Back to Journals » Research and Reports in Tropical Medicine » Volume 9
Loa loa infection detection using biomarkers: current perspectives
Authors Akue JP, Eyang-Assengone ER, Dieki R
Received 10 September 2017
Accepted for publication 21 December 2017
Published 3 April 2018 Volume 2018:9 Pages 43—48
DOI https://doi.org/10.2147/RRTM.S132380
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Thomas Unnasch
Video abstract presented by Jean Paul Akue
Views: 1360
Jean Paul Akue,1 Elsa-Rush Eyang-Assengone,1,2 Roland Dieki1
1Department of Parasitology, Centre International of Medical Research of Franceville, Franceville, Gabon; 2Department of Infectiologie Tropicale, Ecole Doctorale Régionale d’Afrique Centrale, Franceville, Gabon
Abstract: Loa loa is originally a restricted filarial worm from central Africa and some west African countries. However, numerous imported cases are being reported throughout the world due to human movement. Traditionally, its diagnosis is based on identification of microfilariae in the peripheral blood or the passage of the adult worm under the conjunctiva. However, few patients have microfilariae in their peripheral blood, while the majority of infected people are amicrofilaremic (without microfilariae in their blood), despite clinical symptoms suggesting L. loa infection. This situation suggests that diagnoses based on the presence of microfilariae in the blood or the ocular passage of an adult worm, are not sensitive. Therefore, it seems necessary to search for biomarkers to remedy this situation. Furthermore, L. loa is a major obstacle in the control of other filarial worms in areas where these filariae are co-endemic. To develop a diagnostic tool based on a biomarker, several approaches have been considered using antibodies, antigens or nucleic acid detection. However, none of the diagnostic techniques in loiasis based on biomarkers has reached the point of care as have microscopic detection of microfilariae or observation of ocular passage of a worm.
Keywords: Loa loa, diagnosis, antibody, antigen, DNA
Introduction
Loa loa filaria is a round worm discovered for the first time in the eye of a slave from the Caribbean in 1770. Later this filarial worm was described in the Gulf of Guinea. Although restricted to central African and some west African countries (Figure 1), this filarial disease is now emerging as a public health problem due to increasing human movement throughout the world. Loiasis is now frequently reported in America, Europe, Australia1 and Asia.2 These imported cases cause several problems to the clinician in areas of the world where L. loa is not endemic.3 Therefore, appropriate care is not given to the patient at the appropriate time. Clinically, symptoms are in general mild in indigenous populations,4 but characterized by an allergic manifestation among nonindigenous populations.5 Three main features characterize infection by L. loa:
- an angioedema known as a Calabar edema, which in general appears on the arms, assumed to be caused by migration of the adult L. loa worm under the skin, and disappears a few days later;
- ocular passage of the adult worm (also known as an eye worm) under the conjunctiva;6
- encephalitis due to heavy microfilaremia (>30,000 microfilaria/mL), generally seen during the treatment of this filarial disease with diethylcarbamazine (DEC) or ivermectin;7–9
- other symptoms involving deep organs: lungs (e.g., pulmonary infiltrates), brain (e.g., encephalopathy in the absence of treatment), heart (e.g., endomyocardial fibrosis) and renal complications (e.g., renal failure), as well as neurological and psychiatric disorders.10 Recently, although the study did not take into account other factors of mortality, excessive mortality was shown in individuals with a heavy microfilaria load.11 Nevertheless, loiasis is still considered a benign disease and does not appear on the World Health Organization’s official list of neglected tropical diseases.10 These clinical features urgently require an accurate diagnosis of L. loa for residents and immigrants from areas where L. loa is endemic, in order to initiate appropriate care excluding confounding factors that are ubiquitous in endemic areas such as malaria, trypanosomiasis, bilharziosis and intestinal parasites.
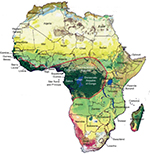  | Figure 1 Natural distribution of Loa loa. Map of Africa showing the area of endemicity for L. loa (circled in red). Adapted from Sayre et al, A New Map of Standardized Terrestrial Ecosystems of Africa. NatureServe. 2013.37 |
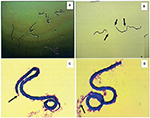
Diagnosis of L. loa still depends on the presence of microfilariae in blood (Figure 2), but these are present in only 30% of infections. Individuals with occasional passage of adult worms under conjunctive tissue or people with occult infection but without microfilaremia account for 70% of infected individuals. The presence of microfilaremia or adult worms are two specific signs, but they are not sensitive for the detection of all cases of loiasis. It is, therefore, necessary to detect biomarkers that could indicate the presence of L. loa with enough sensitivity to detect all cases of loiasis and to follow up L. loa-infected patients (Figure 1).
