Back to Journals » Adolescent Health, Medicine and Therapeutics » Volume 14
Long-Acting Reversible Contraception for Adolescents: A Review of Practices to Support Better Communication, Counseling, and Adherence
Authors Durante JC, Sims J, Jarin J, Gold MA, Messiah SE, Francis JK
Received 31 January 2023
Accepted for publication 12 April 2023
Published 5 May 2023 Volume 2023:14 Pages 97—114
DOI https://doi.org/10.2147/AHMT.S374268
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Alastair Sutcliffe
Julia C Durante,1,2 Jessica Sims,1,2 Jason Jarin,2,3 Melanie A Gold,4 Sarah E Messiah,5– 7 Jenny KR Francis1,2,8
1Department of Pediatrics, University of Texas Southwestern Medical Center, Dallas, TX, USA; 2Children’s Health System of Texas, Dallas, TX, USA; 3Department of Obstetrics and Gynecology, University of Texas Southwestern Medical Center, Dallas, TX, USA; 4Department of Pediatrics and Department of Population & Family Health, Columbia University Irving Medical Center, New York, NY, USA; 5University of Texas Health Science Center at Houston, School of Public Health, Dallas Campus, Dallas, TX, USA; 6Center for Pediatric and Population Health, UTHealth School of Public Health, Dallas, TX, USA; 7Department of Pediatrics, McGovern Medical School, Houston, TX, USA; 8Peter O’Donnell School of Public Health, University of Texas Southwestern Medical Center, Dallas, TX, USA
Correspondence: Jenny KR Francis, Department of Adolescent & Young Adult Medicine, University of Texas Southwestern/Children’s Health, 2350 N Stemmons Fwy, Ste F5200, Dallas, TX, 75207, USA, Tel +1 214-456-6790, Fax +1 214-456-2230, Email [email protected]
Abstract: Long-acting reversible contraception (LARC) methods, including levonorgestrel and copper intrauterine devices (IUDs) and the subdermal contraceptive implant, are the most effective reversible forms of contraception and thus are an important aspect of adolescent pregnancy prevention. While LARC efficacy, safety, and appropriateness are supported by major medical organizations and usage rates are increasing, overall LARC uptake among United States (US) adolescents remains lower than uptake of short-acting contraceptive methods. A better understanding of the barriers affecting adolescent LARC uptake and reasons for discontinuation could help facilitate effective communication. For example, learning how to improve adolescent-centered communication, shared decision-making, and motivational counseling strategies may be the first step to improving utilization rates. This narrative review includes three sections. First, this review will describe the history, mechanisms of action, and epidemiology of adolescent LARC use in the US and globally. Next, this review will describe key factors influencing adolescent LARC uptake, reasons for discontinuation, and multilevel barriers specific to adolescent LARC use. Finally, this review will characterize communication techniques and LARC counseling strategies for adolescents in the context of a reproductive justice approach set in the health belief model framework. The distinction between moving away from a presumptive counseling approach towards an adolescent-centered, shared decision-making approach to encourage parent-adolescent sexual health communication to lay the foundation of empowering adolescent reproductive autonomy should be the underpinning of all effective reproductive communication strategies.
Keywords: long-acting reversible contraception, LARC, Contraception, adolescent, pregnancy prevention, birth control, communication, contraception counseling
Introduction
Long-acting reversible contraception (LARC) methods, including levonorgestrel (LNG) and copper (Cu) intrauterine devices (IUDs) and subdermal contraceptive implants (containing etonogestrel or levonorgestrel), are the most effective reversible forms of contraception. In addition to high pregnancy prevention efficacy, the safety and appropriateness of LARC use among adolescents are supported by all major medical societies, including the American College of Obstetricians and Gynecologists (ACOG) and the American Academy of Pediatrics (AAP).1,2
Decreases in adolescent sexual activity and increases in effective contraception use, including major shifts in the rates of adolescent LARC use, have contributed to significant declines in US adolescent pregnancy rates in recent decades.3 Nevertheless, despite increasing LARC use among adolescents, overall uptake is still low compared to short-acting contraceptive methods.4 LARC uptake among adolescents has also been disproportionate across racial and ethnic groups and ages and continues to face many practical constraints.5,6 Although LARC devices are highly effective contraception options for adolescents, historical misperceptions about safety and appropriateness in adolescents may hamper ongoing clinical LARC counseling and provisions. A better understanding of the history of LARC, its mechanism of action, and current adolescent usage patterns in the US and globally may empower healthcare professionals to provide meaningful anticipatory guidance, address concerns, and ultimately support successful contraception initiation according to the preferences and values of adolescents and their families.
Compared to adult women, adolescents have specific and unique concerns about initiating and continuing LARC devices. Understanding the many factors that may affect adolescent LARC uptake, including LARC characteristics, acceptability, the anticipation of discomfort, common myths, and nuanced cultural and psychosocial factors, is necessary to effectively support adolescent contraception decision-making.7 By identifying the specific barriers affecting adolescents, including healthcare provider misconceptions, legal, administrative, and practical constraints, cost, access, and confidentiality, this review will serve to better inform effective LARC communication strategies.8
Lastly, the benefits of LARCs must always be balanced with the role of empowering autonomy and using an adolescent-centered, shared decision-making communication approach. Provider communication should encourage ongoing parent-adolescent sexual health communication at home following the visit. While “LARC-first” counseling may lead to higher uptake rates, the potential for this directive, presumptive approach to overemphasize LARCs over all other methods risks coercion and possible implicit perpetuation of historic reproductive injustices.8 The principles of reproductive justice support more than just empowering adolescent women to access LARC but also to choose non-LARC methods or no method at all, as well as the freedom to have LARC methods removed at will.8 Thus, evidence-based communication strategies for LARC counseling through a reproductive justice framework and health belief model will be reviewed with practical clinical guidance for common counseling pitfalls and best-practice examples of LARC communication strategies.
This narrative review aims to help clinicians develop practices to improve LARC communication, counseling, and adherence by providing an overview and context behind factors that influence adolescent LARC use. First, we provide an overview of the history of LARC, its mechanism of action, and usage trends in the US and globally for adolescents. Next, we describe the factors affecting adolescent LARC uptake, continuation, and barriers to use. Finally, we conclude by characterizing communication practices to support adolescent-centered, shared decision-making to encourage parent-adolescent dyad sexual health communication and empower adolescent LARC uptake, continuation, and adherence for adolescents who desire this method.
