Back to Journals » International Journal of General Medicine » Volume 15
Local-to-Remote Brain Functional Connectivity in Patients with Thyroid-Associated Ophthalmopathy and Assessment of Its Predictive Value Using Machine Learning
Authors Wen Z , Wan X, Qi CX, Huang X
Received 15 December 2021
Accepted for publication 24 March 2022
Published 21 April 2022 Volume 2022:15 Pages 4273—4283
DOI https://doi.org/10.2147/IJGM.S353649
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Zhi Wen,1,* Xin Wan,2,* Chen-Xing Qi,1 Xin Huang1
1Department of Ophthalmology, Jiangxi Provincial People’s Hospital, Nanchang, Jiangxi, 330006, People’s Republic of China; 2Department of Traditional Chinese Medicine, The First Affiliated Hospital of Nanchang University, Nanchang, Jiangxi, 330006, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Chen-Xing Qi; Xin Huang, Department of Ophthalmology, Jiangxi Provincial People’s Hospital, No. 152, Ai Guo Road, Dong Hu District, Nanchang, Jiangxi, People’s Republic of China, Email [email protected]; [email protected]
Purpose: To explore the alterations in both local and remote brain connectivity in patients with thyroid-associated ophthalmopathy (TAO) and to investigate whether the alterations of local neural function could be used to distinguish patients with TAO from healthy controls (HCs) using support vector machine (SVM) classifier.
Materials and Methods: In total, 21 patients with TAO and 21 well-matched HCs were enrolled in our study and underwent resting-state functional magnetic resonance imaging (rs-fMRI) scanning. We employed regional homogeneity (ReHo) algorithm to evaluate local neural function and selected significantly altered brain regions as seed areas for subsequent study of the remote functional connectivity (FC). Moreover, we chose the observed alterations in the ReHo analysis as classification features to differentiate patients with TAO from HCs through SVM classification method.
Results: Compared with the HCs, TAO patients showed significantly lower ReHo values in the right middle occipital gyrus (MOG) and right angular (ANG). In contrast, TAO patients displayed higher ReHo values in the left hippocampus (Hipp). We further found TAO patients exhibited decreased FC between the left and right Hipp, right MOG and left cerebellum (CER), right ANG and left rectus, right superior temporal pole gyrus (PSTG) (voxel-level p < 0.01, Gaussian random field correction, cluster-level p < 0.05). The alterations in local neural function exhibited an accuracy of 78.57% and area under curve of 0.81 for distinguishing the patients from HCs.
Conclusion: We mainly found the results that patients with TAO showed significantly dysfunctional local and remote brain functional connectivity in several brain regions associated with visual and cognitive functions. The ReHo variability has potential value in differentiating patients with TAO from HCs. These findings may provide novel insights into the neurological mechanisms underlying visual and cognitive disorders in patients with TAO.
Keywords: thyroid-associated ophthalmopathy, functional magnetic resonance imaging, regional homogeneity, functional connectivity, machine learning
Introduction
Thyroid-associated ophthalmopathy (TAO), a vision-threatening autoimmune and inflammatory orbital disease, usually presents with lid retraction, exophthalmos, visual impairment, and diplopia. TAO is a common ocular complication in hyperthyroidism, but can also be seen in patients with normal thyroid function and hypothyroidism.1 Clear diagnosis and timely treatment of TAO are extremely important in order to effectively avoid the incidence of visual impairment. Clinically, TAO is divided into active stage and quiescent stage according to patients’ symptoms and examination results. Because of acute inflammatory changes in orbital tissue, active patients with TAO often show typical signs of ocular manifestations, such as extraocular muscle swelling, bulbar conjunctival hyperemia, and optic neuropathy.2 Dysthyroid optic neuropathy is the most serious complication in TAO patients, and the incidence is about 4–8% according to statistics.3 Besides these ophthalmic manifestations, patients with TAO are also closely associated with cognitive disorder, especially in emotional regulation.4
