most common type of malignant glioma is glioblastoma which has the highest lethality and poorest prognosis among glioma.5 The heterogeneity of glioma also increases the complexity of its therapeutic treatment. To search for new strategies responsible for glioma therapy, we are planning to focus on other types of biomolecules other than protein markers.
In human genome, protein coding genes only represent 2% of the whole genome. Long non-coding RNAs (lncRNAs) are kinds of non-coding RNAs which are defined longer than 200nt, distinguished from small non-coding RNAs. Although they lack protein coding ability, lncRNAs are reported to be involved in various cellular processes and tumorigenesis, including glioma, via interactions with other biological molecules due to the diversity of their structures. Aberrant lncRNA expression in glioma correlates with their malignancy grades and histological differentiation.6 Many researchers have demonstrated the importance of lncRNAs in glioma initiation and progression. Zhang et al. found that lncRNA HOTAIR could promote cell cycle process through EZH2 in human glioblastoma cells.7 Another research group suggested that down-regulated expression of lncRNA MEG3 promoted cell proliferation and G0/G1 accumulation of cell cycle in glioma cell lines, while up-regulated MEG3 promoted cell apoptosis.8 Moreover, Katsushima et al. reported that Notch-induced lncRNA TUG1 antagonized miR-145 to promote cell self-renewal and stemness maintenance and recruited polycomb proteins to repress differentiation genes in glioma stem cells.9 They also treated intracranial xenograft mice with antisense oligonucleotides targeting TUG1 and found inducing differentiation and repressing growth of GSCs in vivo, which demonstrated the possible utilization of anti-lncRNA drugs in glioma treatment.
Large intergenic noncoding RNAs (lincRNAs) are kinds of evolutionarily conserved lncRNAs which have similar expression levels with protein coding genes but show lack of protein-coding capacity.10 LINC00515 has been reported to play different roles in several cancer types, including inhibition of melphalan-resistant myeloma autophagy and chemoresistance,11 functions in tumorigenesis of stage I lung adenocarcinoma.12 However, how LINC00515 exerts its function in glioma has not been clearly claimed. Recent research has also revealed that lncRNAs and miRNAs may act as competing endogenous RNAs (ceRNAs) and participate in different biological processes.13
In this study, we explored the function of LINC00515 in glioma and our research may provide new insights into glioma treatment.
Materials and methods
Clinical sample collection
Glioma tissue samples and adjacent normal tissue samples were obtained from Zhejiang Tongde Hospital, during 2015–2018. Tissue samples were frozen immediately after surgery and isolated for RNA in the following experiments. The research involved with human tissue specimens was approved by the Scientific and Ethics Committee of Zhejiang Tongde Hospital. Written informed consent was obtained from all patients and this procedure was conducted in accordance with the Declaration of Helsinki.
Cell culture
Human glioma cell lines U251, U87MG, LN229 and normal human astrocyte (NHA) cells were purchased from the Shanghai Institute of Cell Biology, Chinese Academy of Sciences (Shanghai, China). Cells were cultured in Dulbecco’s modified Eagle Medium (Invitrogen, Carlsbad, CA, USA) supplemented with 10% (v/v) fetal bovine serum (Invitrogen) and 1% (v/v) penicillin-streptomycin (Invitrogen). Cells were maintained in a humidified environment containing 5% CO2 at 37°C.
RNA interference assay and cell transfections
Synthetic LINC00515 and negative control (NC) siRNA, miR-16 and NC mimics were synthesized by GenePharma (Shanghai, China). The sequences used were: miR-16 mimics, 5ʹ-UAGCAGCACGUAAAUAUUGGCG-3ʹ; siLINC00515-1, 5ʹ-GCAAGTACTTTGCAGACAA-3ʹ, siLINC00515-2, 5ʹ-GTAGAAGAATGAAATTTAA-3ʹ. U251 and U87MG cells were transfected with oligonucleotides using Lipofectamine 3000 (Invitrogen) according to the manufacturer’s instructions.