Overview of LARC History, Mechanism of Action, and Adolescent Usage Patterns in the United States and Globally
Intrauterine Device History and Current Models
Public perception of IUD safety and appropriateness in the United States (US) has shifted significantly in recent decades. The first generation of copper (Cu) and levonorgestrel (LNG) IUDs were released in the US in 1968. In the 1970s, the public trust in IUDs was tarnished by poor outcomes associated with the Dalkon Shield, an IUD with a multifilament, braided string that rendered it prone to ascending infections.9 This led to the device’s subsequent withdrawal from the US market in 1974 and left a looming effect on the public perception, acceptability, and usage patterns of IUDs in the US.10 By 1995, IUD usage rates among reproductive-aged US women had declined to between 1–2% in non-Hispanic White women and nearly 0% in non-Hispanic Black women.10,11
The IUD models available in the US include a copper IUD, ParaGard, introduced in 1988 and approved for 10 years of contraception, as well as four hormonal IUDs. The oldest hormonal IUD model, Mirena (introduced in 2001), contains 52 mg of LNG, releasing approximately 20 mcg every 24 hours.12 While approved initially for five years, its duration of effectiveness has been progressively extended by the FDA with access to longer follow-up data, most recently in 2022 to 8 years when used for pregnancy prevention.13 Three newer LNG-IUDs emerged between 2013–2016 of varying doses and durations of efficacy; Skyla (3 years, 13.5 mg LNG), Kyleena (5 years, 19.5 mg LNG), and Liletta (8 years, 52 mg LNG).14 Each of these new IUDs was studied and approved for both nulliparous and parous women, unlike Mirena, which was initially approved by the FDA based on data from women who had previously been pregnant (although extended follow-up of Mirena efficacy has included a greater proportion of nulliparous women).12,14 FDA approval studies for the Liletta IUD were notable for including adolescents aged 16 and 17, whereas other IUD approval trials have included only women aged 18 and older.15 Two hormonal IUDs, Skyla and Kyleena, feature a slightly narrower insertion device with a diameter of 3.8mm (slightly smaller than Mirena’s diameter of 4.4mm and Paragard and Liletta’s diameter of 4.75mm). Regardless of these slight millimeter differences, all devices can be safely inserted in nulliparous women, including adolescents.16
While hormonal IUDs and a range of Cu-IUD models are available internationally, accessibility and price vary widely.17 Some countries provide IUDs and implants at no cost, whereas patients may face costs equivalent to hundreds of US dollars in others.17 International initiatives to address LARC access and affordability in low- and middle-income countries (LMICs) have included public-private partnerships to facilitate manufacturer donation of unbranded LARC devices as well as the non-profit pharmaceutical development of a lower-cost hormonal IUD, Avibela, available in select LMICs.18
Subdermal Implant History and Current Models
The first subdermal implant available in the US, Norplant, was approved in 1991. Despite initially high popularity, the device fell out of favor due to unanticipated side-effects and difficulty removing its multiple rods. Sales stopped in the US in 2002.10 Furthermore, the device was implicated in several examples of reproductive rights coercion that targeted poor, minority, and imprisoned women, including legislation introduced in some states to financially incentivize or compel Norplant use among women receiving public assistance or those convicted of crimes.10,19 This history may have particular relevance for populations that have experienced past or ongoing reproductive coercion, such as forced sterilization of racial and ethnic minority women in the US.10 Reproductive freedom and autonomy should be central in all discussions about choosing when to initiate or discontinue a contraceptive method to avoid perpetuating historical reproductive wrongs.20
The currently available subdermal implant in the US is the single-rod Nexplanon, first introduced in 2010. This device made improvements over the previous single-rod subdermal model (Implanon, introduced in 1998) with the additional benefits of an improved insertion device and radio-opacity.14 Although its initial approval was for three years, subsequent research has supported ongoing efficacy for four years or more.13
Two LNG-releasing subdermal implants are available internationally, each with two rods containing 75 mg of LNG (150 mg total).21 Research suggests differential rates of LNG release between the two devices, supporting a recommended maximum duration of 5 years for Jadelle (manufactured in Finland) and 3 years for Sino-implant (II) (manufactured in China and distributed globally as Levoplant).21
LARC Mechanism of Action and Medical Benefits
With estimated failure rates of less than 1%, LARC methods are the most effective forms of reversible contraception.22 The mechanism of action of LNG-IUDs works by thickening cervical mucus, impairing the mobility of sperm, and creating changes to the uterine environment that reduce gamete viability and decrease the likelihood of fertilization.23 The Cu-IUD works by creating inflammatory and biochemical challenges to sperm mobility, gamete viability, and fertilization in the uterine environment.23 Ovulation is not affected by the Cu-IUD, whereas some LNG-IUD users may experience anovulation.24 Conversely, the mechanism of action of the etonogestrel subdermal implant suppresses ovulation.1 LARC methods do not function by preventing the implantation of fertilized embryos.23
LARC may pose additional medical benefits aside from contraception. While all IUDs are effective for contraception, the 52 mg LNG-IUD (initially releasing approximately 20mcg/day) more reliably reduces or suppresses menstrual bleeding. In contrast, regular menses more often persist among lower-dose LNG-IUD users (such as Skyla, containing 13.5 mg, releasing approximately 14 mcg/day). Progestin-containing LARC typically improves dysmenorrhea.25 Among hormonal contraceptive options, the LNG-IUD has relatively lower potential for drug interactions due to limited systemic hormone exposure.25 For women who have contraindications to using hormone-containing methods, the Cu-IUD has the advantage of providing highly effective nonhormonal contraception, although it conversely tends to worsen both bleeding and cramping.25 Finally, the low risk of medication interactions and lack of evidence for adverse mood effects in both the Cu-IUD and progesterone-containing LARCs make them particularly useful options for women with mood disorders such as depression and anxiety, who are at increased risk for non-adherence to short-term contraception.26
LARC Insertion Timing
For optimal minimization of pregnancy risk from unprotected intercourse, providers should consider a “quick start” LARC insertion when feasible and clinically appropriate.27 Any LARC may be inserted same-day if the patient’s last menstrual period was less than seven days ago or if the patient has a negative urine pregnancy test and has not had any unprotected intercourse since the last menses. Furthermore, a Cu-IUD or 52 mg LNG-IUD may still be inserted after this seven-day interval without waiting for the next menses if the patient has a negative pregnancy test and has only had unprotected intercourse within the past five days.27 Although no LARC is FDA-approved for emergency contraception, research demonstrates that the Cu-IUD is the most effective emergency contraceptive when inserted within five days following intercourse.28 Recent research suggests that the 52 mg LNG-IUD may provide comparable efficacy as emergency contraception during the same time frame.29
Assessing Safety and Medical Appropriateness
Deciding whether a patient’s medical condition permits safe LARC initiation arises across many clinical settings. The US Center for Disease Control and Prevention describes recommendations for contraception safety based on specific medical conditions and related comorbidities in its Medical Eligibility Criteria for Contraceptive Use.30 Both initiation and continuation of LARC are described as either category 1 (without restriction) or category 2 (advantages outweigh risks) for nearly all medical conditions affecting adolescents, with a few notable exceptions. For instance, current and recent breast cancer prohibits progestin-containing LARC (category 4, unacceptable risk), whereas systemic lupus with positive antiphospholipid antibodies and liver tumors disfavor LARC yet still permit use (category 3, risks outweigh benefits).30 While many of the above scenarios uncommonly affect typical adolescents, understanding how to confidently provide LARC safety guidance applicable to specific medical conditions and concerns is essential to inform and reassure adolescent patients and their families.
Epidemiology of Adolescent LARC Use: US Epidemiology
Adolescent LARC use in the US has risen significantly in recent decades, playing a role in recent reductions in adolescent pregnancy rates to a low of 15.4 births per 1000 US adolescents aged 15–19 in 2020.3,31 Nevertheless, significant unmet contraception needs and disparities continue among adolescents. More than one in five (22%) females who began having sexual intercourse before age 20 did not use any form of contraception (including condoms) at first sexual intercourse, pointing to the need for earlier education and proactive discussion about contraception with youth prior to sexual activity.4 While US adolescent LARC use rates have undoubtedly increased in past decades, data differ on the exact degree. The National Survey of Family Growth (NSFG), an interview-based survey of unmarried and married US men and women aged 15–49, observed an increase in LARC use at last intercourse among never-married adolescents aged 15–19 years from 3% in 2006–2010 to 15% in 2015–2019.32 Stratified by individual method, 5% of adolescents used an IUD while 10% used an implant.32 Furthermore, 20% of adolescents reported ever using a LARC method, implying that approximately a quarter of adolescents initiating LARC may seek device removal.4 By comparison, 12.7–13.7% of US women between ages 20–39 were current LARC users in 2017–2019.33 The National Youth Risk Behavior Survey (YRBS), a biennial survey of US high-school students, observed a smaller increase in current IUD and implant use among adolescents aged 15–19 than the NSFG, from 1.6% in 2013 to 4.8% in 2019.5 The discrepancy between these national datasets may have been due to differences in rates of sexual activity between cohorts, question wording, or in disclosure of sexual activity between paper survey and individual interview mediums.34 Nevertheless, both data sources support substantial recent increases in adolescent LARC use.
Racial disparities persist in US adolescent LARC uptake, with higher usage among non-Hispanic White (6.7%) than non-Hispanic Black (2.0%) or Hispanic (1.6%) adolescents aged 15–19.5 This is particularly significant in the setting of racial and ethnic pregnancy rate disparities among non-Hispanic Black and Hispanic adolescents, who experience pregnancy rates over twice as high as non-Hispanic White adolescents.35 By contrast, overall LARC use among adult US women does not differ between Hispanic, non-Hispanic White, or non-Hispanic Black individuals.33 Further work remains in addressing the complex, systemic factors underlying these discrepancies.
