Back to Journals » OncoTargets and Therapy » Volume 13
Long Non-Coding RNA OIP5-AS1 Knockdown Enhances CDDP Sensitivity in Osteosarcoma via miR-377-3p/FOSL2 Axis
Received 29 September 2019
Accepted for publication 10 April 2020
Published 7 May 2020 Volume 2020:13 Pages 3853—3866
DOI https://doi.org/10.2147/OTT.S232918
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Gaetano Romano
Ling Liu,* Shuya Wang*
Department of Surgery, Huaihe Hospital of Henan University, Kaifeng 475000, Henan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Ling Liu
Department of Surgery, Huaihe Hospital of Henan University, No. 115, Ximen Street, Longting District, Kaifeng 475000, Henan, People’s Republic of China
Tel +86-371-23906022
Email [email protected]
Background: Drug resistance is one of big obstacles for the treatment of tumor. Long non-coding RNA Opa-interacting protein 5-antisense RNA 1 (OIP5-AS1) was identified to involve in drug resistance. In this research, the effects of OIP5-AS1 on cisplatin (CDDP) resistance in osteosarcoma (OS) were mainly investigated.
Methods: The levels of OIP5-AS1, microRNA-377-3p (miR-377-3p), and FOS like 2 (FOSL2) were measured by quantitative real-time polymerase chain reaction. The inhibitory concentration 50 (IC50) value of CDDP, cell viability and apoptotic rate was evaluated through Cell Counting Kit-8 and flow cytometry assays, respectively. The levels of multidrug resistance-associated protein 1 (MRP1), P-glycoprotein, B-cell lymphoma 2, Bcl2-associated X, cleaved-caspase-3, and FOSL2 were detected by Western blot assay. The interaction between miR-377-3p and OIP5-AS1 or FOSL2 was verified by Dual-Luciferase Reporter and RNA Immunoprecipitation assays. The function of OIP5-AS1 was detected by a xenograft tumor model in vivo.
Results: OIP5-AS1 and FOSL2 were up-regulated, while miR-377-3p was down-regulated in CDDP-resistant OS tissues and cells. OIP5-AS1 silencing inhibited cell viability and the IC50 value of CDDP, and promoted apoptotic rate in CDDP-resistant OS cells. Mechanically, OIP5-AS1 was verified as a sponge to miR-377-3p and FOSL2 was a target of miR-377-3p. Moreover, OIP5-AS1 knockdown repressed OS tumor growth and enhanced CDDP sensitivity of OS in vivo.
Conclusion: OIP5-AS1 positively modulated FOSL2 expression to decrease CDDP sensitivity in OS by sponging miR-377-3p.
Keywords: lncRNA OIP5-AS1, miR-377-3p, FOSL2, CDDP resistance, osteosarcoma
Introduction
Osteosarcoma (OS) is a common primary bone tumor among children and young adults.1 Because of the improvement in treatment approaches, the 5-year survival rate of OS patients was elevated to about 70%.2 Cisplatin (CDDP), an important non-specific chemical agent for cancer patients, could destroy the function of DNA, repress mitosis, and accelerate cell apoptosis in tumor.3 However, lots of OS patients underwent chemoresistance over time, and this is a new barrier for OS treatments. Therefore, it is crucial to elucidate the molecular mechanism of CDDP resistance in OS and search the novel therapeutic targets for OS patients.
Long non-coding RNAs (lncRNAs), a form of long RNAs (>200 nucleotides (nts)) without protein-coding potentiality, have been identified to affect gene expression at post-transcriptional stage.4 Emerging data indicated that lncRNAs played vital roles in drug resistance of OS. For example, a study implied that lncRNA HOTTIP elevated CDDP resistance in OS.5 Another study disclosed that ANRIL depletion enhanced CDDP sensitivity and apoptosis.6 Opa-interacting protein 5-antisense RNA 1 (OIP5-AS1) was reported to be associated with the progression of various tumors. For instance, Li et al documented that OIP5-AS1 was increased in oral squamous cell carcinoma (OSCC), and its deletion confined cell viability and metastasis.7 The similar results were also reported in gastric cancer,8 hemangioma,9 glioma,10 and OS.11 Another report revealed that OIP5-AS1 was also related to chemoresistance.12 However, the effect of OIP5-AS1 on OS chemoresistance was seldom reported.