Cell proliferation, cell colony formation and cell apoptosis assays
Cell proliferation rate was measured by cell counting kit-8 (CCK-8, Beyotime, Shanghai, China) assay. Transfected cells were seeded into 96-well plates at a density of 3000 cells per well (n=5). During 1, 2, 3, 4, 5 days culturing, 10% (v/v) CCK-8 solution was added into each well. After incubating at 37°C under light protection for 2 hrs, the values of OD 450 nm were read on machine. 1000 cells were plated into 6-well plates per well for colony formation assay (n=3). After two-week culturing under previously described conditions, the colonies were fixed with methanol and stained with 0.1% crystal violet. Transfected cells were harvested to analyze apoptosis using Apoptosis Detection Kit (BD Biosciences, San Jose, CA, USA) according to manufacturer’s instructions. Apoptotic cells were detected by FACS Calibur flow cytometer (BD Biosciences).
RNA isolation and real-time PCR
Total RNA from frozen tissue samples and cell lines was isolated using TRIzol reagent (Invitrogen). RNA from nuclear and cytoplasmic fractions was separated following described method of Abcam. Briefly, harvested U251 cell pellets were suspended into 500 μL fractionation buffer (20 mM HEPES pH 7.4, 10 mM KCl, 2 mM MgCl2, 1 mM EDTA, 1 mM EGTA) and incubated for 15 min on ice. 1-mL syringe was used to pass cell suspension through a 27-gauge needle 10 times and then left on samples on ice for 20 mins and centrifuged at 3,000 rpm for 5 mins at 4°C to obtain the supernatant containing cytoplasmic fraction and pellet containing nuclei. RNA purification kit (ZYMO research, Orange, CA, USA) was used to purify RNA from cytoplasmic supernatant and TRIzol reagent was used to extract RNA from nuclear pellet. The levels of GAPDH (cytoplasm), U6 (nucleus) and LINC00515 were detected using real-time PCR. HiScript 1st Strand cDNA Synthesis Kit (Vazyme Biotech, Nanjing, China) was used to synthesize cDNA for following real-time PCR assays. Real-time PCR was performed using AceQ qPCR SYBR Green Master Mix (Vazyme Biotech) on an ABI 7900 system (Applied Biosystems, Foster City, CA, USA) in triplicate. The primer sequences used were as follows: LINC00515, forward, 5ʹ-GGTTTTCAAGGCAGCAGTGG-3ʹ, reverse, 5ʹ-GACTACGGAGCCCCCAAAAA-3ʹ. GAPDH, forward, 5ʹ-GTCTCCTCTGACTTCAACAGCG-3ʹ, reverse, 5ʹ-ACCACCCTGTTGCTGTAGCCAA-3ʹ.
RNA fluorescent in situ hybridization (FISH) assay
U251 cells were plated onto 4-well chamber slide (Sigma-Aldrich) and performed FISH assay using the Fluorescent In Situ Hybridization Kit (Ribobio, Guangzhou, China) according to manufacturer’s instructions after culturing cells were at 60–70% confluence. LINC00515 probes were synthesized by Ribobio. Cells were fixed in 4% paraformaldehyde for 10 mins and washed with PBS three times. PBS containing 0.5% Triton X-100 was used for cell permeabilization. After washing, pre-hybridization buffer were added into each well and incubated at 37°C for 30 mins and pre-hybridization buffer and supplement with LINC00515 FISH probe mix in hybridization buffer were discarded. The slide was hybridized at 37°C overnight protected from light and all the following steps were also performed in a dark circumstance. The slide was washed by 0.1% Tween-20/4× Saline Sodium Citrate (SSC) at 42°C for three times. 2× SSC, 1× SSC were then used to wash the slide at 42°C. After washing with PBS, cells were stained with DAPI for 10 mins. Fluorescent signal detection was performed using microscope (Nikon, Tokyo, Japan).
Luciferase assay
Wild type (WT) and mutant fragments of LINC00515 or PRMT5 3ʹ-UTR containing the sites targeting miR-16 were synthesized by Genscript (Nanjing, China) and cloned into luciferase reporter vector pGL3-basic (Promega, Madison, WI, USA). The WT and mutant vectors were individually co-transfected with a β-galactosidase (β-gal) expression plasmid (Invitrogen) which was set as transfection efficiency control and miRNA mimics using Lipofectamie 3000 (Invitrogen). Relative luciferase activity was measured using Dual-Luciferase Assay Kit (Promega) after 48 hrs transfection.