Global Epidemiology
Adolescents in LMICs face particular hurdles to obtaining and using contraception, including barriers to uptake and continuation.36 Of the 16 million adolescents 15–19 who give birth each year worldwide, 95% live in LMICs, incurring increased complication rates and associated health risks.36 Estimates of unmet contraceptive needs vary globally, ranging from 34–67% among unmarried adolescents ages 15–19 and 7–62% among married adolescents.36 Adolescent-specific estimates of LARC use are challenging to obtain in many regions, particularly countries that refrain from asking unmarried women about contraceptive use.37 Among all women aged 15–49 worldwide, LARC use has increased over the past 25 years, with total IUD users increasing from 133 to 159 million (8.4% of women aged 15–49) and implant users increasing from 2 million to 23 million (1.2% of women aged 15–49).37 Rates of IUD use are lower among women living in low-income (3.0%) and low-middle-income (3.6%) countries than among women in upper-middle-income (16.3%) and high-income (6.5%) countries.37 Geographically, IUD use is highest among women aged 15–49 in Eastern and South-Eastern Asia (18.6%) and lowest in the regions of Sub-Saharan Africa (0.7%) and Oceania (0.3%).37 Conversely, women in low-income countries are more likely to use the implant (3.7%) than other regions (0.6–1.2%).37 The Sub-Saharan Africa region has the highest rates of implant use worldwide (4.5%); this rate has increased substantially from 1.1% since 2011 in the context of public health initiatives to improve implant affordability, access, and delivery practices in this region.37,38
LARC Uptake, Continuation, and Barriers to Use
Many factors affect how adolescents perceive the role of LARC in pregnancy prevention and decide whether or not to use one.39 The Health Belief Model (HBM) is a health promotion framework that incorporates individual assessment of the perceived threat of a health problem (including susceptibility and severity), the expected benefits and barriers involved in taking action to avoid the problem, and factors that influence decisions to act or not act.40 Interventions to facilitate contraception use in adolescents and young adults often incorporate HBM constructs in modeling patient contraception decision-making and as predictors of behaviors.41 In Figure 1, we apply the constructs of the HBM to contextualize and summarize the factors affecting LARC use in adolescents discussed in this review. For the purposes of this review, we will focus primarily on factors affecting adolescents in the US. The multifaceted experiences and attitudes about LARC use among adolescents in different regions worldwide deserve specific attention and further investigation.42–46
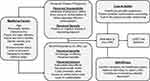 |
Figure 1 Application of the Health Belief Model Constructs to LARC Use in Adolescents. |
Factors Affecting Adolescent LARC Uptake
The factors impacting adolescent LARC decision-making involve a delicate balance between contraception characteristics and preferences, acceptability, anticipation of discomfort during insertion, health myths, and the role of shared cultural and interpersonal stories.47 Many factors affecting adolescent LARC uptake discussed below mirror those affecting adults, except for romantic partners playing a smaller role in adolescent contraception decisions compared to adult women.39
Acceptability
Adolescents are more likely to use less effective contraception methods than adult women. Many complex reasons underlie this discrepancy, often centering on perceived device acceptability. Overall, fewer adolescents have heard of IUDs (77.3%) and implants (78.6%) compared to short-acting methods such as oral contraceptive pills (97.2%) and Depo-Provera (DMPA) injections (82.1%).48 While LARC acceptability tends to be relatively similar for IUDs (37%) and implants (43%) among adolescents and young adults, the distribution may be polarized, with some adolescents either highly interested or highly disinterested.48 This suggests that while a lack of awareness of LARC methods among adolescents may contribute to low overall acceptability, awareness-building may be a powerful tool to boost adolescent knowledge and interest. Conversely, other adolescents view the implant and IUD as entirely unacceptable (24.4% and 18.5%, respectively), emphasizing the importance of maintaining an individualized adolescent-centered focus in contraceptive counseling.48
Contraception Characteristics and Preferences
Efficacy is critically important to most adolescents choosing a contraception method. Individual endorsement of LARC efficacy is associated with a higher likelihood of LARC uptake, as is personal acceptance of method-specific factors, including convenience and duration of LARC devices.49 However, other important considerations impact adolescent contraceptive choices, including non-contraceptive benefits, anticipated side-effects, and changes to menstruation, with some preferring regular menses and others amenorrhea.47 All LARC methods typically involve an adjustment period of irregular uterine bleeding after insertion, which may last weeks or months. Nearly all LARC methods affect cycle regularity; Mirena is the most likely to promote amenorrhea, whereas low-dose IUDs and the Cu-IUD are more likely to preserve monthly cycles. Nexplanon often leads to breakthrough bleeding that can last weeks or months; while this typically self-resolves by six months, some adolescents continue to have unwanted bleeding beyond this timeframe. Given these possibilities, for some individuals, the high efficacy of LARC may not outweigh the benefits of user-controlled contraception that satisfy other valued criteria, such as more predictable, lighter, or suppressed menstruation.
Age may impact which specific LARC device is preferred. In the Contraceptive CHOICE project, a multilevel prospective intervention conducted in St. Louis between 2008 and 2013, more than 70% of adolescents initiated a LARC method.50 This intervention was notable for using a “LARC-first” counseling protocol, providing same-day insertion, and guaranteeing no patient cost-sharing for any contraception method. Among LARC adopters, adolescents ages 14–17 most often chose a subdermal implant (63%), whereas adolescents ages 18–19 more often chose a hormonal IUD (71%).51 The perceived invasiveness of a pelvic exam and anticipated discomfort may underlie the preference of younger adolescents for the subdermal implant over the IUD.
Anticipation of Discomfort with Insertion
Insertion of a LARC device entails some degree of discomfort or pain for most patients.52 Anxiety, fear of pain, and concerns about the procedural experience are widespread, even among adolescents who elect to obtain LARC.52 Insertion of the subdermal implant typically entails minimal discomfort, involving a brief stinging sensation from an injection of a local anesthetic. The remaining insertion procedure is experienced as a dull pressure without acute discomfort and is typically completed within one minute. The healing process often involves a mild bruise at the procedure site that may last several days.
Conversely, inserting an IUD requires approximately five minutes of discomfort to accommodate speculum insertion, application of the tenaculum to the cervix, sounding of the uterus depth, and finally, the insertion of the IUD itself.52 The procedure may be further prolonged by complex anatomy or stenosis of the internal cervical os. While providers may attempt to minimize this challenge by timing placement during menses when the cervix is naturally dilated, research does not suggest that this practice improves outcomes.53 Importantly, nulliparity is not a risk factor for failed IUD insertion.54 Patient experiences of discomfort or pain vary wildly from minimal to severe.55 There are few reliable indicators of which patients will experience severe IUD insertion pain; a history of dysmenorrhea, nulliparity or low parity, and high anticipated pain may serve as predictors.52,56 Research into pain reduction interventions for IUD insertion has so far not established an optimal evidence-based analgesia approach.57 Nevertheless, fear of the potential for significant discomfort or pain is real and often amplified on social media platforms, stoking adolescents’ anxiety surrounding the procedure, despite the vast majority of adolescents reporting satisfaction after LARC insertion.58
LARC Health Myths
Misconceptions and fears often dissuade adolescents from choosing a LARC method.47 Both adolescents and their parents often have significant concerns about the health effects, safety, and efficacy of LARC.48 Common myths include fears of infertility, cancer, ectopic pregnancy, and risk of damage to the pelvic organs.9 Furthermore, despite failure rates of <0.5%, a survey of over one-thousand urban adolescents and young adults found that 11.5% reported knowing someone else who got pregnant on the IUD.48 Internet-based LARC information is of variable quality and may contribute to knowledge gaps and inaccurate perceptions of risk.59 Concerns about hormonal side-effects persist for both the implant and hormonal IUDs, despite relatively lower systemic absorption from the IUD compared to other progestin-only methods.47 Table 1 discusses communication approaches to the specific myths described in this section, including guidance on addressing LARC misconceptions and accessibility barriers.
 |
Table 1 How to Address LARC Myths and Barriers with Adolescents and Rationale for Response |
Family, Cultural, and Shared Interpersonal Experiences
Interpersonal, cultural, and historical factors influence how individuals and their communities value LARC characteristics and personally assess the acceptability and desirability of LARC.47,60 In particular, social context and shared experiences of friends and family can either positively or negatively influence LARC choice.47,61 Ongoing misperceptions shared among adolescents and their parents include the idea that nulliparous women cannot use IUDs or that insertion must occur during menstruation.9 Research demonstrates greater maternal acceptance of short-acting contraception than LARC.62 The misperceptions relayed by stories from family and friends, who serve as important sources of medical information for adolescents, may dissuade adolescents from LARC use, as adolescents with low interest in uptake more often have a friend or family member who disliked a given LARC method.48
Furthermore, racial and ethnic communities that have experienced historic reproductive coercion may have specific concerns about LARC.10 Qualitative research has found that the history of prior reproductive injustice impacted patient concerns about being steered towards LARC based on race, ethnicity, socioeconomic status, and educational background.63 In particular, Hispanic and non-Hispanic Black women described experiences of feeling steered to choose to begin LARC and pressured to continue LARC, with some observing providers resisting requests for device removal.63 Awareness of potential coercion and reproductive autonomy concerns is crucial to adolescent-centered contraceptive care.
Factors Affecting Adolescent LARC Continuation and Discontinuation
Many factors influence LARC continuation and discontinuation rates, including considerations for specific populations, medical comorbidities, drawbacks leading to early removal, and IUD expulsion.