Life cycle
The cycle starts when a female Chrysops takes microfilariae from the blood of an infected individual during a blood meal. Then the microfilariae mature toward infective larvae (L3), which become infective and can be transmitted to another human during the next blood meal. In humans, filarial worms will develop to adult stage and then can produce microfilariae, which can be transmitted to the next individual during another blood meal. The microfilariae have a diurnal periodicity, appearing in the peripheral blood in the day time, and reach their maximum at around midday (~11:00 am to 1:00 pm).
L. loa as a public health problem
L. loa was a highly restricted filarial disease, found in some west African countries and in most central African countries, as shown on the map (Figure 1). However, due to population movement, cases of L. loa are now being reported worldwide. This filarial disease has had an impact on the WHO program on control of filarial disease by mass chemotherapy, using ivermectin and diethylcarbamazine (DEC), given that the administration of the drug in areas where this filarial disease is endemic may induce fatal side effects such as encephalitis.12 This side effect is usually observed in individuals with microfilariae up to 30,000 microfilariae/mL. This suggests that accurate diagnosis is required to evaluate the number of microfilariae in a given individual before drug administration. This task is difficult with large populations using microscopic counts and therefore a potential biomarker is needed to facilitate the evaluation of the number of microfilariae. Furthermore, accurate diagnosis will help assess the clinical outcome after treatment, mapping the spread of L. loa in a large region, as well as the assessment of control programs. In loiasis infection, the biomarkers evaluated so far can be classified as originating with the human host (antibodies) or parasites (circulating antigen, nucleic acid) (Figure 2).
Antibody detection of loiasis infections: current status
Many antibody classes have been evaluated for the detection of loiasis using many techniques such as electrosyneresis13 and enzyme-linked immunosorbent assay (ELISA).14 These approaches used either heterologous species15 or homologous species.16 In most of these cases, antibody detection does not distinguish active (currently infected with the parasite) from passive infection (having been infected in the past but infection has been cleared; antibodies remain present for long period of time). Furthermore, cross-reactivity among filarial antigens does not guarantee the specificity of these tests, resulting in misinterpretation of the results. As a consequence, it is difficult to assume that elevated antibodies are caused by loiasis in co-endemic areas between several filarial nematode species. However, it was noted that one specific subclass of immunoglobulin G4 (IgG4) was elevated in this infection and might be used as a marker of L. loa infection.16 This observation was extended to a large population with crude extract of microfilaria antigens.17 The use of crude adult or microfilaria antigen product with an elevated level of IgG4 as well as an elevated level of this subclass was noted in many filarial infections, suggesting that this phenomenon is a hallmark of filarial infection. In addition, it was shown that in loiasis 70% of infected people are amicrofilaremic,18 and the elevation-specific IgG4 is still present, indicating that stimulation of IgG4 is not necessarily linked to the presence of microfilariae. This observation indicates that a specific IgG4 test will detect more infected people than the microscopic detection of microfilaria. One limitation of this approach is the source of antigenic material, making it difficult to standardize the technique. A solution to overcome this situation was the use of a recombinant molecule of L. loa. The recombinant antigen called Ll-SXP-1 was used in an ELISA:19 the sensitivity of this assay was 56% and the specificity 98%. Use of this test for the detection IgG revealed that 20 out of 24 loiasis patients, one out of 20 patients with lymphatic filariae and four out of 20 patients infected with Onchocerca volvulus were also detected. An attempt to distinguish active from past infection using this test with IgG4 showed that four out of eight patients followed after treatment remained with IgG4. The luciferase immunoprecipitation system (LIPS),20 using the same Ll-SXP-1, was able to achieve 100% specificity against uninfected patients and 97% specificity with regard to other filarial infections when using its rapid format (QLIPS/IgG). With the same recombinant antigen, a lateral flow assay (LFA) was developed recently to detect antibodies with sensitivity up to 94% and specificity at 100%21 compared to nonendemic controls, and the specificity was 82%, 87%, and 88% compared to O. volvulus, Wuchereria bancrofti and Mansonella perstans, respectively. The latter test is about to go to point-of-care evaluation.