MicroRNAs (miRNAs), a type of small RNAs without translation ability, can regulate gene expression through silencing or degrading message RNAs (mRNAs).13 Recent studies reported that miRNAs were implicated in the processes of chemoresistance. For instance, miR-34c was dramatically decreased in chemoresistance OS, and its overexpression impeded cell metastasis, and improved drug sensitivity.14 Another study indicated that miR-340 mitigated CDDP resistance in OS by targeting ZEB1.15 Notably, a recent study reported that miR-377-3p was associated with tumor progression.16,17
FOS like 2 (FOSL2), located on chromosome 2p23.3, is a leucine zipper DNA-binding FOS-type nuclear phosphoprotein and plays vital roles in fat metabolism, bone development and the occurrence of diseases and cancers.18 Emerging evidence demonstrated that FOSL2 was involved in the progression and chemoresistance of OS.19,20 However, the mechanisms of miR-377-3p and FOSL2 on CDDP resistance were rarely documented in OS. In the current exploration, we mainly explored the effects of OIP5-AS1 on CDDP resistance in OS.
Materials and Methods
Tissues Collection
The study was approved by the Ethics Committee of Huaihe Hospital of Henan University and executed in accordance with the Declaration of Helsinki Principles. Moreover, OS tissue samples (n=47) were collected from Huaihe Hospital of Henan University, and these samples were classified as CDDP-resistant OS tissue samples (n=30) and CDDP-sensitive OS tissue samples (n=17) based on the previous study.21 Meanwhile, we have verified these CDDP-resistant cells in Figure S1. All tissue samples were frozen at −80°C refrigerator until further used. Written informed consents were provided by all patients.
Cell Culture and Treatment
Two OS cell lines MG63 and Saos-2 were obtained from Procell (Wuhan, China). Through the stepwise increasing CDDP concentrations (0–60 μg/mL, Sigma, Shanghai, China), the corresponding OS-resistant cell lines (MG63/CDDP and Saos-2/CDDP) were established from parental cell lines MG63 and Saos-2, as previously described.22 All cells were cultivated in Dulbecco’s modified Eagle’s medium (DMEM) high-glucose (4.5g/L) (Solarbio, Beijing, China) containing 10% fetal bovine serum (FBS; Thermo Fisher Scientific, Rockville, MD, USA). All cells were incubated in an incubator with 5% CO2 at 37°C.
Cell Transfection
Small interference RNA (siRNA) against OIP5-AS1 (si-OIP5-AS1, 5ʹ-GCTCCTAGGATTCCAGTTA-3ʹ) and its negative control (si-NC), miR-377-3p mimic (miR-377-3p) and its control (miR-NC), miR-377-3p inhibitor (anti-miR-377-3p) and its scramble (anti-miR-NC) were purchased from GenePharma (Shanghai, China). The sequences of OIP5-AS1 and FOSL2 were inserted into pcDNA3.1 vector (pcDNA-NC; Invitrogen, Carlsbad, CA, USA) to construct overexpression plasmid (pcDNA-OIP5-AS1 or pcDNA-FOSL2). Whereafter, MG63/CDDP and Saos-2/CDDP cells (2 × 105 cells/well) were seeded in 6-well plates, and transfected with these plasmids and oligonucleotides for 48 h according to the producer’s instructions of Lipofectamine 2000 (Invitrogen).
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
Total RNA from CDDP-resistant OS tissues or cells was extracted using an RNA extraction kit (Solarbio). The random primers (Solarbio) were used to perform reverse transcription, and the qRT-PCR was carried out on Thermal Cycler CFX6 System (Bio-Rad, California, USA) with SYBR mix (TaKaRa, Dalian, China). The amplification parameters were: denaturation at 95°C for 10 min, followed by 40 cycles of denaturation at 95°C for 30s, annealing at 60°C for 30s and extension at 72°C for 1min. The levels of OIP5-AS1 and FOSL2 were normalized by glyceraldehyde 3-phosphate dehydrogenase (GAPDH), and the level of miR-377-3p was standardized via small nuclear RNA U6, then calculated with the method of 2−ΔΔCt. The primer sequences were presented as follows: OIP5-AS1 (F, 5ʹ-TGCGAAGATGGCGGAGTAAG-3ʹ, R, 5ʹ-TAGTTCCTCTCCTCTGGCCG-3ʹ); miR-377-3p: (F, 5ʹ-GGGAGGCAGTGTATTGTTA-3ʹ, R, 5ʹ-CAGTGCGTGTCGTGGAGT-3ʹ); FOSL2 (F, 5ʹ-GAGAGGAACA AGCTGGCTGC-3ʹ, R, 5ʹ-GCTTCTCCTTCTCCTTCTGC-3ʹ); GAPDH (F, 5ʹ-TGTTCGTCATGGGTGTGAAC-3ʹ, R, 5ʹ-ATGGCATGGACTGTGGTCAT-3ʹ), and U6 (F, 5ʹ-ATTGGAACGATACAGAGAAGATT-3ʹ, and R, 5ʹ-GGAACGCTTCACGAATTTG-3ʹ).