Continuation and Discontinuation Rates
While the adolescent continuation of LARC is higher than non-LARC methods, adolescents may have higher discontinuation rates than adult women. In the CHOICE project, adolescent continuation rates of the copper IUD (75.6%), LNG-IUD (80.6%), and implant (82.2%) were lower than continuation rates among women over age 26 (between 84–86% for each LARC method).64 Nevertheless, adolescents are much more likely to continue LARC than short-acting contraception methods. Reviews have demonstrated adolescent 12-month LARC continuation rates (86.4% for IUDs and 85.3% for implants) significantly higher than the 39.8% continuation rate of oral contraception, the most common form of short-acting contraception.65 Similarly, adolescents may be more likely to discontinue short-term methods than adults, as demonstrated in lower 12-month oral contraceptive continuation among adolescents compared to adults 26 and older (44% vs. 53%).64 Overall, most adolescents have high LARC satisfaction rates and intentions to continue LARC one year after insertion.64 Nevertheless, adolescent satisfaction rates may be lower than those of adults. In the CHOICE project, 75% of adolescents aged 14–19 were very satisfied with LARC, compared to 83% of adults 26 and older.64
Postpartum and Postabortion LARC Placement
Postpartum LARC placement is associated with reduced repeat pregnancy and is recommended as a best practice by ACOG.1 Adolescents who received the LNG-IUD postpartum reported 100% satisfaction at 12 months and had much higher 12-month continuation rates (77%) than postpartum adolescents receiving Depo-Provera (43%).66 Although postpartum IUD insertion is associated with a slightly elevated rate of expulsion (10.2% for immediate post-placental placement and 13.2% for placement within 72 hours), this practice provides a powerful tool to meet the needs of the 40–70% of postpartum women who initially intend to obtain an IUD that do not ultimately obtain one.67,68 Postpartum implant placement additionally leads to higher initiation rates than delayed insertion.69 Adolescents receiving the implant postpartum have higher initiation rates than those receiving placement at 6 weeks and were more likely to have the device in place at 3 months.70
Immediate post-abortion LARC provision is a safe and effective option for adolescents following a medical or surgical abortion to reduce the high risk of repeat pregnancy in this population.1 Immediate postabortion LARC insertion is generally acceptable to adolescents and nulliparous women, although rates of LARC uptake in these groups may be lower than in parous and adult women.71,72 Importantly, immediate LARC initiation does not increase the risk of medication-induced abortion failure.73 Rates of IUD complications and expulsions following first-trimester uterine aspiration and medication-induced abortions are low.74 Immediate postabortion IUD insertion is associated with higher initiation rates (96%) than interval insertion (23%), primarily due to failure to return for follow-up.75
Disadvantages and Reasons for Early Removal
While relatively few adolescents opt for early IUD removal, many factors underlie these decisions. A systematic review on IUD continuation in adolescents and young adults under 25 found that bleeding and pain were the most reported side-effects.65 These complications, however, rarely required device removal. Unpredictable post-IUD bleeding was generally viewed as acceptable by the vast majority of adolescent IUD users.65,66 Most post-IUD insertion pain was experienced shortly after insertion and was responsive to oral analgesics, while IUD-related pain leading to removal mainly occurred within the first three months after insertion.65
Bothersome “nuisance” bleeding is the most commonly cited reason for discontinuing the subdermal implant.76 Proactive anticipatory guidance about possible adverse effects is critical to inform reasonable expectations for post-insertion LARC experiences. Users should remain aware of the normalcy of post-insertion abnormal uterine bleeding and be reassured that this does not represent a sign of danger or that the LARC is harming their bodies. In particular, offering an ameliorating short-term medication course, such as oral contraceptives, may help reduce bleeding and avert discontinuation for patients willing to try a temporizing treatment.76
Medical Indications to Discontinue LARC
Most common LARC sequelae, such as irregular bleeding or cramping, do not necessitate LARC removal unless requested by the patient. The emergence of any of the rare conditions that generally prohibit progestin-containing LARC, as discussed previously, would typically prompt a recommendation for LARC removal.30 Otherwise, continuing a pre-existing LARC is generally acceptable in most medical situations, including several notable scenarios where initiation would not be recommended, such as active pelvic inflammatory disease, cervical or endometrial cancer, or in the setting of complicated solid organ transplantation.30 Counseling on the benefits and risks of continuing LARC in the setting of comorbid medical complications should involve an adolescent-centered, shared decision-making approach.
IUD Expulsion in Adolescents
Spontaneous expulsion occurs in a relatively small percentage of IUD recipients, estimated to be between 2–10% in adult women.1 Risk factors for adolescents and adults include heavy menstrual bleeding, higher BMI, and postpartum insertion.77 Most but not all adolescents recognize IUD expulsion when it happens.65 Although not a contraindication to future IUD use, adolescents should be counseled that it is a risk factor for repeat expulsion. The rate of subsequent IUD expulsion rate in adolescents with a history of IUD expulsion is nearly 30%, similar to that of adult women.1,78
While younger age may be a risk factor for IUD expulsion, rates among adolescents are not known to be definitively higher than those of adult women.77 Studies in adolescents have observed expulsion rates ranging from as low as 1% to approximately 15%.65 Adolescents in the CHOICE project between ages 14–19 did experience expulsion rates of 10.5% at 12 months and 18.8% at 36 months, approximately twice as high as rates for women over age 20 (5.7% and 9.3%, respectively). Importantly, neither nulliparity nor IUD type was a risk factor for expulsion in this study.77 Systematic reviews have observed pooled IUD expulsion rates of 8.0% (95% CI 4.0–11.0%); however, studies exhibited significant heterogeneity.79
Barriers to Adolescent LARC Use
Despite recent increases, LARC use rates among adolescents still trail behind those of short-term contraception. The multiple barriers to adolescent LARC use involve several overlapping domains of care, including but not limited to healthcare provider misconceptions, legal, administrative, practical constraints, and patient concerns about cost, access, and confidentiality. Several key successful systems-change interventions have addressed multilevel adolescent LARC barriers. Understanding the many hurdles for adolescents deciding to use a LARC method may help explain ongoing areas for improvement in provider counseling and the healthcare system.
Healthcare Provider Knowledge Gaps
Healthcare providers may serve as a barrier to adolescent LARC use due to lack of knowledge, comfort, or training in LARC provision.6 Deficient LARC education spans both medical school and residency training and continues into independent practice.80,81 Adolescents may be intentionally or unintentionally dissuaded from using a LARC based on myths and misconceptions propagated by some healthcare providers.60 In particular, until recent recommendations from the AAP and ACOG in the past decade, IUDs were perceived by many providers as non-options for nulliparous adolescents.2,82
Some providers may not counsel adolescents about or provide LARC options at all.1,19 Limited LARC counseling by providers may contribute to relatively low levels of LARC awareness among adolescents, given that nearly three-quarters of adolescents view healthcare providers as the primary source of LARC information.83
Legal, Administrative, and Practical Constraints
While practical and economic difficulties may affect any patient, adolescents are particularly vulnerable and require additional support to counteract logistic constraints to obtaining a LARC outside their control. Many states or institutions require parental consent for any outpatient procedure, overriding adolescents’ unique and compelling needs to access LARC.84 Adolescents may not be aware of resources such as Title X-funded clinics to receive free and confidential care in most US states.85 Their ability to access services independently may further be hampered by logistics such as the need for transportation, geographic proximity to adolescent-friendly clinics, or lack of privacy. Finally, offices often ask patients to return for a separate procedure appointment due to scheduling constraints or the need to order the device itself, leading nearly half of adult women to fail to return for IUD insertion.86
Cost and Access
Another commonly identified barrier is the costs associated with LARCs for both providers and patients. While US health insurance companies must pay 100% of LARC device and insertion costs under the Affordable Care Act, commercially insured patients often have associated out-of-pocket costs.87 Uninsured youth who are either unable to access or unaware of free or low-cost family planning services may be deterred by the prospect of paying hundreds of dollars for a LARC device.6,88 Cost is also a factor for providers, including the upfront cost of purchasing LARC devices for their office, associated clinical costs with procedural equipment, longer appointment times, and the risk of device expiration or non-reimbursement. Failing to stock sufficient devices in clinics impacts adolescents who desire LARC by limiting providers’ ability to offer same-day insertion services.86 Dismantling these barriers will help enable adolescents who desire LARC to receive a device using evidence-based same-day insertion practices.6,80
Confidentiality
Confidentiality is an important consideration when discussing contraceptive care for adolescents. Most US states allow minors to consent to contraception.85 Title X and Medicaid funding sources typically carry federal protections within the Code for Federal Regulation that enable minors to access contraception.85 Despite these protections, state regulations may conflict with or fail to provide guidance on protections for confidential adolescent contraception access.85 Furthermore, adolescents may fear unintentional disclosure of contraceptive services to parents or caregivers from insurance benefit explanations, electronic health record releases, or telehealth settings where parents may read or overhear.89 Guidance is available to maintain confidentiality with billing in published recommendations by the American Academy of Pediatrics and Society for Adolescent Health and Medicine.90
Multilevel Interventions to Address Adolescent LARC Barriers
Research into evidence-based interventions addressing LARC access and uptake is ongoing.91 Several notable multilevel interventions have sought to address barriers to adolescent and adult LARC use. The CHOICE project, as mentioned previously, utilized “LARC-first” counseling alongside multilevel systemic interventions that led to LARC uptake by 72% of adolescents, greatly exceeding national adolescent LARC use rates between 5–15%.5,32,51,92 This seminal intervention reduced birth rates in adolescents aged 15–19 years to 19.4 per 1000 and abortion rate to 9.7 per 1000 (vs. 94.0 per 1000 and 41.5 per 1000, respectively, in sexually experienced US adolescents in the year 2008).92 Another prominent large-scale intervention is the Colorado Family Planning Initiative, which similarly addressed multilevel barriers to adolescent LARC uptake. Following implementation, IUD use among ages 15–19 rose from 1.6% in 2008 to 9.8% in 2019, whereas hormonal implant use rose from 0.9% in 2008 to 23.1% in 2019.93 This further coincided with reductions in birth rate from 39.6 per 1000 to 13.5 per 1000 and abortion rate from 11.2 per 1000 to 3.9 per 1000 among cohort adolescents ages 15–19.93 Dozens of other local and state-run initiatives are ongoing, many receiving grants through the Teen Pregnancy Prevention Program, established in 2010 and run by the Office of Population Affairs.94
Practices to Support Better Communication, Counseling to Empower Uptake, Continuation, and Adherence
Effective contraceptive counseling approaches that promote parent-youth communication and proactively address discontinuation may facilitate adolescent LARC uptake, continuation, and adherence. Central goals of effective communication include developing adolescent-provider relationships, building trust, and supporting shared decision-making.95 A shift from presumptive counseling to an adolescent-centered, shared decision-making approach while encouraging parent-adolescent sexual health communication may empower providers to view contraceptive counseling practices through a reproductive justice framework. Table 2 discusses components of the key communication strategies discussed below, including clinical guidance about common pitfalls and improved ways to address the factors affecting LARC decision-making sensitively and effectively.96
 |
Table 2 Communication Strategies When Considering LARC Placement with Adolescents |
Presumptive Counseling and the Potential for Coercion and Bias
Numerous prominent interventions seeking to increase adolescent LARC uptake have employed a directive “LARC-first” counseling approach, which aims to discuss the most effective contraception methods (LARC) before less-effective methods in order to maximize patient attention and consideration, presuming that the patient desires the most effective method.92,97 In this approach, LARC methods are discussed first for all patients instead of using an individualized, open-ended approach. As discussed above, the Contraceptive CHOICE project used a “LARC-first” counseling approach alongside no-cost, same-day services, yielding 72% LARC uptake among adolescents aged 15–19 and drastically reducing pregnancy rates.50,92
While effective, presumptive “LARC-first” counseling may threaten to prove coercive if providers fail to center adolescent autonomy and the possibility of alternative contraceptive priorities that might value characteristics other than efficacy “first”.27,98 Furthermore, racial bias may affect how providers recommend specific contraceptive methods. Providers may be more likely to recommend LARC to low-income Hispanic and non-Hispanic Black women than low-income White women.99 The benefits of maximizing patient attention and prioritization must be balanced against the potential for unintentional reproductive coercion due to differential LARC recommendation patterns across demographic groups.