Circulating antigens for the detection of loiasis infection
An attempt was made to develop a circulating antigen test through a polyethylene glycol ELISA (PEG-ELISA).22 Although detection of infected individuals was shown, there was a cross-reaction with M. perstans-infected individuals and the nature of the antigen implicated in this immunocomplex was not determined. Another attempt was made after identification of a 38-kDa antigen of L. loa,23 by co-electrosyneresis, but the sensitivity seemed low: 24 out of 47 microfilaremic and 11 out of 13 amicrofilaremic individuals. Despite its success in other regions, this approach using antigen detection is limited in areas endemic for L. loa and in co-infected individuals. Antigenemia was claimed to be specific for W. bancrofti. However, this test was found to be cross-reactive with L. loa in the Democratic Republic of Congo24 and Cameroon25 where L. loa is endemic. This suggests great homology between lymphatic filaria and L. loa antigens. Despite the simplicity of the test,26 and the availability at the point of care, this approach is limited by cross-reactivity. This is an important issue because the presence of parasite antigen suggests a current or active infection with the presence of the specific parasite from which antigen is derived. Antigen quantitation is necessary in loiasis infection and requires a specific test because the numbers of blood microfilariae are the major cause of encephalitis in loiasis infection. An attempt to develop such a test was made with identification of quantifiable circulating biomarkers.35 Using the LOAG-16297 antigen LIPS assay, the sensitivity was 76.9% while the specificity reached 96% with a predictive positive value of 95% and a negative predictive value of 80%. Furthermore, a competitive LIPS assay using another marker (LOAG-17808) showed a sensitivity of 80.7%, a specificity of 37.5% and positive and negative predictive values of 59.4% and 64.3%, respectively. A significant correlation was shown between these two biomarkers and the circulating blood microfilaria examined under microscope.35
Use of nucleic acid as a biomarker in loiasis infection
Three approaches are being used:
- a classical amplification technique using the L. loa immunodominant gene for diagnosis of the infection.27 The main reports on this approach were based on the use of the 15-kDa L. loa gene:27 this gene presents different repeat sequences with some variability, which were used to define a specific species sequence in its region 3.28 Using this method they were able to detect more cases of L. loa infection. The performance of this assay was improved by a nested PCR, 29 which can detect more occult infections because this status is prevalent in loiasis infections;
- restriction fragment length polymorphism (RFLP)-PCR:30 in addition, since the quantification of microfilariae is crucial to avoid a fatal side effect caused by drug treatment, a quantitative PCR(qPCR) was developed.31 However, the logistics surrounding the use of PCR for diagnostic purposes require equipment such as a thermocycler as well as personnel trained in the technique for the visualization of the result (agarose gel, staining, etc.);
- detection of parasite DNA by loop-mediated isothermal amplification.32 This approach shows promise for diagnosis and quantification of microfilariae, essential to avoid the fatal side effect observed during the treatment of hypermicrofilaremic loiasis.32 The LOOP method uses eight primers that recognize a parasite’s DNA fragments and a Bst polymerase (enzyme) with strand displacement activity, under isothermal conditions. One set of such primers was designed using a L. loa repeat DNA sequence,33 and it showed very high specificity compared to other filarial parasites. The sensitivity of this technique is very high in detecting up to 5 Ag/mL of L. loa DNA. Although the source of the DNA is not clear. It is important to note that the filarial worm is a multicellular organism that contains nuclear DNA and mitochondrial DNA. Mitochondrial DNA is smaller and more susceptible to destruction, and its life span can be shorter than 1 h. Nuclear DNA is larger and more stable. One plausible explanation for the circulating DNA of this parasite in the body fluid may be the release of nuclear DNA by the parasite, in either adult worm or microfilaria form. This is why even an amicrofilaremic individual may be positive with the nucleic acid-based test. This presence of DNA also suggests the presence of the parasite in current infection. However, the field evaluation of this technique is still ongoing and its performance at the point of care has not yet been determined. To quantify microfilariae, a LOOP technique was developed with microfilaria-specific genes. This technique is able to distinguish high microfilaremic individuals and was improved by staining, which makes the reaction visible with the naked eye.34
Biomarkers in urine samples
Using urine is a noninvasive method that is easily accepted by patients. Recently, antigen-based assays have been developed using protein identified in urine by the combination of proteomic and bioinformatic techniques.35 These techniques use reverse LIPS to quantify microfilaria in a blood sample. This technique will be useful for microfilaremic individuals who need quantification before treatment. It is worth mentioning that this study specifically targets microfilariae, but the LIPS technique is also capable of detecting amicrofilaremic individuals. This is substantiated because protein isolated from urine may contain antigens that can be used as potential biomarkers,36 and the reaction of these antigens with human specific IgG4 is significantly elevated in L. loa-infected microfilaremic and amicrofilaremic individuals compared to those with M. perstans microfilariae alone. The identity of this antigen needs to be clarified.