Cell Counting Kit-8 (CCK-8) Assay
The cell viability of MG63/CDDP and Saos-2/CDDP cells was conducted using CCK-8 kit (Solarbio). For CDDP sensitivity, the cells (2 × 104 cells/well) in 96-well plates were firstly transfected with si-NC or si-OIP5-AS1, then the cells incubated with different concentrations of CDDP (0, 0.625, 1.25, 2.5, 5, 10, 20, 40, and 60 μg/mL) for 48 h. The CCK-8 was added into each well and incubated for another 4 h. The absorbance at 450 nm was detected using a microplate reader. The inhibitory concentration 50 (IC50) value of CDDP was calculated by the survival curve.
For cell viability, the cells were cultivated in 96-well plate for 24 h. Following transfection, the cells were cultured for another 0 h, 24 h, 48 h, and 72 h, then CCK-8 solution was added, and the absorbance at 450 nm was measured.
Flow Cytometry Analysis of Cell Apoptosis
The Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) apoptosis detection kit (Solarbio) was used to assess the apoptotic rate. The MG63/CDDP and Saos-2/CDDP cells (1×105) were re-suspended in binding buffer and then incubated with Annexin V-FITC for 10 min, PI for 5 min at room temperature in dark condition. The apoptotic rate was evaluated through flow cytometry (BD Bioscience, San Jose, CA, USA) and analyzed with Cell Quest software (BD Biosciences, Franklin Lakes, NJ, USA)
Western Blot Assay
The protein in CDDP-resistant tissues and cells was extracted using RIPA reagent (Solarbio) and the concentration of protein samples was tested using a BCA detection kit (Beyotime, Shanghai, China). Then sodium dodecyl sulfonate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed to separate protein samples and then the bands were transferred onto a polyvinylidene fluoride (PVDF) membrane (GE Healthcare, Piscataway, NJ, USA). Subsequently, the membrane was blocked in 5% skim milk (dissolved in TBST buffer) for 4 h, and then incubated with primary antibody for 12 h at 4°C. The membrane was incubated with secondary antibody for 2 h at 37°C. The bands’ intensities were examined using an ECL kit (Beyotime) and analyzed with Quantity One software (Bio-Rad). The multidrug resistance-associated protein 1 (MRP1, 1:1000), P-glycoprotein (P-gp, 1:1000), B-cell lymphoma 2 (Bcl-2, 1:1000), Bcl2-associated X (Bax, 1:1000), cleaved-caspase-3 (1:500), FOSL2 (1:1000), and GAPDH (1:10,000) primary antibodies and goat anti-rabbit secondary antibody (1:10,000) were obtained from Abcam (Cambridge, MA, USA).
Dual-Luciferase Reporter Assay
The interaction between miR-377-3p and OIP5-AS1 or FOSL2 was predicted by starbase v3.0 (http://starbase.sysu.edu.cn/). The sequences of OIP5-AS1 and FOSL2 3ʹ-untranslated regions (3ʹUTR) possessing putative miR-377-3p binding sites were amplified and inserted into pmirGLO vector (Promega, Madison, WI, USA), generating OIP5-AS1-wild type (WT)/mutant type (MUT) and FOSL2 3ʹUTR-WT/MUT reporter plasmids. Whereafter, with the help of Lipofectamine 2000 (Invitrogen), MG63/CDDP and Saos-2/CDDP cells (1×104 cells/well) in 96-well plates were co-transfection with the constructed reporter plasmids and miR-377-3p or miR-NC. The Dual-Lucy Assay Kit (Solarbio) was used to detect luciferase activity.
RNA Immunoprecipitation (RIP) Assay
In this assay, to further demonstrate the underlying binding between OIP5-AS1 and miR-377-3p, RIP assay was conducted based on the instruction guidelines of Magna RIP kit (Millipore, Bedford, MA, USA). Firstly, the complete RIP lysis buffer was used to lyse the collected MG63/CDDP and Saos-2/CDDP cells, followed by incubation with the RIP buffer containing magnetic beads conjugated with Anti-Argonaute2 (Anti-Ago2) antibody or Anti-IgG (as a control) (Millipore). And then, proteinase K was applied to digest the complexes, and qRT-PCR assay was performed to analyze the enrichments of OIP5-AS1 and miR-377-3p.
Tumor Xenograft Assay
GenePharma (Shanghai, China) provided the Lentiviral vector (Lenti-short hairpin OIP5-AS1, named as sh-OIP5-AS1) for stable OIP5-AS1 expression, and scrambled shRNA control (sh-NC). Synchronously, six-week-old male Balb/c mice (n=3 per group) were collected from the National Laboratory Animal Center (Beijing, China). This research was approved by the Institutional Committee for Animal Research of the Huaihe Hospital of Henan University. All the animal studies were performed in compliance with the ARRIVE guidelines and the Basel Declaration. All animals received humane care according to the National Institutes of Health (USA) guidelines. MG63 cells (5 × 106) stably transfected with sh-OIP5-AS1 or sh-NC were subcutaneously injected into the left flank of the nude mice. At 7 days after injection, mice were administrated with phosphate-buffered saline (PBS, Invitrogen) or 3 mg/kg CDDP (Sigma) each 7 days. Tumor volume was measured every week. All mice were sacrificed on 28 days after injection, and the resected tumor was weighed, followed by utilization for qRT-PCR and Western blot assays.