Adolescent-Centered, Shared Decision-Making Approach
A patient-centered, or specifically, an adolescent-centered approach seeks to avoid the potential for coercion risked by a narrower, directive, or presumptive approach. While providers may take a range of overlapping approaches to communicate about LARC benefits and risks, counseling with adolescents must be particularly sensitive to reinforce and support autonomy. Healthcare providers often combine the approach of relational communication, involving building understanding and trust, alongside task-oriented communication, which seeks to provide practical solutions to help patients attain their goals.95
Shared decision-making seeks to provide the best scientific evidence available after identifying the patient’s own values and preferences to find the best contraception method for that individual.47,95 Striking this delicate balance between adolescent autonomy and ushering youth towards a shared and informed decision involves not imposing the healthcare provider’s opinion of what the patient “should” select because the method is most effective. Rather, shared decision-making provides the best scientific evidence available within the context of individual and family preferences in order to prioritize adolescent autonomy and informed choice of a desired method.95,100 Adolescent-centered, shared decision-making contraceptive counseling should further seek to provide unbiased care that acknowledges the history of systemic injustice, discrimination, and racial disparities notoriously found in reproductive healthcare.101
Encouraging Parent-Adolescent Sexual Health Communication
Empowering providers to engage parents to start conversations about contraception at many time points at home with their adolescent is a crucial first step to addressing improved sexual health communication. Parental knowledge, attitudes, and beliefs about LARC, as well as their motivations and concerns about adolescent contraception, are key targets for counseling. Parent views on LARC strongly influence how adolescents view their acceptability and desirability.102 Additionally, parent-youth sexual and reproductive health conversations are effective in promoting safer sexual practices among adolescents.103 Cultural concerns that discussing contraception could encourage adolescent sexual activity often influence parental avoidance of discussing contraception with adolescents, which may lead providers to avoid the conversation due to anticipated resistance.104,105
Finally, advance timing is essential. Providers should begin proactively counseling adolescents and families about contraception, including LARC, prior to the onset of sexual activity.106 In the context of United States reproductive healthcare laws becoming more restrictive following the overturning of Roe v. Wade, communication and counseling about contraception is even more critical to support primary pregnancy prevention.107 Given that ongoing legal challenges are potentially threatening access to confidential contraception services in many regions of the US, providers should treat each communication opportunity with preteen and adolescent-aged patients as a key moment to discuss access to essential reproductive healthcare services.108
Using a Reproductive Justice Framework
The foundation of empowering adolescent reproductive autonomy should be the underpinning of all effective reproductive communication strategies. Providers of reproductive health services should always aim to consider the impact of reproductive health advancements with the effects they might have on a structurally disadvantaged population. Integrating a reproductive justice approach into a “LARC promotion toolkit” should be the basis for all LARC conversations with families and patients.8 First, LARC should not be touted as the singlehanded “solution” to unintended pregnancy. Some have incorrectly heralded LARC as a magic bullet to “solve” societal sequelae of adolescent pregnancy, such as poverty. Yet, without more extensive consideration of the cultural and structural factors that contribute to adolescent pregnancies, LARC cannot alone diminish the inequities that undermine reproductive healthcare access.8,109
Second, a reproductive justice framework focuses on bodily autonomy, affirming that a woman, even an adolescent woman, has the right to choose the best method for herself, rather than simply following a directive “first-line” paradigm where LARC methods are recommended over all others.110 Providers should celebrate and incorporate all methods, including less-effective methods, to match the desires of each woman, including adolescents, and follow the reproductive justice framework that rejects efforts to direct patients towards a specific contraceptive method.
Lastly, at its core, a reproductive justice framework recognizes the inadvertent failure to acknowledge prior injustices to poor women of color. The Sister Song Women of Color Reproductive Justice Collective and the National Women’s Health Network suggest that healthcare providers directly address concerns about reproductive injustice and acknowledge racist and eugenicist legacies that might explain why poor women of color may feel socio-demographically targeted when a provider recommends LARC.111 A reproductive justice framework seeks to enable adolescents to access and use LARC if they desire and to remove LARC when they desire, while directly acknowledging prior reproductive abuses to socially disadvantaged groups.111 The ultimate goal is to enhance the health, social well-being, and bodily autonomy and integrity of all individuals, including marginalized women who have been historically oppressed, and to enable individuals to feel safe when making the best reproductive decision for their health and circumstances without pressure, coercion or judgment from their healthcare provider.
Conclusion
In sum, LARC is an essential tool that should be available to adolescents seeking pregnancy prevention. Deftly navigating hurdles to adolescent LARC use, such as myths and access barriers, may help providers facilitate uptake, continuation, and adherence. Adolescent-centered, shared decision-making communication strategies within a reproductive justice and health belief model framework can increase LARC awareness and empower uptake while fostering adolescent autonomy and incorporating family values in contraceptive method choice.
Abbreviations
LARC, Long-Acting Reversible Contraception; DMPA, Depot medroxyprogesterone acetate (Depo-Provera); IUD, Intrauterine device; Cu-IUD, Copper IUD; LNG-IUD, Levonorgestrel IUD; STI, Sexually Transmitted Infection; US, United States.
Disclosure
This work was funded by the National Institutes of Health (K23 HD097291) and the UT FOCUS award from the American Heart Association, Doris Duke Charitable Foundation, and the Harry S. Moss Heart Trust. The content is solely the authors’ responsibility and does not necessarily represent the official views of the study sponsors. The sponsors did not have a role in the review design, analysis, interpretation of data, writing of the report, or decision to submit the article for publication. Dr Melanie A Gold reports grants from Merck/Organon, consulting fees from Bayer, outside the submitted work. Dr Jenny KR Francis reports grants from the National Institutes of Health (K23 HD097291), grants from the UT FOCUS award funded by the American Heart Association, the Doris Duke Charitable Foundation, and the Harry S. Moss Heart Trust, and grants from an Organon Investigator-Initiated Study during the conduct of the study. The authors have no other relevant conflicts of interest to disclose.
References
1. Committee on Adolescent Health Care, Long-Acting Reversible Contraception Work Group. ACOG committee opinion no. 735: adolescents and long-acting reversible contraception: implants and intrauterine devices. Obstet Gynecol. 2018;131(5):e130–e139. DOI:10.1097/aog.0000000000002632
2. Braverman PK, Adelman WP, Alderman EM, et al.; Committee on Adolescence. Contraception for Adolescents. Pediatrics. 2014;134(4):e1244–e1256. doi:10.1542/peds.2014-2299
3. Lindberg LD, Santelli J, Desai S. Understanding the decline in adolescent fertility in the United States, 2007–2012. J Adolesc Health. 2016;59(5):577–583. doi:10.1016/j.jadohealth.2016.06.024
4. Martinez GM, Abma JC. Sexual activity and contraceptive use among teenagers aged 15–19 in the United States, 2015–2017. NCHS Data Brief. 2020;2020(366):1–8.