The main pathological changes of TAO were extraocular muscle swelling and periorbital fat increase, while the main physiological mechanism of TAO is related to the abnormal metabolism of thyroid hormones. Previous studies have proved that thyroid hormones play an important role in neuron differentiation, myelination, neuron formation and synaptic production.5 Therefore, thyroid hormones are essential for maintaining the normal structures and functions of adult visual pathways, which was clearly elaborated by Tu et al.6 In a rat study, optic nerve function showed significant abnormalities due to the decrease in serum thyroid hormone level.1 Moreover, previous clinical epidemiological investigations have suggested that abnormal thyroid hormone level was a high risk factor for open-angle glaucoma and age-related macular degeneration.7,8 Related neuroimaging research further revealed that patients who develop TAO exhibited statistically significant thinning of the gray matter, which might be related to cognitive changes and visual impairment reported by TAO cases.4 Based on the magnetic-resonance spectroscopy study, Bhataraet et al reported that the Cho/Cr ratio of prefrontal cortex in TAO patients was lower than that of healthy controls (HCs).9 In another functional magnetic resonance imaging (fMRI) research, the hyperthyroid patients accompanied by dysthyroid optic neuropathy were reported to show aberrant neural activity and brain function in the limbic system and middle frontal gyrus.10 According to the data reported by a combined voxel-based morphometry and diffusion tensor imaging study, TAO cohort was found to have decreased grey matter volume and increased diffusivities in the brain cortex that connected with visual and cognitive dysfunction.11 Investigations thus far have shown that individuals with TAO commonly have visual and cognitive dysfunction, with the structural and metabolic abnormalities in the corresponding brain regions. Considering that previous neuroimaging studies primarily focused on either local brain regions or global functional connectivity (FC) of networks, systematically investigating the neuropathological mechanism of visual and neurocognitive deficits of TAO subjects from local to global may shed some new light on the disease.
Resting-state fMRI provides a promising and non-invasive neuroimaging technique to measure spontaneous or intrinsic brain activity. Synchronous neuronal activity occurs in the normal human brain, where it has an important role in learning and memory.12 Bayati et al found that the key characteristic of neuronal information processing is the reliable propagation of synchronous neuronal activity.13 In addition, several previous fMRI studies showed that synchronous neuronal activity may play a critical role in neurophysiological activity.14,15 In 2004, Zang et al described ReHo, a new technique for assessment of rs-fMRI data, for the evaluation of synchronous neuronal activity.16 ReHo is thought to be a reliable and sensitive measurement; it reflects the temporal homogeneity of regional blood oxygen level-dependent (BOLD) signals by measuring the ReHo values of the time series of regional BOLD signals.
FC analysis based on rs-fMRI data is an algorithm of the spatial temporal correlations and synchrony of the BOLD signals among anatomically different regions.17 Among the rs-fMRI analysis techniques, FC is the most extensively applied algorithm to explore interregional connectivity because of its high sensitivity and reliability.
Machine learning, the core algorithm of artificial intelligence, has provided a systematic approach to learn from a set of high-dimensional data and then make predictions. As a supervised learning algorithm, support vector machine (SVM) shows the unique ability to find small differences in spatial patterns of structural and functional brain changes between subjects and controls. A reliable conclusion could be drawn from recent rs-fMRI studies that the SVM approach offered relatively high accuracy in classifying patients and predicting their future disease progress.18,19 To the best of our knowledge, this is the first study that the SVM technique based on neuroimaging data to perform predictive investigations in patients with TAO till now.
In the current study, we first investigated changes in synchronous neuronal activity in patients with TAO by using the ReHo method. We subsequently applied the method of seed-based FC to detect if there were alterations in interregional connectivity between the regions showing abnormal ReHo values and other brain areas. We further chose the observed alterations in the ReHo analysis as classification features to examine whether these alterations could differentiate subjects with TAO from HCs through SVM classification method.
Materials and Methods
Subjects
The present study recruited 21 inpatients with TAO from endocrinology and ophthalmology departments of the Jiangxi Provincial People’s Hospital and 21 HCs (14 males and 7 females) matched for sex, age and education from medical examination center of the same hospital. These subjects enlisted in the work all met the following criteria1: no clear contraindications to MRI, such as metal implants2; without cardiovascular and psychiatric disorders3; right-handed4; no cerebral diseases evaluated by structural MRI.
All the patients were due to hyperthyroidism and fulfilled the criteria for TAO diagnosed by two experienced ophthalmologists according to the diagnostic criteria from the American Academy of Ophthalmology,20 and these patients were all in the active stage of TAO with vision loss and definite ocular symptoms.