Statistical Analysis
GraphPad Prism 7 (GraphPad Inc., La Jolla, CA, USA) was used to process the experiment data. All quantitative data from three independent experiments were presented as the mean ± standard deviation (SD). The differences between two groups were analyzed by Student’s t-test, while one-way analysis of variance (ANOVA) was used to evaluate the differences among multiple groups. P<0.05 was considered as statistical significance.
Results
OIP5-AS1 Was Highly Expressed in CDDP-Resistant OS Tissues and Cells
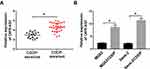
In order to explore the effects of OIP5-AS1 on CDDP-resistant OS, the level of OIP5-AS1 was firstly tested in CDDP-resistant OS tissues. As presented in (Figure 1A), the level of OIP5-AS1 was obviously enhanced in CDDP-resistant OS tissues related to that in CDDP-sensitive OS tissues. Also, OIP5-AS1 was dramatically increased in CDDP-resistant MG63 and Saos-2 cells (MG63/CDDP and Saos-2/CDDP) in contrast to that in MG63 and Saos-2 cells (Figure 1B). These data implied that OIP5-AS1 might associate with CDDP resistance in OS.
OIP5-AS1 Knockdown Enhanced CDDP Sensitivity in MG63/CDDP and Saos-2/CDDP Cells
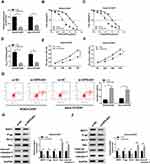
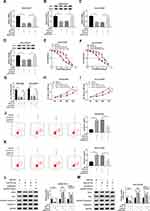
Based on the above results, the effects of OIP5-AS1 silencing on CDDP resistance were further researched. As exhibited in (Figure 2A), the knockdown efficiency was confirmed, indicated by the apparent decrease of OIP5-AS1 level in MG63/CDDP and Saos-2/CDDP cells transfected with si-OIP5-AS1. The IC50value of CDDP was strikingly lower in si-OIP5-AS1-transfectedMG63/CDDP and Saos-2/CDDP cells than that in si-NC group (Figure 2B–D). Furthermore, the transfection of si-OIP5-AS1 resulted in the remarkable decline of cell viability of MG63/CDDP and Saos-2/CDDP cells (Figure 2E and F). While the apoptotic rate was distinctly elevated in si-OIP5-AS1 group compared with that in si-NC group (Figure 2G). Since Bcl-2, Bax, and cleaved-caspase-3 as apoptosis-related factors, the levels of these protein were detected in MG63/CDDP and Saos-2/CDDP cells. As displayed in (Figure 2H and I), the protein level of Bcl-2 was dramatically decreased in MG63/CDDP and Saos-2/CDDP cells transfected with si-OIP5-AS1, while Bax and cleaved-caspase-3 were notably up-regulated in si-OIP5-AS1 group. Meanwhile, as multidrug resistance-related proteins, MRP1 and P-gp have confirmed to exert the vital roles in the chemotherapy resistance of OS,23 thus, Western blot assay was applied to detect the impact of OIP5-AS1 downregulation on the protein levels of MRP1 and P-gp. Data suggested that the protein levels of MRP1 and P-gp were markedly decreased in response to transfection of si-OIP5-AS1 in MG63/CDDP and Saos-2/CDDP cells (Figure 2H and I). Taken together, these results indicated that the depletion of OIP5-AS1 decreased CDDP resistance in MG63/CDDP and Saos-2/CDDP cells.