5. Szucs L, Lowry R, Fasula A, et al. Condom and contraceptive use among sexually active high school students — youth risk behavior survey, United States, 2019. MMWR Supplements. 2020;69:11–18. doi:10.15585/mmwr.su6901a2
6. Pritt NM, Norris AH, Berlan ED. Barriers and facilitators to adolescents’ use of long-acting reversible contraceptives. J Pediatr Adolesc Gynecol. 2017;30(1):18–22. doi:10.1016/j.jpag.2016.07.002
7. Roderique-Davies G, McKnight C, Jonn B, Faulkner S, Lancastle D. Models of health behaviour predict intention to use long acting reversible contraception use. Womens Health. 2016;12(6):507–512. doi:10.1177/1745505716678231
8. Higgins JA. Celebration meets caution: LARC’s boons, potential busts, and the benefits of a reproductive justice approach. Contraception. 2014;89(4):237–241. doi:10.1016/j.contraception.2014.01.027
9. Russo JA, Miller E, Gold MA. Myths and misconceptions about long-acting reversible contraception (LARC). J Adolesc Health. 2013;52(4 Suppl):S14–S21. doi:10.1016/j.jadohealth.2013.02.003
10. Jacobs Institute of Women’s Health. History of Long-Acting Reversible Contraception (LARC) in the United States; 2016. Available from: https://publichealth.gwu.edu/sites/default/files/downloads/projects/JIWH/LARC_History.pdf.
11. Piccinino LJ, Mosher WD. Trends in contraceptive use in the United States: 1982-1995. Fam Plann Perspect. 1998;30(1):4–10, 46. doi:10.2307/2991517
12. Bayer HealthCare Pharmaceuticals Inc. Highlights of Prescribing Information, Mirena (levonorgestrel-releasing intrauterine system); 2022. Available from: https://labeling.bayerhealthcare.com/html/products/pi/Mirena_PI.pdf.
13. McNicholas C, Maddipati R, Zhao Q, Swor E, Peipert JF. Use of the etonogestrel implant and levonorgestrel intrauterine device beyond the U.S. Food and Drug Administration-approved duration. Obstet Gynecol. 2015;125(3):599–604. doi:10.1097/aog.0000000000000690
14. Kaiser Family Foundation. Intrauterine devices (IUDs): access for women in the U.S. Available from: https://www.kff.org/womens-health-policy/fact-sheet/intrauterine-devices-iuds-access-for-women-in-The-u-s.
15. AbbVie. Highlights of Prescribing Information, Liletta (levonorgestrel-releasing intrauterine system); 2019. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/206229s008lbl.pdf.
16. Foran T, Butcher BE, Kovacs G, Bateson D, O’Connor V. Safety of insertion of the copper IUD and LNG-IUS in nulliparous women: a systematic review. Eur J Contracept Reprod Health Care. 2018;23(5):379–386. doi:10.1080/13625187.2018.1526898
17. Buhling KJ, Zite NB, Lotke P, Black K. Worldwide use of intrauterine contraception: a review. Contraception. 2014;89(3):162–173. doi:10.1016/j.contraception.2013.11.011
18. Danna K, Jaworski G, Rahaivondrafahitra B, et al. Introducing the hormonal intrauterine device in Madagascar, Nigeria, and Zambia: results from a pilot study. Reprod Health. 2022;19(1):4. doi:10.1186/s12978-021-01300-x
19. Menon S, Alderman EM, Chung RJ. Committee on adolescence. long-acting reversible contraception: specific issues for adolescents. Pediatrics. 2020;146(2):e2020007252. doi:10.1542/peds.2020-007252
20. Kumar MM. Long-acting reversible contraceptives for adolescents: more complex than “first-line”. JAMA Pediatr. 2018;172(1):6–7. doi:10.1001/jamapediatrics.2017.3520
21. Fuchs R, Taylor D, Jenkins DW, et al. Levonorgestrel release rates measured through analysis of two-rod contraceptive explants. Contracept X. 2020;2:100039. doi:10.1016/j.conx.2020.100039
22. Trussell J. Contraceptive failure in the United States. Contraception. 2011;83(5):397–404. doi:10.1016/j.contraception.2011.01.021
23. Ortiz ME, Croxatto HB. Copper-T intrauterine device and levonorgestrel intrauterine system: biological bases of their mechanism of action. Contraception. 2007;75(6 Suppl):S16–S30. doi:10.1016/j.contraception.2007.01.020
24. Apter D, Gemzell-Danielsson K, Hauck B, Rosen K, Zurth C. Pharmacokinetics of two low-dose levonorgestrel-releasing intrauterine systems and effects on ovulation rate and cervical function: pooled analyses of Phase II and III studies. Fertil Steril. 2014;101(6):1656–62.e1–e4. doi:10.1016/j.fertnstert.2014.03.004
25. Francis JKR, Gold MA. Long-acting reversible contraception for adolescents: a review. JAMA Pediatr. 2017;171(7):694–701. doi:10.1001/jamapediatrics.2017.0598
26. Hall KS, Steinberg JR, Cwiak CA, Allen RH, Marcus SM. Contraception and mental health: a commentary on the evidence and principles for practice. Am J Obstet Gynecol. 2015;212(6):740–746. doi:10.1016/j.ajog.2014.12.010
27. Reproductive Health Access Project. Quick start algorithm for hormonal contraception. Available from: https://www.reproductiveaccess.org/wp-content/uploads/2014/12/QuickstartAlgorithm.pdf.
28. Harper CC, Speidel JJ, Drey EA, Trussell J, Blum M, Darney PD. Copper intrauterine device for emergency contraception: clinical practice among contraceptive providers. Obstet Gynecol. 2012;119(2 Pt 1):220–226. doi:10.1097/AOG.0b013e3182429e0d
29. Turok DK, Gero A, Simmons RG, et al. Levonorgestrel vs. copper intrauterine devices for emergency contraception. N Engl J Med. 2021;384(4):335–344. doi:10.1056/NEJMoa2022141
30. Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. medical eligibility criteria for contraceptive use, 2016. MMWR Recomm Rep. 2016;65(3):1–103. doi:10.15585/mmwr.rr6503a1
31. Osterman M, Hamilton B, Martin JA, Driscoll AK, Valenzuela CP. Births: final Data for 2020. Natl Vital Stat Rep. 2021;70(17):1–50.
32. Lindberg LD, Firestein L, Beavin C. Trends in U.S. adolescent sexual behavior and contraceptive use, 2006–2019. Contracept X. 2021;3:100064. doi:10.1016/j.conx.2021.100064
33. Daniels K, Abma JC. Current contraceptive status among women aged 15–49: United States, 2017–2019. NCHS Data Brief. 2020;388:1–8.
34. Lindberg LD, Scott RH, Desai S, Pleasure ZH. Comparability of estimates and trends in adolescent sexual and contraceptive behaviors from two national surveys: National Survey of Family Growth and the Youth Risk Behavior Survey. PLoS One. 2021;16(7):e0253262. doi:10.1371/journal.pone.0253262
35. Centers for Disease Control and Prevention. Reproductive health: teen pregnancy - birth rates for females aged 15 to 19 years, by race and Hispanic origin of mother: United States, 2018 and 2019. Available from: https://www.cdc.gov/teenpregnancy/about/index.htm.
36. Chandra-Mouli V, McCarraher DR, Phillips SJ, Williamson NE, Hainsworth G. Contraception for adolescents in low and middle income countries: needs, barriers, and access. Reprod Health. 2014;11(1):1. doi:10.1186/1742-4755-11-1
37. United Nations, Department of Economic and Social Affairs. Contraceptive use by method 2019 - data booklet; 2019. Available from: https://www.un.org/development/desa/pd/sites/www.un.org.development.desa.pd/files/files/documents/2020/Jan/un_2019_contraceptiveusebymethod_databooklet.pdf.