Subjects with any of the following conditions would be removed from the study:1 additional eye diseases which would show vision decline and limited eye movement similar to TAO;2 no ocular surgical history (retinal laser photocoagulation, vitrectomy, etc);3 women who were pregnant or breastfeeding during the study.
The whole research process followed the normative requirements in the Declaration of Helsinki and was approved by the Ethical Committee for Medicine of the Jiangxi Provincial People’s Hospital. All participants participated in the study voluntarily and were informed of the study objectives, methods and potential risks before signing the informed consent.
MRI Data Acquisition
MRI scanning was performed on a 3-Tesla MR scanner (GE Healthcare, Milwaukee, WI, USA) with an eight-channel head coil. All participants were required to close their eyes without falling asleep when undergoing MRI scanning. The structural MRI parameters and functional image parameters were elaborated in detail in our last article.17
fMRI Data Preprocessing
All preprocessing was performed using the toolbox for Data Processing & Analysis of Brain Imaging (DPABI, http://www.rfmri.org/dpabi),21 which is based on Statistical Parametric Mapping (SPM8) (http://www.fil.ion.ucl.ac.uk) implemented in MATLAB 2013a (MathWorks, Natick, MA, USA) and briefly following the steps as the previous neuroimaging studies.22,23
The Calculation of ReHo Signal Value
The ReHo metrics were calculated using DPABI software. The analysis of ReHo signal value followed the calculating formula of the Kendall’s coefficient of concordance in the present research.16 All ReHo maps of each voxel were z-transformed with Fisher’s R-to-Z transformation to reduce the influence of individual differences on the statistical comparisons between the two groups. Finally, the remaining zReHo maps were spatially smoothed using a Gaussian kernel of 6 mm × 6 mm × 6 mm full width at half maximum.
The Calculation of FC Value
To investigate whether abnormal FC existed between the brain regions that exhibited altered localized temporal synchronization (measured by ReHo) and other brain regions. Three seed regions of interest (ROIs) were used for FC analysis, which were derived from those showing significant abnormality of the ReHo values. The Montreal Neurological Institute coordinates of the ROIs were L-Hipp (−36, −24, −12), R-MOG (30, −75, 33), and R-ANG (30, −60, 42).
Statistical Analysis
The cumulative clinical measurements were analyzed in this study using SPSS 20.0 (SPSS Inc, Chicago, IL, USA). Chi-square test was adopted to evaluate categorical variables, while Independent two-sample t-test was applied to analyse continuous variables. A p value <0.05 was considered statistically significant.
A two-sample t-test was used to investigate the difference in the zReHo maps and z-value FC maps between the TAO group and the HC group with DPABI toolkit after controlling for the effects of age and sex. Gaussian random field (GRF) method was utilized to correct for multiple comparisons of ReHo differences between the two groups (two-tailed, voxel-level p < 0.01, GRF correction, cluster-level p < 0.05). These results were shown by applying the BrainNet Viewer software (https://www.nitrc.org./projects/bnv/).
SVM Analysis
To evaluate whether the ReHo metrics alterations could serve as potential diagnostic indices for TAO, we performed machine learning analyses using SVM algorithm with the average ReHo values of all clusters showing significant among-group differences as the features. In the present study, individual participant’s ReHo maps served as inputs for the machine learning algorithm.
Mapping nonlinear data to the high-dimensional feature space and finding a linear separating hyperplane to separate the two sets of data were the core idea of the SVM algorithm. The present work adopted the Gaussian radial basis function kernel support vector machines (RBF-SVM), to study the potential diagnostic of the ReHo indicators. The radial basis function kernel parameter γ was optimized in the values of 2N, with the C in the SVM algorithm set to 1, and the performance of the proposed method was verified by a leave-one-out cross-validation. The number of samples in our study was assumed to be n. In each leave-one-out cross-validation fold, ReHo data from n-1 samples were selected as the training dataset to train the classification model; ReHo data from the remaining sample were regarded as a test dataset to test the ability of the classifier to classify new cases reliably18. This process was repeated for each subject to assess the overall accuracy of the SVM algorithm.