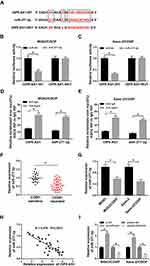
MiR-377-3p Was a Direct Target of OIP5-AS1 in MG63/CDDP and Saos-2/CDDP Cells
To further elucidate the mechanism of OIP5-AS1 in CDDP-resistant OS, starBase v3.0 was used to predict the potential target of OIP5-AS1. The results showed that miR-377-3p had complementary base pairing with OIP5-AS1 (Figure 3A). The following dual-luciferase reporter assay indicated that the introduction of miR-377-3p in MG63/CDDP and Saos-2/CDDP cells led to a marked reduction in the luciferase activity of OIP5-AS1-WT reporter in comparison with that in miR-NC group, while the luciferase activity of OIP5-AS1-MUT reporter had no change in any group (Figure 3B and C). To further prove the direct binding between OIP5 and miR-377-3p, RIP assay was performed in CDDP-resistant OS cells. In agreement with bioinformatics analysis and dual-luciferase assay, data exhibited that OIP5 and miR-377-3p were obviously enriched in Anti-Ago2 group versus Anti-IgG control group in MG63/CDDP and Saos-2/CDDP cells (Figure 3D and E). Besides, miR-377-3p was significantly down-regulated in CDDP-resistant OS tissues and cells (Figure 3F and G). The scatter plot presented that the level of OIP5-AS1 was negatively correlated with the level of miR-377-3p (Figure 3H). The level of miR-377-3p was conspicuously up-regulated in si-OIP5-AS1-transfectedMG63/CDDP and Saos-2/CDDP cells, while notably reduced in MG63/CDDP and Saos-2/CDDP cells transfected with pcDNA-OIP5-AS1 (Figure 3I). These data demonstrated that OIP5-AS1sponged miR-377-3p in MG63/CDDP and Saos-2/CDDP cells.
OIP5-AS1 Depletion Decreased CDDP Resistance in MG63/CDDP and Saos-2/CDDP Cells by Regulating miR-377-3p
To explore whether OIP5-AS1 silencing improved CDDP sensitivity was mediated by miR-377-3p in OS, si-OIP5-AS1 and anti-miR-377-3p were transfected into MG63/CDDP and Saos-2/CDDP cells. The level of miR-377-3p was significantly enhanced in MG63/CDDP and Saos-2/CDDP cells transfected with si-OIP5-AS1, while partly mitigated by the re-introduction of anti-miR-377-3p (Figure 4A). Whereafter, the IC50 values of CDDP in MG63/CDDP and Saos-2/CDDP cells were detected by an MTT cell viability assay. As shown in (Figure 4B–D), OIP5-AS1 knockdown effectively improved CDDP sensitivity in MG63/CDDP and Saos-2/CDDP cells, while the re-introduction of anti-miR-377-3p obviously overturned si-OIP5-AS1-induced increases in CDDP sensitivity. Also, the cell viability was dramatically reduced in MG63/CDDP and Saos-2/CDDP cells transfected with si-OIP5-AS1, while regained in si-OIP5-AS1 + anti-miR-377-3p group (Figure 4E and F). However, the apoptotic rate showed the opposite trend. Briefly, the transfection of si-OIP5-AS1 distinctly elevated the apoptotic rate in MG63/CDDP and Saos-2/CDDP cells, while the re-introduction of anti-miR-377-3p weakened this promoted effect (Figure 4G and H). Besides, anti-miR-377-3p counteracted the constraint impact on the protein level of Bcl-2 and the facilitated impacts on the protein levels of Bax and cleaved-caspase-3 in MG63/CDDP and Saos-2/CDDP cells caused by si-OIP5-AS1 (Figure 4I and J). To sum up, OIP5-AS1 silencing enhanced CDDP sensitivity in MG63/CDDP and Saos-2/CDDP cells by modulating miR-377-3p.
FOSL2 Was a Candidate Target of miR-377-3p in MG63/CDDP and Saos-2/CDDP Cells
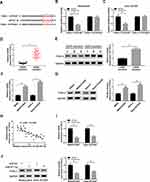
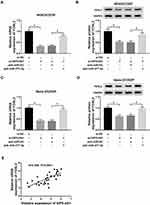
To further explore the mechanism of miR-377-3p in CDDP resistance of OS, the potential targets of miR-377-3p were predicted by starBase v3.0. The results showed that 3ʹUTR of FLOS2 had complementary binding sites with miR-377-3p (Figure 5A). The luciferase activity of FOSL2 3ʹUTR-WT reporter was drastically declined in MG63/CDDP and Saos-2/CDDP cells transfected with miR-377-3p, while the luciferase activity of FOSL2 3ʹUTR-MUT reporter had no apparent fluctuation in any group (Figure 5B and C). In addition, the mRNA and protein levels of FOSL2 were significantly increased in CDDP-resistant OS tissues and cells (Figure 5D–G). The scatter diagram indicated that miR-377-3p was negatively correlated with FOSL2 (Figure 5H). Moreover, the mRNA and protein level of FOSL2 was strikingly down-regulated in MG63/CDDP and Saos-2/CDDP cells transfected with miR-377-3p (Figure 5I and J). These results demonstrated that miR-377-3p could interact with FOSL2 to inhibit its expression in MG63/CDDP and Saos-2/CDDP cells.