38. Jacobstein R. Liftoff: the blossoming of contraceptive implant use in Africa. Glob Health Sci Pract. 2018;6(1):17–39. doi:10.9745/ghsp-d-17-00396
39. Hendrick CE, Cone JN, Cirullo J, Maslowsky J. Determinants of Long-acting Reversible Contraception (LARC) initial and continued use among adolescents in the United States. Adolesc Res Rev. 2020;5(3):243–279. doi:10.1007/s40894-019-00126-w
40. Skinner CS, Tiro J, Champion VL. The health belief model. In: Health Behavior: Theory, Research, and Practice.
41. Lopez LM, Grey TW, Chen M, Tolley EE, Stockton LL. Theory-based interventions for contraception. Cochrane Database Syst Rev. 2016;11(11):Cd007249. doi:10.1002/14651858.CD007249.pub5
42. Stokholm Baekgaard R, Gjaerevold Damhaugh E, Mrema D, Rasch V, Khan K, Linde DS. Training of healthcare providers and use of long-acting reversible contraception in low- and middle-income countries: a systematic review. Acta Obstet Gynecol Scand. 2021;100(4):619–628. doi:10.1111/aogs.14127
43. Bolarinwa OA, Afaya A, Ajayi KV, Ojo A, Alawode OA. Prevalence and factors associated with the use of long-acting reversible and permanent contraceptive methods among women who desire no more children in high fertility countries in sub-saharan Africa. BMC Public Health. 2022;22(1):2141. doi:10.1186/s12889-022-14575-x
44. Apanga PA, Kumbeni MT, Ayamga EA, Ulanja MB, Akparibo R. Prevalence and factors associated with modern contraceptive use among women of reproductive age in 20 African countries: a large population-based study. BMJ Open. 2020;10(9):e041103. doi:10.1136/bmjopen-2020-041103
45. Kungu W, Khasakhala A, Agwanda A. Use of long-acting reversible contraception among adolescents and young women in Kenya. PLoS One. 2020;15(11):e0241506. doi:10.1371/journal.pone.0241506
46. Ngo TD, Nuccio O, Pereira SK, Footman K, Reiss K. Evaluating a LARC expansion program in 14 Sub-Saharan African Countries: a service delivery model for meeting FP2020 goals. Matern Child Health J. 2017;21(9):1734–1743. doi:10.1007/s10995-016-2014-0
47. Ti A, Soin K, Rahman T, Dam A, Yeh PT. Contraceptive values and preferences of adolescents and young adults: a systematic review. Contraception. 2021. doi:10.1016/j.contraception.2021.05.018
48. Hoopes AJ, Teal SB, Akers AY, Sheeder J. Low acceptability of certain contraceptive methods among young women. J Pediatr Adolesc Gynecol. 2018;31(3):274–280. doi:10.1016/j.jpag.2017.11.008
49. Cohen R, Sheeder J, Kane M, Teal SB. Factors associated with contraceptive method choice and initiation in adolescents and young women. J Adolesc Health. 2017;61(4):454–460. doi:10.1016/j.jadohealth.2017.04.008
50. Secura GM, Allsworth JE, Madden T, Mullersman JL, Peipert JF. The Contraceptive CHOICE Project: reducing barriers to long-acting reversible contraception. Am J Obstet Gynecol. 2010;203(2):115.e1–115.e7. doi:10.1016/j.ajog.2010.04.017
51. Mestad R, Secura G, Allsworth JE, Madden T, Zhao Q, Peipert JF. Acceptance of long-acting reversible contraceptive methods by adolescent participants in the Contraceptive CHOICE Project. Contraception. 2011;84(5):493–498. doi:10.1016/j.contraception.2011.03.001
52. Callahan DG, Garabedian LF, Harney KF, DiVasta AD. Will it hurt? The intrauterine device insertion experience and long-term acceptability among adolescents and young women. J Pediatr Adolesc Gynecol. 2019;32(6):615–621. doi:10.1016/j.jpag.2019.08.004
53. Whiteman MK, Tyler CP, Folger SG, Gaffield ME, Curtis KM. When can a woman have an intrauterine device inserted? A systematic review. Contraception. 2013;87(5):666–673. doi:10.1016/j.contraception.2012.08.015
54. Teal SB, Romer SE, Goldthwaite LM, Peters MG, Kaplan DW, Sheeder J. Insertion characteristics of intrauterine devices in adolescents and young women: success, ancillary measures, and complications. Am J Obstet Gynecol. 2015;213(4):515.e1–5. doi:10.1016/j.ajog.2015.06.049
55. Friedman JO. Factors associated with contraceptive satisfaction in adolescent women using the IUD. J Pediatr Adolesc Gynecol. 2015;28(1):38–42. doi:10.1016/j.jpag.2014.02.015
56. Maguire K, Davis A, Rosario Tejeda L, Westhoff C. Intracervical lidocaine gel for intrauterine device insertion: a randomized controlled trial. Contraception. 2012;86(3):214–219. doi:10.1016/j.contraception.2012.01.005
57. Lopez LM, Bernholc A, Zeng Y, et al. Interventions for pain with intrauterine device insertion. Cochrane Database Syst Rev. 2015;2015(7):Cd007373. doi:10.1002/14651858.CD007373.pub3
58. Higgins JA, Sanders JN, Palta M, Turok DK. Women’s sexual function, satisfaction, and perceptions after starting long-acting reversible contraceptives. Obstet Gynecol. 2016;128(5):1143–1151. doi:10.1097/aog.0000000000001655
59. Harris K, Byrd K, Engel M, Weeks K, Ahlers-Schmidt CR. Internet-based information on long-acting reversible contraception for adolescents. J Prim Care Community Health. 2016;7(2):76–80. doi:10.1177/2150131915621058
60. Rubin SE, Felsher M, Korich F, Jacobs AM. Urban adolescents’ and young adults’ decision-making process around selection of intrauterine contraception. J Pediatr Adolesc Gynecol. 2016;29(3):234–239. doi:10.1016/j.jpag.2015.09.001
61. Brown BP, Chor J, Hebert LE, Webb ME, Whitaker AK. Shared negative experiences of long-acting reversible contraception and their influence on contraceptive decision-making: a multi-methods study. Contraception. 2019;99(4):228–232. doi:10.1016/j.contraception.2019.01.002
62. O’Rourke-Suchoff DK, Arora KS, Hildebrand VM, Singer ME. Exploring maternal attitudes towards adolescent contraception: implications for use of LARC. Int J Adolesc Med Health. 2017;30(6). doi:10.1515/ijamh-2016-0120
63. Higgins JA, Kramer RD, Ryder KM. Provider bias in Long-Acting Reversible Contraception (LARC) promotion and removal: perceptions of young adult women. Am J Public Health. 2016;106(11):1932–1937. doi:10.2105/ajph.2016.303393
64. Rosenstock JR, Peipert JF, Madden T, Zhao Q, Secura GM. Continuation of reversible contraception in teenagers and young women. Obstet Gynecol. 2012;120(6):1298–1305. doi:10.1097/aog.0b013e31827499bd
65. Usinger KM, Gola SB, Weis M, Smaldone A. Intrauterine contraception continuation in adolescents and young women: a systematic review. J Pediatr Adolesc Gynecol. 2016;29(6):659–667. doi:10.1016/j.jpag.2016.06.007
66. Howard DL, Wayman R, Strickland JL. Satisfaction with and intention to continue depo-provera versus the mirena IUD among post-partum adolescents through 12 months of follow-up. J Pediatr Adolesc Gynecol. 2013;26(6):358–365. doi:10.1016/j.jpag.2013.07.013
67. American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice. Committee opinion no. 670: immediate postpartum long-acting reversible contraception. Obstet Gynecol. 2016;128(2):e32–e37. DOI:10.1097/aog.0000000000001587
68. Averbach SH, Ermias Y, Jeng G, et al. Expulsion of intrauterine devices after postpartum placement by timing of placement, delivery type, and intrauterine device type: a systematic review and meta-analysis. Am J Obstet Gynecol. 2020;223(2):177–188. doi:10.1016/j.ajog.2020.02.045
69. Sothornwit J, Werawatakul Y, Kaewrudee S, Lumbiganon P, Laopaiboon M. Immediate versus delayed postpartum insertion of contraceptive implant for contraception. Cochrane Database Syst Rev. 2017;4(4):Cd011913. doi:10.1002/14651858.CD011913.pub2
70. Bryant AG, Bauer AE, Stuart GS, et al. Etonogestrel-releasing contraceptive implant for postpartum adolescents: a randomized controlled trial. J Pediatr Adolesc Gynecol. 2017;30(3):389–394. doi:10.1016/j.jpag.2016.08.003
71. Rose SB, Garrett SM. Postabortion initiation of long-acting reversible contraception by adolescent and nulliparous women in New Zealand. J Adolesc Health. 2016;58(2):160–166. doi:10.1016/j.jadohealth.2015.09.025
72. Buckingham P, Moulton JE, Subasinghe AK, Amos N, Mazza D. Acceptability of immediate postpartum and post-abortion long-acting reversible contraception provision to adolescents: a systematic review. Acta Obstet Gynecol Scand. 2021;100(4):629–640. doi:10.1111/aogs.14129
73. Raymond EG, Weaver MA, Tan YL, et al. Effect of immediate compared with delayed insertion of etonogestrel implants on medical abortion efficacy and repeat pregnancy: a randomized controlled trial. Obstet Gynecol. 2016;127(2):306–312. doi:10.1097/aog.0000000000001274