Results
Demographics and Disease Characteristics
We found no statistically significant differences between the TAO and HC groups in gender (P>0.999), education (P=0.861) or age (P=0.745), but significant differences in BCVA of right eye (P=0.026), left eye (P=0.023). The results of these data are listed in Table 1.
 |
Table 1 Characteristics of Participants Included in the Study |
Comparisons of ReHo Values
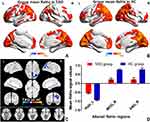
Compared with the HCs, TAO patients showed significantly lower ReHo values in the right middle occipital gyrus (MOG) and right angular (ANG). In contrast, TAO patients displayed higher ReHo values in the left hippocampus (Hipp). The differences in ReHo signal values are clearly shown in Table 2 and Figure 1.
 |
Table 2 Abnormal Distance-Related Brain Functional Connectivity in TAO Patients |
Differences in FC
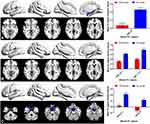
Three seed ROIs were used for FC analysis (Figure 2), which were derived from those showing significant abnormality of the ReHo values. We finally found TAO patients exhibited decreased FC between the left and right Hipp, right MOG and left cerebellum (CER), right ANG and left rectus, right superior temporal pole gyrus (PSTG) (voxel-level p<0.01, GRF correction, cluster-level p<0.05).
SVM Classification Results
The classification results using machine learning analysis and the ROC curve of the classifier are shown in Figure 3. The curve was drawn using ROC assistant software. The performance of the classifier achieved an accuracy of 78.57% and area under curve of 0.81 for TAO group vs HC group.
Discussion
In recent years, the ReHo method has been successfully used to investigate the regional synchronization of spontaneous fMRI signals in several eye diseases and shows huge development prospect.24,25 The SVM approach, the most commonly used supervised machine learning algorithm, offered relatively high accuracy in classifying patients and predicting their future disease progress on the basis of fMRI data. To the best of our knowledge, this work is the first research to systematically investigate the neuropathological mechanism of visual and neurocognitive deficits of TAO subjects from local to global. Compared with the HCs, TAO patients showed significantly lower ReHo values in the right MOG and right ANG. In contrast, TAO patients displayed higher ReHo values in the left Hipp. We further found TAO patients exhibited decreased FC between the left and right Hipp, right MOG and left CER, right ANG and left rectus, right PSTG (voxel-level p<0.01, GRF correction, cluster-level p<0.05). The alterations in local neural function exhibited an accuracy of 78.57% and area under curve of 0.81 for distinguishing the patients from HCs.
The occipital lobe contains most of the anatomical areas of the visual cortex, where the three Brodmann areas17–19 related to vision could be found.26 The occipital lobe is associated with visual information processing, and plays a critical role in the perception of facial emotion.27 Brodmann area,19 the MOG, is considered to be critical part of the dorsal visual stream, which is mainly related to visual formation and neuronal activities of visual perception.28 Disrupted local neural function of the MOG has been reported in fMRI studies concerning late blindness,29 retinal detachment,30 diabetic retinopathy,31 and retinitis pigmentosa.32 Chen and colleges revealed that active TAOs exhibited significantly decreased spontaneous brain activity in the MOG, which is associated with alteration in visual function.33 In line with these studies, our current research showed that TAO patients had significantly lower ReHo values in the right MOG, which might be a reasonable finding due to the common symptoms with active-stage TAO patients, including extraocular muscle swelling, diplopia, and optic neuropathy. Reduction in visual input and the presence of visual pathway damage may result in decreased brain activity in the occipital lobe. Additionally, we found TAO patients exhibited decreased remote cortical connectivity between the right MOG and left CER by applying the FC approach. The CER, anatomically located in the posterior fossa, plays a critical role in visuospatial processing, eye movement, and higher cognitive function.34,35 Our previous research has reported that patients with TAO patients showed abnormal spontaneous brain activity in CER using the ALFF algorithm, which might reflect the neuropathological mechanism of visual impairment in patients.20 Therefore, we speculated that the altered ReHo value in the right MOG and decreased FC between the right MOG and left CER might reflect the abnormal local and remote cortical connectivity related to visual impairment in patients with TAO.