MiR-377-3p Overexpression Enhanced CDDP Sensitivity in MG63/CDDP and Saos-2/CDDP Cells by Modulating FOSL2
To investigate the effects of miR-377-3p and FOSL2 on CDDP-resistant of OS, miR-377-3p and pcDNA-FOSL2 were transfected into MG63/CDDP and Saos-2/CDDP cells. Firstly, the mRNA and protein levels of FOSL2 were distinctly decreased in miR-377-3p-transfected MG63/CDDP and Saos-2/CDDP cells, while partly reversed by the emergence of pcDNA-FOSL2 (Figure 6A–D). Furthermore, the transfection of pcDNA-FOSL2 recovered the restraint effect on IC50 value of CDDP in MG63/CDDP and Saos-2/CDDP cells confined by miR-377-3p mimics (Figure 6E–G). Also, the cell viability showed the same trends. In brief, cell viability was firstly reduced in MG63/CDDP and Saos-2/CDDP cells transfected with miR-377-3p, and then partially alleviated in MG63/CDDP and Saos-2/CDDP cells co-transfected with miR-377-3p and pcDNA-FOSL2 (Figure 6H and I). However, the emergence of pcDNA-FOSL2 neutralized the accelerated impact on apoptotic rate in MG63/CDDP and Saos-2/CDDP cells constrained by miR-377-3p (Figure 6J and K). Besides, the introduction of pcDNA-FOSL2 undermined the suppressive impact on the protein level of Bcl-2 and the promoted impact on the protein levels of Bax and cleaved-caspase-3 in MG63/CDDP and Saos-2/CDDP cells induced by miR-377-3p (Figure 6L and M). These data revealed that miR-377-3p elevated CDDP sensitivity in MG63/CDDP and Saos-2/CDDP cells by regulating FOSL2.
OIP5-AS1 Silencing Decreased FOSL2 Expression in MG63/CDDP and Saos-2/CDDP Cells by Sponging miR-377-3p
To elucidate the relationship among OIP5-AS1, miR-377-3p, and FOSL2, we measured the level of FOSL2 in MG63/CDDP and Saos-2/CDDP cells transfected with si-OIP5-AS1 and anti-miR-377-3p. As exhibited in (Figure 7A–D), the mRNA and protein levels of FOSL2 were obviously declined in si-OIP5-AS1-transfected MG63/CDDP and Saos-2/CDDP cells, while rescued in MG63/CDDP and Saos-2/CDDP cells co-transfected with si-OIP5-AS1 and anti-miR-377-3p. In addition, FOSL2 was positively linear correlated with OIP5-AS1 (Figure 7E). These data unraveled that OIP5-AS1 knockdown down-regulated the expression of FOSL2 in MG63/CDDP and Saos-2/CDDP cells via miR-377-3p.
OIP5-AS1 Knockdown Suppressed OS Tumor and Increased CDDP Sensitivity Growth in vivo
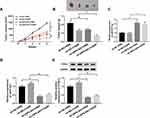
Next, we further explored the impact of OIP5-AS1 deficiency on tumor growth, mice xenograft models of OS were established in vivo. As presented in (Figure 8A and B), tumor volume and tumor weight were reduced in presence of CDDP treatment or OIP5-AS1 downregulation, implying that CDDP treatment or OIP5-AS1 silencing hindered OS tumor growth in vivo. Meanwhile, combined sh-OIP5-AS1 and CDDP resulted in a more overt suppression on tumor growth, suggesting that OIP5-AS1 deletion elevated CDDP-caused anti-tumor effect in vivo.
Apart from that, our data suggested that the levels of OIP5-AS1 and FOSL2 were declined in tumor tissues from sh-OIP5-AS1-transfected MG63 cells, and miR-377-3p level was increased (8C and 8D). These data manifested that OIP5-AS1 knockdown could impede OS tumor growth and enhanced CDDP sensitivity partly through regulating the miR-377-3p/FOSL2 axis.
Discussion
Despite CDDP-based chemotherapy is an effective treatment for cancer, the development of CDDP resistance is becoming a big impediment for cancer patients.24 Convincing evidence demonstrated that lncRNAs were implicated in drug resistance of diverse cancers. In the current study, the mechanism of OIP5-AS1 on CDDP-resistant in OS was mainly explored. These data disclosed that OIP5-AS1 regulated FOSL2expressionto aggravate CDDP resistance by sponging miR-377-3p.
Recent studies uncovered that OIP5-AS1 played vital roles in tumor progression and chemoresistance. For example, Wang et al indicated that the high expression of OIP5-AS1 contributed to tumor progression in bladder cancer.25 Another study in OS discovered that OIP5-AS1 was remarkably increased in doxorubicin (DXR)-resistant OS tissues and cells, and OIP5-AS1 inhibition alleviated DXR resistance via miR-200b-3p/FN1 axis.12 In the present study, OIP5-AS1 was highly expressed in CDDP-resistant OS tissues and cells. OIP5-AS1 depletion-retarded cell proliferation, elevated CDDP sensitivity, and facilitated apoptosis in OS. The results of OIP5-AS1 on CDDP resistance in OS were consistent with the previous report.26 Furthermore, the chemoresistance of OIP5-AS1 was validated on OS xenografts in nude mice. These data implied that OIP5-AS1 played crucial roles in CDDP resistance of OS in vitro and in vivo.