74. Committee on Practice Bulletins-Gynecology, Long-Acting Reversible Contraception Work Group. Practice bulletin no. 186: long-acting reversible contraception: implants and intrauterine devices. Obstet Gynecol. 2017;130(5):e251–e269. DOI:10.1097/aog.0000000000002400
75. Fox MC, Oat-Judge J, Severson K, et al. Immediate placement of intrauterine devices after first and second trimester pregnancy termination. Contraception. 2011;83(1):34–40. doi:10.1016/j.contraception.2010.06.018
76. Green S, Sheeder J, Richards M. The etonogestrel implant in adolescents: factors associated with removal for bothersome bleeding in the first year after insertion. J Pediatr Adolesc Gynecol. 2021;34(6):825–831. doi:10.1016/j.jpag.2021.05.011
77. Madden T, McNicholas C, Zhao Q, Secura GM, Eisenberg DL, Peipert JF. Association of age and parity with intrauterine device expulsion. Obstet Gynecol. 2014;124(4):718–726. doi:10.1097/aog.0000000000000475
78. Keenahan L, Bercaw-Pratt JL, Adeyemi O, Hakim J, Sangi-Haghpeykar H, Dietrich JE. Rates of intrauterine device expulsion among adolescents and young women. J Pediatr Adolesc Gynecol. 2021;34(3):362–365. doi:10.1016/j.jpag.2020.11.003
79. Diedrich JT, Klein DA, Peipert JF. Long-acting reversible contraception in adolescents: a systematic review and meta-analysis. Am J Obstet Gynecol. 2017;216(4):364.e1–364.e12. doi:10.1016/j.ajog.2016.12.024
80. Coles CB, Shubkin CD. Effective, recommended, underutilized: a review of the literature on barriers to adolescent usage of long-acting reversible contraceptive methods. Curr Opin Pediatr. 2018;30(5):683–688. doi:10.1097/mop.0000000000000663
81. Smith AJB, Harney KF, Singh T, Hurwitz AG. Provider and health system factors associated with usage of long-acting reversible contraception in adolescents. J Pediatr Adolesc Gynecol. 2017;30(6):609–614. doi:10.1016/j.jpag.2017.05.001
82. Berlan ED, Pritt NM, Norris AH. Pediatricians’ attitudes and beliefs about long-acting reversible contraceptives influence counseling. J Pediatr Adolesc Gynecol. 2017;30(1):47–52. doi:10.1016/j.jpag.2016.09.001
83. Coates C, Gordon CM, Simpson T. A qualitative study exploring contraceptive practices and barriers to long-acting reversible contraceptive use in a sample of adolescents living in the Southern United States. J Pediatr Adolesc Gynecol. 2018;31(6):605–609. doi:10.1016/j.jpag.2018.07.006
84. Behmer Hansen RT, Arora KS. Consenting to invasive contraceptives: an ethical analysis of adolescent decision-making authority for long-acting reversible contraception. J Med Ethics. 2018;44(9):585–588. doi:10.1136/medethics-2018-104855
85. Guttmacher Institute. An overview of consent to reproductive health services by young people; 2021. Available from: https://www.guttmacher.org/state-policy/explore/overview-minors-consent-law.
86. Bergin A, Tristan S, Terplan M, Gilliam ML, Whitaker AK. A missed opportunity for care: two-visit IUD insertion protocols inhibit placement. Contraception. 2012;86(6):694–697. doi:10.1016/j.contraception.2012.05.011
87. Weisman CS, Chuang CH, Snyder AH, Liu G, Leslie DL. ACA’s contraceptive coverage requirement: measuring use and out-of-pocket spending. Health Aff. 2019;38(9):1537–1541. doi:10.1377/hlthaff.2018.05484
88. Swan L. Policy impacts on contraceptive access in the United States: a scoping review. J Popul Res. 2023;40. DOI:10.1007/s12546-023-09298-8
89. Carlson JL, Goldstein R. Using the electronic health record to conduct adolescent telehealth visits in the time of COVID-19. J Adolesc Health. 2020;67(2):157–158. doi:10.1016/j.jadohealth.2020.05.022
90. Society for Adolescent Health and Medicine. American academy of pediatrics. confidentiality protections for adolescents and young adults in the health care billing and insurance claims process. J Adolesc Health. 2016;58(3):374–377. DOI:10.1016/j.jadohealth.2015.12.009
91. Matsushita T, Hasegawa T, Noma H, Ota E, Chou VB, Okada Y. Interventions to increase access to long‐acting reversible contraceptives. Cochrane Database Syst Rev. 2021;(11). doi:10.1002/14651858.CD014987
92. Secura GM, Madden T, McNicholas C, et al. Provision of no-cost, long-acting contraception and teenage pregnancy. N Engl J Med. 2014;371(14):1316–1323. doi:10.1056/NEJMoa1400506
93. Romer SE, Kennedy KI. The Colorado initiative to reduce unintended pregnancy: contraceptive access and impact on reproductive health. Am J Public Health. 2022;112(S5):S532–S536. doi:10.2105/ajph.2022.306891
94. Office of Population Affairs, United States Department of Health and Human Services. Teen pregnancy prevention program - grant programs; 2023.
95. Dehlendorf C, Krajewski C, Borrero S. Contraceptive counseling: best practices to ensure quality communication and enable effective contraceptive use. Clin Obstet Gynecol. 2014;57(4):659–673. doi:10.1097/grf.0000000000000059
96. Kendall-Taylor N, Ginsburg KR. Framing strategies to shape parent and adolescent understandings of development. Pediatrics. 2021;148(3). doi:10.1542/peds.2021-050735
97. Gomez AM, Fuentes L, Allina A. Women or LARC first? Reproductive autonomy and the promotion of long-acting reversible contraceptive methods. Perspect Sex Reprod Health. 2014;46(3):171–175. doi:10.1363/46e1614
98. Pindar C, Lee SH, Meropol SB, Lazebnik R. The role of reproductive autonomy in adolescent contraceptive choice and acceptance of long-acting reversible contraception. J Pediatr Adolesc Gynecol. 2020;33(5):494–499. doi:10.1016/j.jpag.2020.06.013
99. Dehlendorf C, Ruskin R, Grumbach K, et al. Recommendations for intrauterine contraception: a randomized trial of the effects of patients’ race/ethnicity and socioeconomic status. Am J Obstet Gynecol. 2010;203(4):319.e1–8. doi:10.1016/j.ajog.2010.05.009
100. American College of Obstetricians and Gynecologists. ACOG committee opinion no. 710: counseling adolescents about contraception. Obstet Gynecol. 2017;130(2):e74–e80. DOI:10.1097/aog.0000000000002234
101. American College of Obstetricians and Gynecologists. Patient-centered contraceptive counseling: ACOG committee statement number 1. Obstet Gynecol. 2022;139(2):350–353. DOI:10.1097/aog.0000000000004659
102. Hoopes AJ, Gilmore K, Cady J, Akers AY, Ahrens KR. A qualitative study of factors that influence contraceptive choice among adolescent school-based health center patients. J Pediatr Adolesc Gynecol. 2016;29(3):259–264. doi:10.1016/j.jpag.2015.09.011
103. Widman L, Choukas-Bradley S, Noar SM, Nesi J, Garrett K. Parent-adolescent sexual communication and adolescent safer sex behavior: a meta-analysis. JAMA Pediatr. 2016;170(1):52–61. doi:10.1001/jamapediatrics.2015.2731
104. Lantos H, Manlove J, Wildsmith E, Faccio B, Guzman L, Moore KA. Parent-teen communication about sexual and reproductive health: cohort differences by race/ethnicity and nativity. Int J Environ Res Public Health. 2019;16(5):833. doi:10.3390/ijerph16050833
105. Flores D, Barroso J. Century parent-child sex communication in the United States: a process review. J Sex Res. 2017;54(4–5):532–548. doi:10.1080/00224499.2016.1267693
106. Boekeloo BO. Will you ask? Will they tell you? Are you ready to hear and respond?: barriers to physician-adolescent discussion about sexuality. JAMA Pediatr. 2014;168(2):111–113. doi:10.1001/jamapediatrics.2013.4605
107. Hillard PJA. Reproductive justice and adolescents in a post-roe United States. J Pediatr Adolesc Gynecol. 2022;35(6):607–608. doi:10.1016/j.jpag.2022.10.009
108. Wilkinson TA, Kottke MJ, Berlan ED. Providing contraception for young people during a pandemic is essential health care. JAMA Pediatr. 2020;174(9):823–824. doi:10.1001/jamapediatrics.2020.1884
109. Gubrium AC, Mann ES, Borrero S, et al. Realizing reproductive health equity needs more than long-acting reversible contraception (LARC). Am J Public Health. 2016;106(1):18–19. doi:10.2105/ajph.2015.302900
110. Moskowitz E, Jennings B. Directive counseling on long-acting contraception. Am J Public Health. 1996;86(6):787–790. doi:10.2105/ajph.86.6.787
111. National Women’s Health Network, Sister Song. Long-acting reversible contraception statement of principles; 2017. Available from: https://www.nwhn.org/wp-content/uploads/2017/02/LARCStatementofPrinciples.pdf.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