The Hipp is a critical hub in the default mode network (DMN), a network responsible for spontaneous cognition.36 Characterized as an integrated network, the brain’s DMN has been defined as a distributed set of regions in association cortices that showed elevated spontaneous activity in rest situations.37 The Hipp is also a key structure of the limbic system, which is involved in learning, episodic memory, and spatial navigation.38 Prior fMRI studies have demonstrated the dysfunction of the Hipp may result in cognitive deficits in a variety of diseases, including depression,39 Parkinson’s disease,40 and Alzheimer’s disease.41 In recent years, clinical observational studies have reported that TAO was involved in functional and psychological alterations in the brain (eg, depression, memory impairment, emotional instability and personality irregularities).42,43 Another neuroimaging research indicated that subjects with major depression had elevated FC between the left Hipp and left MOG, which may reflect memory deficits and sleep disorders in these patients.27 Consistent with these studies, our current research found that, in comparison with HCs, TAO patients displayed higher ReHo values in the left Hipp, and decreased FC between the left and right Hipp. We speculated that increased ReHo in the Hipp might reflect compensation for cognitive impairment in patients with TAO. We could deduce a credible conclusion from these results that the dysfunction of the Hipp was closely related to psychological abnormalities in patients with TAO.
In comparison with HCs, patients with TAO showed lower ReHo values in the right ANG, and decreased FC between right ANG and left rectus, right PSTG in our study. Anatomically, the ANG, located just above the Wernicke area, at the parietal-occipital junction, is an important joint area at the back of the brain. For its specific position, the ANG is thought to play a critical role in integrating information between multiple input modalities and brain networks.44 Accumulated evidence has indicated that the ANG is involved in reading, comprehension, visuospatial attention, spatial cognition, and episodic memory.45,46 Humphreys et al proposed a unifying model to explain complicated cognitive functions in the ANG and considered the ANG could combine different information from local regions and brain networks as an integrative dynamic buffer.47 In addition, the ANG is a constituent part of the DMN, which was not only associated with spontaneous activity in rest situations but also the unconscious processing of working memory tasks and implicit memory.48 Thus, the decreased ReHo values in the right ANG might contribute to a range of cognitive deficits in patients with TAO. With respect to PSTG, it has been found to be highly involved in the essential role of the identification of biological motion and social interactions in connection with a direct gaze.49,50 Tu and colleges revealed that patients diagnosed with TAO exhibited abnormal FC density between the extensive temporal gyrus and the frontal lobe.6 Therefore, combined with the above findings, we speculated that the decreased regional neural function and remote cortical connectivity in the ANG were highly associated with alteration in cognitive function in patients with TAO.
Considering that understanding the cognitive deficits associated with TAO is meaningful to the diagnosis, management and treatment of this disease, performing ReHo and FC analysis within TAO patients is important. At present, clinical physicians for the diagnosis of TAO mostly rely on clinical symptoms and computerized tomography, but some patients did not show typical symptoms. Therefore, we applied an objective method, supervised machine learning, to obtain higher reliability for the diagnosis of TAO. And to evaluate the classification efficiency of the SVM classifier, we performed the ROC method as well. The overall identification accuracy of the SVM was 78.57%, and the area under the curve was 0.81 based on the leave-one-out cross-validation technique. These findings offered strong support for the hypothesis that the ReHo values had credible potential value in discriminating the two groups. Machine learning techniques have the advantage of objectivity and sensitivity, and can alleviate the shortage of experienced clinicians to some extent. Although the application of machine learning technology in the medical field is still in the initial stage, with the further development and extension of machine learning technology, it will become a trend for clinicians to use machine learning to diagnose and manage patients’ health in the near future.
However, the research had some potential limitations that should be ameliorated in the following studies. The first one is the relatively small sample size, although the results were encouraging. Due to the small sample size in the study, the generalizability of the findings was uncertain. Secondly, our study should include both the active and inactive patients with TAO, which help understanding the neurological mechanisms underlying visual and cognitive disorders in these patients. In the present study, we could not confirm whether patients during quiescent stage will show altered local and remote cortical connectivity in brain regions associated with visual and cognitive functions. Thirdly, a 78.57% accurate classification was not high enough. And we will expand the sample size and combine various machine learning methods including such as random forest or deep neural network to improve the accuracy in the next study. Additionally, the effect of physiological noise when performing rs-fMRI scanning was not completely eliminated, which might interfere with BOLD signals. Therefore, a combination of structural MRI, electroencephalogram, diffusion tensor imaging and fMRI might make the findings more convincing.
Conclusion
In conclusion, we mainly found the results that patients with TAO showed significantly dysfunctional local and remote brain functional connectivity in several brain regions associated with visual and cognitive functions. The ReHo variability has potential value in differentiating patients with TAO from HCs with a 78.57% accurate classification. These findings may provide novel insights into the neurological mechanisms underlying visual and cognitive disorders in patients with TAO.