Cell lysates preparation and western blot analysis
Cells were harvested and lysed in cell lysis buffer (Beyotime). Cell lysates were incubated on ice for 15 mins and centrifuged at 12,000 rpm for 10 mins at 4°C. Protein levels were measured by BCA Protein Assay Kit (Thermo Fisher Scientific, San Jose, CA, USA). Boiled samples were loaded equally into SDS-PAGE and transferred onto PDVF membranes (Roche, Basel, Switzerland). After 1 hr blocking in 5% milk at room temperature, membranes were incubated with primary antibodies and rotated overnight at 4°C. After washing with PBS containing 0.1% Tween-20 and incubation of secondary antibodies, ECL Western Blotting Substrate (Thermo Fisher Scientific) was used to detect protein bands. Antibodies used for western blot assay were: PRMT5 (Abcam, USA, ab109451) and GAPDH (Abcam, USA, ab181602).
Statistical analysis
All experiments were performed at least three times. Data were analyzed with GraphPad Prism (version 8, La Jolla, CA, USA) and were expressed as the mean ± standard deviation. The means between two groups were compared using Student’s t-test. P<0.05 was considered to be significant.
Results
LncRNA LINC00515 was overexpressed in glioma tissues and cell lines
It was reported that LINC00515 expression was up-regulated in multiple myeloma.11 However, there is no study on the function of LINCR00515 in glioma. So, we first examined the mRNA level of LINC00515 in glioma tissues and adjacent normal tissues. Real-time PCR results showed that LINC00515 was overexpressed in glioma tissues (Figure 1A). In addition, LINC00515 expression was correlated with the grade of glioma (Figure 1B). Meanwhile, LINC00515 mRNA levels were much higher in U251, U87MG and LN229 glioma cells compared with NHAs (Figure 1C). These data indicated that LINC00515 expression was elevated in glioma tissues and may play a role in glioma progression.
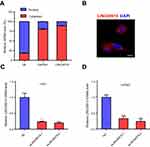
Cellular distribution of LINC00515 in glioma cells
Since there are few studies on LINC00515, we first decided to determine the subcellular localization of LINC00515. We fractionated U251 glioma cells into cytoplasmic and nuclear fractions and measured the mRNA level of LINC00515. The results revealed that LINC00515 was mainly distributed in the cytoplasm (Figure 2A). Strand-specific RNA fluorescence in situ hybridization (FISH) detection of LINC00515 showed similar results (Figure 2B). To further explore the function of LINC00515 in glioma cells, we used siRNAs to knockdown LINC00515 expression in U251 and U87MG glioma cells. The interference effect was verified by real-time PCR analysis (Figure 2C and D).
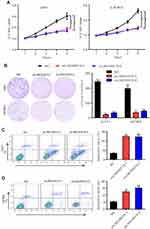
LINC00515 promotes tumor progression of glioma cells
We then performed a series of experiments to assess the effects of LINC00515 knockdown on glioma progression. Cell Counting Kit-8 (CCK-8) assay revealed that LINC00515 knockdown significantly impacted cell proliferation rate of U251 and U87MG glioma cells compared with NC cells (Figure 3A). Similarly, colony formation assay showed that U251 and U87MG glioma cells formed fewer colonies after LINC00515 silencing (Figure 3B). Further, LINC00515 deficiency increased apoptosis of glioma cells (Figure 3C and D). These results demonstrated that LINC00515 was involved in proliferation and apoptosis of glioma cells.
LINC00515 sponged miR-16 in glioma cells
The cytoplasmic distribution of LINC00515 indicated that it may function through a ceRNA network in glioma cells. To explore the potential target of LINC00515, we predicted the binding partners of LINC00515 using online bioinformatics programs and we found that miR-16 may bind with LINC00515 (Figure 4A). Dual luciferase reporter assay showed that miR-16 mimics could reduce luciferase activity of wild type (WT) LINC00515, while mutation of the binding site could eliminate this effect (Figure 4B). In U251, U87MG and LN229 glioma cells, miR-16 expression was decreased compared with NHAs, which is negatively correlated with LINC00515 expression (Figure 4C). Additionally, miR-16 expression levels were increased in LINC00515 silencing U251 and U87MG glioma cells (Figure 4D). These data suggested that miR-16 can bind with LINC00515 and negatively regulate its expression. Thus, LINC00515 sponged miR-16 in glioma cells.