Accumulating data disclosed that the dysregulation of miR-377 was associated with tumor progression and chemoresistance in OS. For instance, the overexpression of miR-377-facilitated apoptosis in OS via HAT1/Wnt axis.27 Another study found that miR-377 was decreased in CDDP resistance OS tissues and cells, and its upregulation improved CDDP sensitivity by regulating XIAP.28 In this research, our data proved that OIP5-AS1 served as a sponge of miR-377-3p, and miR-377-3p was decreased in CDDP-resistant OS tissues and cells. Functionally, OIP5-AS1 silencing enhanced CDDP sensitivity and apoptosis, and suppressed cell viability in CDDP-resistant cells by modulating miR-377-3p. These results unraveled that OIP5-AS1 knockdown improved CDDP sensitivity in OS by sponging miR-377-3p.
Convincing evidence manifested that FOSL2 was also involved in tumor development and chemoresistance. For example, a report in OS indicated that FOSL2 was elevated, and promoted cell viability and mobility mediated by miR-143-3p.19 Another study suggested that FOSL2 was increased in ovarian cancer tissues and cells, and UCA1 accelerated CDDP resistance in ovarian cancer via miR-143/FOSL2 axis.20 In this exploration, FOSL2 was negatively interacted with miR-377-3p, and FOSL2 was augmented in chemoresistance OS tissues and cells. MiR-377-3p refrained cell proliferation, decreased IC50 value of CDDP, but promoted apoptosis in CDDP-resistant OS cells by regulating FOSL2. More importantly, OIP5-AS1 decreased FOSL2 expression in CDDP-resistant OS cells via miR-377-3p. Furthermore, our data further revealed that OIP5-AS1 deletion could inhibit OS tumor growth and improved CDDP sensitivity in vivo partly through the miR-377-3p/FOSL2 axis. These data manifested that OIP5-AS1 contributed to CDDP resistance in OS by regulating FOSL2 expression via miR-377-3p in vitro and in vivo.
In conclusion, OIP5-AS1, FOSL2 were increased while miR-377-3p was reduced in CDDP-resistant OS tissues and cells. OIP5-AS1 depletion declined FOSL2 expression to improve CDDP sensitivity in OS by sponging miR-377-3p in vitro and in vivo. The novel regulatory network may shed light on the mechanism of chemoresistance in OS.
Disclosure
The authors have no conflict of interest to declare.
References
1. Picci P. Osteosarcoma (osteogenic sarcoma). Orphanet J Rare Dis. 2007;2(1):6. doi:10.1186/1750-1172-2-6
2. Mankin HJ, Hornicek FJ, Rosenberg AE, et al. Survival data for 648 patients with osteosarcoma treated at one institution. Clin Orthopaed Rel Res. 2004;429:
3. Dasari S, Tchounwou PB. Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol. 2014;740:
4. Yang G, Lu X, Yuan L. LncRNA: a link between RNA and cancer. BBA Gene Reg Mech. 2014;1839(11):1097–1109. doi:10.1016/j.bbagrm.2014.08.012
5. Li Z, Zhao L, Wang Q. Overexpression of long non-coding RNA HOTTIP increases chemoresistance of osteosarcoma cell by activating the Wnt/β-catenin pathway. Am J Transl Res. 2016;8(5):2385.
6. Li G, Zhu Y. Effect of lncRNA ANRIL knockdown on proliferation and cisplatin chemoresistance of osteosarcoma cells in vitro. Pathol Res Prac. 2019;215(5):931–938. doi:10.1016/j.prp.2019.01.042
7. Li M, Ning J, Li Z, et al. Long noncoding RNA OIP5-AS1 promotes the progression of oral squamous cell carcinoma via regulating miR-338-3p/NRP1 axis. Biomed Pharmacother. 2019;118:109259. doi:10.1016/j.biopha.2019.109259
8. Bai Y, Li S. Long noncoding RNA OIP5‐AS1 aggravates cell proliferation, migration in gastric cancer by epigenetically silencing NLRP6 expression via binding EZH2. J Cell Biochem. 2019.