Acknowledgment
We acknowledge the assistance provided by the Natural Science Foundation of Jiangxi Province (20212BAB216058) and Jiangxi Provincial Health Technology Project (SKJP_202210012).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Özkan B, Anik Y, Katre B, Ö A, Gençtürk M, Yüksel N. Quantitative assessment of optic nerve with diffusion tensor imaging in patients with thyroid orbitopathy. Ophthalmic Plast Reconstr Surg. 2015;31(5):391–395. doi:10.1097/IOP.0000000000000359
2. Huh HD, Kim JH, Kim SJ, Yoo JM, Seo SW. The change of lacrimal gland volume in Korean patients with thyroid-associated ophthalmopathy. Korean J Ophthalmol. 2016;30(5):319–325. doi:10.3341/kjo.2016.30.5.319
3. Yu B, Gong C, Ji YF, Xia Y, Tu YH, Wu WC. Predictive parameters on CT scan for dysthyroid optic neuropathy. Int J Ophthalmol. 2020;13(8):1266–1271. doi:10.18240/ijo.2020.08.13
4. Silkiss RZ, Wade AR. Neuroanatomic variations in graves’ dysthyroid ophthalmopathy as studied with MRI. Trans Am Ophthalmol Soc. 2016;114:T9.
5. Demeneix BA. Evidence for prenatal exposure to thyroid disruptors and adverse effects on brain development. Eur Thyroid J. 2019;8(6):283–292. doi:10.1159/000504668
6. Tu Y, Huang P, Mao C, Liu X, Gao J. Abnormal functional connectivity density in patients with dysthyroid optic neuropathy. Ophthalmic Res. 2020. doi:10.1159/000512755
7. Lin HC, Kang JH, Jiang YD, Ho JD. Hypothyroidism and the risk of developing open-angle glaucoma: a five-year population-based follow-up study. Ophthalmology. 2010;117(10):1960–1966. doi:10.1016/j.ophtha.2010.02.005
8. Gopinath B, Liew G, Kifley A, Mitchell P. Thyroid dysfunction and ten-year incidence of age-related macular degeneration. Invest Ophthalmol Vis Sci. 2016;57(13):5273–5277. doi:10.1167/iovs.16-19735
9. Bhatara VS, Tripathi RP, Sankar R, Gupta A, Khushu S. Frontal lobe proton magnetic-resonance spectroscopy in Graves’ disease: a pilot study. Psychoneuro Endocrinol. 1998;23(6):605–612. doi:10.1016/s0306-4530(98)00028-6
10. Jiang YP, Yang YC, Tang LY, et al. Altered spontaneous brain activity patterns in dysthyroid optic neuropathy: a resting-state fMRI study. J Integr Neurosci. 2021;20(2):375–383. doi:10.31083/j.jin2002037
11. Wu Q, Hu H, Chen W, et al. Morphological and microstructural brain changes in thyroid-associated ophthalmopathy: a combined voxel-based morphometry and diffusion tensor imaging study. J Endocrinol Invest. 2020;43(11):1591–1598. doi:10.1007/s40618-020-01242-4
12. Jutras MJ, Buffalo EA. Synchronous neural activity and memory formation. Curr Opin Neurobiol. 2010;20(2):150–155. doi:10.1016/j.conb.2010.02.006
13. Bayati M, Valizadeh A, Abbassian A, Cheng S. Self-organization of synchronous activity propagation in neuronal networks driven by local excitation. Front Comput Neurosci. 2015;9:69. doi:10.3389/fncom.2015.00069
14. Li R, Li Y, An D, Gong Q, Zhou D, Chen H. Altered regional activity and inter-regional functional connectivity in psychogenic non-epileptic seizures. Sci Rep. 2015;5:11635. doi:10.1038/srep11635
15. Spencer KM, Nestor PG, Perlmutter R, et al. Neural synchrony indexes disordered perception and cognition in schizophrenia. Proc Natl Acad Sci USA. 2004;101(49):17288–17293. doi:10.1073/pnas.0406074101
16. Zang Y, Jiang T, Lu Y, He Y, Tian L. Regional homogeneity approach to fMRI data analysis. Neuroimage. 2004;22(1):394–400. doi:10.1016/j.neuroimage.2003.12.030
17. Qi CX, Huang X, Tong Y, Shen Y. Altered functional connectivity strength of primary visual cortex in subjects with diabetic retinopathy. Diabetes Metab Syndr Obes. 2021;14:3209–3219. doi:10.2147/DMSO.S311009