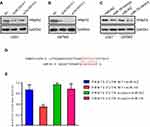
PRMT5 was the downstream target of LINC00515 through sponging miR-16 in glioma cells
PRMT5 was reported as an oncogene in glioma. We found that knockdown LINC00515 expression could increase PRMT5 expression in U251 and U87MG glioma cells (Figure 5A and B). Meanwhile, U251 and U87MG glioma cells transfected with miR-16 mimics significantly inhibited PRMT5 expression (Figure 5C). The binding prediction result showed that miR-16 could bind to the 3ʹ untranslated region (3ʹUTR) of PRMT5 (Figure 5D). Dual luciferase reporter assay confirmed that miR-16 mimics could repress luciferase activity of WT PRMT5 3ʹUTR and the mutation of the binding sequence could dispel the inhibitory effect (Figure 5E). Overall, these data indicated that LINC00515 regulated PRMT5 expression through sponging miR-16 in glioma cells.
Discussion
In recent years, with the development of sequencing technology, more and more non-coding RNAs have been discovered and confirmed to be involved in a variety of physiological and pathological processes.14,15 In this study, we demonstrated that the expression of lncRNA LINC00515 was abnormally elevated in glioma tissues and cell lines and its expression was correlated with tumor grade. Furthermore, we found that LINC00515 played a vital role in the proliferation and apoptosis of glioma cells. Molecular mechanisms that contribute to the effect of LINC00515 in glioma progression were revealed by bioinformatics prediction and subsequent experiments. In glioma cells, LINC00515 could act as a sponge for miR-16 and their expression is negatively correlated. Also, LINC00515 could regulate the expression of an epigenetic modulator, PRMT5, and this regulation was, at least partially, through miR-16 in glioma cells. To sum up, our results indicate that LINC00515 promotes gliomagenesis and may act as a sponge of miR-16 to regulate PRMT5 expression in glioma.
Previously, hundreds of studies have shown that lncRNA plays an important role in glioma initiation and progression. For instance, lncRNA TUG1 was reported to be essential in the maintenance of stemness of glioma cells. TUG1 can recruit polycomb complex to the promoters of differentiation-related genes and repress their expression to promote self-renewal of glioma cells.9 LncRNA NEAT1 was up-regulated in glioma and it could promote cell viability, migration and invasion by activating the expression of SOX2 through suppressing miR-132.16 LINC00515 is a newly reported 557bp length lncRNA. There are few studies on LINC00515 and its role in human cancers is still unclear. It was reported that in multiple myeloma LINC00515 could activate ATG14 expression via sponging miR-140-5p, which mediated increased autophagy levels and chemoresistance of melphalan.11 Here, our study confirmed the role of LINC00515 in glioma progression which will be beneficial to expand our understanding on the function of LINC00515 in tumors.
It has been proven that many deregulated lncRNAs were strongly linked to the development stages of various cancers. One of the most investigated lncRNA, MALAT1 has been proven as a prominent biomarker of lung cancer and prostate cancer patients. Our data suggested that LINC00515 expression was correlated with the grade of glioma patients which indicated that LINC00515 might be a potential biomarker for glioma. We did not examine LINC00515 expression in the blood of glioma patients and further research is needed.
PRMT5 is the most important type II protein arginine methyltransferase. Numerous literature have demonstrated that PRMT5 was an oncogene in a large number of cancers including glioma.17,18 Braun et al. used an in vivo shRNA screen in glioma and found that inhibition of PRMT5 suppressed tumor progression in vivo including patient-derived xenograft models.19 Another study revealed that PRMT5 inhibitor could induce apoptosis of glioma cells which suggested that PRMT5 might be a potential druggable target for clinical therapy of glioma.20 In our study, we demonstrated a new lncRNA–miRNA–mRNA axis in PRMT5 regulation in glioma cells which provided a novel molecular mechanism of PRMT5 regulation.
In conclusion, our study reveals a critical LINC00515/miR-16/PRMT5 cascade in glioma progression. LINC00515 is overexpressed in glioma and acts as an oncogenic lncRNA that promotes PRMT5 expression via a ceRNA network. These findings can help explain the abnormal expression of PRMT5 in glioma and may provide a theoretical basis for clinical treatment of glioma.
Disclosure
The authors report no conflicts of interest in this work.