9. Li Y. LncRNA OIP5-AS1 promotes proliferation of the hemangioma vascular endothelial cells via regulating miR-195-5p/NOB1 axis. Front Pharmacol. 2019;10:449. doi:10.3389/fphar.2019.00449
10. Sun W-L, Kang T, Wang Y-Y, et al. Long noncoding RNA OIP5-AS1 targets Wnt-7b to affect glioma progression via modulation of miR-410. Biosci Rep. 2019;39(1):BSR20180395. doi:10.1042/BSR20180395
11. Dai J, Xu L, Hu X, et al. Long noncoding RNA OIP5-AS1 accelerates CDK14 expression to promote osteosarcoma tumorigenesis via targeting miR-223. Biomed Pharmacother. 2018;106:1441–1447. doi:10.1016/j.biopha.2018.07.109
12. Kun‐Peng Z, Chun‐Lin Z, Xiao‐Long M, et al. Fibronectin‐1 modulated by the long noncoding RNA OIP5‐AS1/miR‐200b‐3p axis contributes to doxorubicin resistance of osteosarcoma cells. J Cell Physiol. 2019;234(5):6927–6939. doi:10.1002/jcp.27435
13. Garzon R, Calin GA, Croce CM. MicroRNAs in cancer. Annu Rev Med. 2009;60:167–179. doi:10.1146/annurev.med.59.053006.104707
14. Xu M, Jin H, Xu C-X, et al. MiR-34c inhibits osteosarcoma metastasis and chemoresistance. Med Oncol. 2014;31(6):972. doi:10.1007/s12032-014-0972-x
15. Yan H, Zhang B, Fang C, et al. miR-340 alleviates chemoresistance of osteosarcoma cells by targeting ZEB1. Anticancer Drugs. 2018;29(5):440–448. doi:10.1097/CAD.0000000000000614
16. Sun C, Li S, Zhang F, et al. Long non-coding RNA NEAT1 promotes non-small cell lung cancer progression through regulation of miR-377-3p-E2F3 pathway. Oncotarget. 2016;7(32):51784.
17. Zhan W, Liao X, Chen Z, et al. Circular RNA hsa_circRNA_103809 promoted hepatocellular carcinoma development by regulating miR‐377‐3p/FGFR1/ERK axis. J Cell Physiol. 2019.
18. Tulchinsky E. Fos family members: regulation, structure and role in oncogenic transformation. Histol Histopathol. 2000;15(3):921–928. doi:10.14670/HH-15.921
19. Sun X, Dai G, Yu L, et al. miR-143-3p inhibits the proliferation, migration and invasion in osteosarcoma by targeting FOSL2. Sci Rep. 2018;8(1):606. doi:10.1038/s41598-017-18739-3
20. Li Z, Niu H, Qin Q, et al. lncRNA UCA1 mediates resistance to cisplatin by regulating the miR-143/FOSL2-signaling pathway in ovarian cancer. Mol Ther Nuc Acids. 2019;17:92–101. doi:10.1016/j.omtn.2019.05.007
21. Zhou Y, Huang Z, Wu S, Zang X, Liu M, Shi J. miR-33a is up-regulated in chemoresistant osteosarcoma and promotes osteosarcoma cell resistance to cisplatin by down-regulating TWIST. J Exp Clin Cancer Res. 2014;33:12. doi:10.1186/1756-9966-33-12
22. Song L, Duan P, Gan Y, et al. Silencing LPAATβ inhibits tumor growth of cisplatin-resistant human osteosarcoma in vivo and in vitro. Int J Oncol. 2017;50(2):535–544. doi:10.3892/ijo.2016.3820
23. Chou AJ, Gorlick R. Chemotherapy resistance in osteosarcoma: current challenges and future directions. expert review of anticancer therapy. J Exp Clin Cancer Res. 2006;6(7):1075–1085.
24. Siddik ZH. Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene. 2003;22(47):7265. doi:10.1038/sj.onc.1206933
25. Wang Y, Shi F, Xia Y, et al. LncRNA OIP5‐AS1 predicts poor prognosis and regulates cell proliferation and apoptosis in bladder cancer. J Cell Biochem. 2019;120(5):7499–7505. doi:10.1002/jcb.28024
26. Song L, Zhou Z, Gan Y, et al. Long noncoding RNA OIP5‐AS1 causes cisplatin resistance in osteosarcoma through inducing the LPAATβ/PI3K/AKT/mTOR signaling pathway by sponging the miR‐340‐5p. J Cell Biochem. 2019;120(6):9656–9666. doi:10.1002/jcb.28244
27. Xia P, Gu R, Zhang W, et al. MicroRNA‐377 exerts a potent suppressive role in osteosarcoma through the involvement of the histone acetyltransferase 1‐mediated Wnt axis. J Cell Physiol. 2019;234:22787–22798. doi:10.1002/jcp.28843
28. Liu X, Xu J, Li F, et al. Down-regulation of miR-377 contributes to cisplatin resistance by targeting XIAP in osteosarcoma. Eur Rev Med Pharmacol Sci. 2018;22(5):1249–1257. doi:10.26355/eurrev_201803_14465
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.