18. Tong Y, Huang X, Qi CX, Shen Y. Altered functional connectivity of the primary visual cortex in patients with iridocyclitis and assessment of its predictive value using machine learning. Front Immunol. 2021;12:660554. doi:10.3389/fimmu.2021.660554
19. Wang Y, Zhang L, Qi L, et al. Machine learning: applications and advanced progresses of radiomics in endocrine neoplasms. J Oncol. 2021;2021:8615450. doi:10.1155/2021/8615450
20. Qi CX, Wen Z, Huang X. Spontaneous brain activity alterations in thyroid-associated ophthalmopathy patients using amplitude of low-frequency fluctuation: a resting-state fMRI study. Neuroreport. 2021;32(18):1416–1422. doi:10.1097/WNR.0000000000001745
21. Yan CG, Wang XD, Zuo XN, Zang YF. DPABI: data processing & analysis for (Resting-State) brain imaging. Neuroinformatics. 2016;14(3):339–351. doi:10.1007/s12021-016-9299-4
22. Van Dijk KR, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59(1):431–438. doi:10.1016/j.neuroimage.2011.07.044
23. Goto M, Abe O, Aoki S, et al. Japanese Alzheimer’s disease neuroimaging initiative. diffeomorphic anatomical registration through exponentiated lie algebra provides reduced effect of scanner for cortex volumetry with atlas-based method in healthy subjects. Neuroradiology. 2013;55(7):869–875. doi:10.1007/s00234-013-1193-2
24. Dan HD, Zhou FQ, Huang X, Xing YQ, Shen Y. Altered intra- and inter-regional functional connectivity of the visual cortex in individuals with peripheral vision loss due to retinitis pigmentosa. Vision Res. 2019;159:68–75. doi:10.1016/j.visres.2019.02.013
25. Wen SM, Min YL, Yuan Q, et al. Altered spontaneous brain activity in retinal vein occlusion as determined by regional homogeneity: a resting-state fMRI study. Acta Radiol. 2019;60(12):1695–1702. doi:10.1177/0284185119845089
26. Flores LP. Occipital lobe morphological anatomy: anatomical and surgical aspects. Arq Neuropsiquiatr. 2002;60(3–A):566–571. doi:10.1590/s0004-282x2002000400010
27. Teng C, Zhou J, Ma H, et al. Abnormal resting state activity of left middle occipital gyrus and its functional connectivity in female patients with major depressive disorder. BMC Psychiatry. 2018;18(1):370. doi:10.1186/s12888-018-1955-9
28. Tu S, Qiu J, Martens U, Zhang Q. Category-selective attention modulates unconscious processes in the middle occipital gyrus. Conscious Cogn. 2013;22(2):479–485. doi:10.1016/j.concog.2013.02.007
29. Renier LA, Anurova I, De Volder AG, Carlson S, VanMeter J, Rauschecker JP. Preserved functional specialization for spatial processing in the middle occipital gyrus of the early blind. Neuron. 2010;68(1):138–148. doi:10.1016/j.neuron.2010.09.021
30. Huang X, Li D, Li HJ, et al. Abnormal regional spontaneous neural activity in visual pathway in retinal detachment patients: a resting-state functional MRI study. Neuropsychiatr Dis Treat. 2017;13:2849–2854. doi:10.2147/NDT.S147645
31. Qi CX, Huang X, Shen Y. Altered intrinsic brain activities in patients with diabetic retinopathy using amplitude of low-frequency fluctuation: a resting-state fMRI study. Diabetes Metab Syndr Obes. 2020;13:2833–2842. doi:10.2147/DMSO.S259476
32. Dan HD, Shen Y, Huang X, Zhou F, Xing Y. Arterial spin labeling perfusion magnetic resonance imaging reveals resting cerebral blood flow alterations specific to retinitis pigmentosa patients. Curr Eye Res. 2019;44(12):1353–1359. doi:10.1080/02713683.2019.1649702