References
1. McGuire S. World cancer report 2014. Geneva, Switzerland: World Health Organization, International Agency for Research on Cancer, WHO Press, 2015. Adv Nutr. 2016;7(2):418–419. doi:10.3945/an.116.012211
2. Lapointe S, Perry A, Butowski NA. Primary brain tumours in adults. Lancet. 2018;392(10145):432–446. doi:10.1016/S0140-6736(18)30990-5
3. Komori T. The 2016 WHO classification of tumours of the central nervous system: the major points of revision. Neurol Med Chir (Tokyo). 2017;57(7):301–311. doi:10.2176/nmc.ra.2017-0010
4. Omuro A, DeAngelis LM. Glioblastoma and other malignant gliomas: a clinical review. Jama. 2013;310(17):1842–1850. doi:10.1001/jama.2013.280319
5. Hanif F, Muzaffar K, Perveen K, Malhi SM, Simjee SU. Glioblastoma multiforme: a review of its epidemiology and pathogenesis through clinical presentation and treatment. Asian Pac J Cancer Prev. 2017;18(1):3. doi:10.22034/APJCP.2017.18.1.23
6. Peng Z, Liu C, Wu M. New insights into long noncoding RNAs and their roles in glioma. Mol Cancer. 2018;17(1):61. doi:10.1186/s12943-018-0812-2
7. Zhang K, Sun X, Zhou X, et al. Long non-coding RNA HOTAIR promotes glioblastoma cell cycle progression in an EZH2 dependent manner. Oncotarget. 2015;6(1):537.
8. Wang P, Ren Z, Sun P. Overexpression of the long non‐coding RNA MEG3 impairs in vitro glioma cell proliferation. J Cell Biochem. 2012;113(6):1868–1874. doi:10.1002/jcb.24055
9. Katsushima K, Natsume A, Ohka F, et al. Targeting the Notch-regulated non-coding RNA TUG1 for glioma treatment. Nat Commun. 2016;7:13616. doi:10.1038/ncomms13616
10. Khalil AM, Guttman M, Huarte M, et al. Many human large intergenic noncoding RNAs associate with chromatin-modifying complexes and affect gene expression. Proc Natl Acad Sci. 2009;106(28):11667–11672. doi:10.1073/pnas.0904715106
11. Lu D, Yang C, Zhang Z, Cong Y, Xiao M. Knockdown of Linc00515 inhibits multiple myeloma autophagy and chemoresistance by upregulating miR-140-5p and downregulating ATG14. Cellular Physiol Biochem. 2018;48(6):2517–2527. doi:10.1159/000492690
12. Tian Z, Wen S, Zhang Y, et al. Identification of dysregulated long non-coding RNAs/microRNAs/mRNAs in TNM I stage lung adenocarcinoma. Oncotarget. 2017;8(31):51703. doi:10.18632/oncotarget.18512
13. Ebert MS, Neilson JR, Sharp PA. MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells. Nat Methods. 2007;4(9):721. doi:10.1038/nmeth1079
14. Beermann J, Piccoli M-T, Viereck J, Thum T. Non-coding RNAs in development and disease: background, mechanisms, and therapeutic approaches. Physiol Rev. 2016;96(4):1297–1325. doi:10.1152/physrev.00041.2015
15. Esteller M. Non-coding RNAs in human disease. Nature RevGenet. 2011;12(12):861. doi:10.1038/nrg3074
16. Zhou K, Zhang C, Yao H, et al. Knockdown of long non-coding RNA NEAT1 inhibits glioma cell migration and invasion via modulation of SOX2 targeted by miR-132. Mol Cancer. 2018;17(1):105. doi:10.1186/s12943-018-0849-2
17. Stopa N, Krebs JE, Shechter D. The PRMT5 arginine methyltransferase: many roles in development, cancer and beyond. Cell Mol Life Sci. 2015;72(11):2041–2059. doi:10.1007/s00018-015-1847-9
18. Karkhanis V, Hu Y-J, Baiocchi RA, Imbalzano AN, Sif S. Versatility of PRMT5-induced methylation in growth control and development. Trends Biochem Sci. 2011;36(12):633–641. doi:10.1016/j.tibs.2011.09.001
19. Braun CJ, Stanciu M, Boutz PL, et al. Coordinated splicing of regulatory detained introns within oncogenic transcripts creates an exploitable vulnerability in malignant glioma. Cancer Cell. 2017;32(4):411–26. e11. doi:10.1016/j.ccell.2017.08.018
20. Banasavadi-Siddegowda YK, Welker AM, An M, et al. PRMT5 as a druggable target for glioblastoma therapy. Neuro-Oncology. 2017;20(6):753–763. doi:10.1093/neuonc/nox206
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.