33. Chen W, Wu Q, Chen L, et al. Disrupted spontaneous neural activity in patients with thyroid-associated ophthalmopathy: a resting-state fMRI study using amplitude of low-frequency fluctuation. Front Hum Neurosci. 2021;15:676967. doi:10.3389/fnhum.2021.676967
34. Kralj-Hans I, Baizer JS, Swales C, Glickstein M. Independent roles for the dorsal paraflocculus and vermal lobule VII of the cerebellum in visuomotor coordination. Exp Brain Res. 2007;177(2):209–222. doi:10.1007/s00221-006-0661-x
35. Izawa J, Criscimagna-Hemminger SE, Shadmehr R. Cerebellar contributions to reach adaptation and learning sensory consequences of action. J Neurosci. 2012;32(12):4230–4239. doi:10.1523/JNEUROSCI.6353-11.2012
36. Persson J, Pudas S, Nilsson LG, Nyberg L. Longitudinal assessment of default-mode brain function in aging. Neurobiol Aging. 2014;35(9):2107–2117. doi:10.1016/j.neurobiolaging.2014.03.012
37. Kucyi A, Esterman M, Riley CS, Valera EM. Spontaneous default network activity reflects behavioral variability independent of mind-wandering. Proc Natl Acad Sci USA. 2016;113(48):13899–13904. doi:10.1073/pnas.1611743113
38. Ekstrom AD, Ranganath C. Space, time, and episodic memory: the hippocampus is all over the cognitive map. Hippocampus. 2018;28(9):680–687. doi:10.1002/hipo.22750
39. Price RB, Duman R. Neuroplasticity in cognitive and psychological mechanisms of depression: an integrative model. Mol Psychiatry. 2019;25(3):530–543. doi:10.1038/s41380-019-0615-x
40. Das T, Hwang JJ, Poston KL. Episodic recognition memory and the hippocampus in Parkinson’s disease: a review. Cortex. 2019;113:191–209. doi:10.1016/j.cortex.2018.11.021
41. Mu Y, Gage FH. Adult hippocampal neurogenesis and its role in Alzheimer’s disease. Mol Neurodegener. 2011;6:85. doi:10.1186/1750-1326-6-85
42. Han JS, Seo HS, Lee YH, et al. Fractional anisotropy and diffusivity changes in thyroid-associated orbitopathy. Neuroradiology. 2016;58(12):1189–1196. doi:10.1007/s00234-016-1764-0
43. Du B, Wang Y, Yang M, He W. Clinical features and clinical course of thyroid-associated ophthalmopathy: a case series of 3620 Chinese cases. Eye. 2021;35(8):2294–2301. doi:10.1038/s41433-020-01246-7
44. Seghier ML. The angular gyrus: multiple functions and multiple subdivisions. Neuroscientist. 2013;19(1):43–61. doi:10.1177/1073858412440596
45. Rugg MD, King DR. Ventral lateral parietal cortex and episodic memory retrieval. Cortex. 2018;107:238–250. doi:10.1016/j.cortex.2017.07.012
46. Wang J, Conder JA, Blitzer DN, Shinkareva SV. Neural representation of abstract and concrete concepts: a meta-analysis of neuroimaging studies. Hum Brain Mapp. 2010;31(10):1459–1468. doi:10.1002/hbm.20950
47. Humphreys GF, Lambon Ralph MA, Simons JS. A unifying account of Angular Gyrus contributions to episodic and semantic cognition. Trends Neurosci. 2021;44(6):452–463. doi:10.1016/j.tins.2021.01.006
48. Zuber P, Gaetano L, Griffa A, et al. Additive and interaction effects of working memory and motor sequence training on brain functional connectivity. Sci Rep. 2021;11(1):23089. doi:10.1038/s41598-021-02492-9
49. Sokołowski A, Folkierska-żukowska M, Jednoróg K, Wypych M, Dragan WŁ. It is not (Always) the mismatch that beats you-on the relationship between interaction of early and recent life stress and emotion regulation, an fMRI study. Brain Topogr. 2021:14. doi:10.1007/s10548-021-00880-y
50. Zhao T, Hu A, Su R, Lyu C, Wang L, Yan N. Phonetic versus spatial processes during motor-oriented imitations of visuo-labial and visuo-lingual speech: a functional near-infrared spectroscopy study. Eur J Neurosci. 2021;55:154–174. doi:10.1111/ejn.15550
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.