Conclusion and perspectives for the use of biomarkers to detect L. loa infection
One general advantage of the biomarkers (antibodies, antigens, DNA) used now is the possibility of collecting samples for experimentation at any time of day. Although the technique using nucleic acid needs a supplementary challenging step with DNA extraction, loop-mediated isothermal amplification seems to be more promising because it allies the power of the amplification technique, the simplicity of isothermal amplification and a visual end result that is accessible to naïve individuals. However, the perspective of developing a rapid format of protein expressed at all stages of parasite development would be ideal for point-of-care diagnosis.
Disclosure
The authors report no conflicts of interest in this work.
References
Antinori S, Schifanella L, Million M, et al. Imported Loa loa filariasis: three cases and a review of cases reported in non-endemic countries in the past 25 years. Int J Infect Dis. 2012;16(9):e649–662. | ||
Ushirogawa H, Okino T, Hatsushika R, Tabuchi A. Morphological studies of the adult female Loa loa (Nematoda: Filarioidea) and a review of the literature on imported human loiasis in Japan. Kawasaki Med J. 2003;29(3–4):53–59. | ||
Yoshikawa M, Ouji Y, Hayashi N, et al. Diagnostic problems in a patient with amicrofilaremic Loa loa. J Travel Med. 2008;15(1):53–57. | ||
Klion AD, Massougbodji A, Sadeler BC, et al. Loiasis in endemic and nonendemic populations: immunologically mediated differences in clinical presentation. J Infect Dis. 1991;163(6):1318–1325. | ||
Nutman TB, Miller KD, Mulligan M, Ottesen EA. Loa loa infection in temporary residents of endemic regions: recognition of a hyperresponsive syndrome with characteristic clinical manifestations. J Infect Dis. 1986;154(1):10–18. | ||
Moussala M, Fobi G, Ongolo Zogo P, Bella Hiag LA, Bengono G, McMoli TE. Retinal hemorrhages after ivermectin treatment for onchocerciasis in a patient with Loa loa microfilaremia. J Fr Ophtalmol. 2004;27(1):63–66. | ||
Boussinesq M, Gardon J, Gardon-Wendel N, Kamgno J, Ngoumou P, Chippaux JP. Three probable cases of Loa loa encephalopathy following ivermectin treatment for onchocerciasis. Am J Trop Med Hyg. 1998;58(4):461–469. | ||
Gardon J, Gardon-Wendel N, Demanga-Ngangue, Kamgno J, Chippaux JP, Boussinesq M. Serious reactions after mass treatment of onchocerciasis with ivermectin in an area endemic for Loa loa infection. Lancet. 1997;350(9070):18–22. | ||
Lukiana T, Mandina M, Situakibanza NH, et al. A possible case of spontaneous Loa loa encephalopathy associated with a glomerulopathy. Filaria J. 2006;5:6. | ||
Metzger WG, Mordmüller B. Loa loa—does it deserve to be neglected? Lancet Infect Dis. 2014;14(4):353–357. | ||
Chesnais CB, Takougang I, Paguele M, Pion SD, Boussinesq M. Excess mortality associated with loiasis: a retrospective population-based cohort study. Lancet Infect Dis. 2017;17(1):108–116. | ||
Ducorps M, Gardon-Wendel N, Ranque S, et al. Secondary effects of the treatment of hypermicrofilaremic loiasis using ivermectin. Bull Soc Pathol Exot. 1995;88(3):105–112. | ||
Walker-Deemin A, Kombila M, Mouray H, et al. Detection of circulating antigens in Gabonese patients with Loa loa filariasis. Trop Med Int Health. 1996;1(6):772–778. | ||
Goussard B, Ivanoff B, Frost E, Garin Y, Bourderio C. Age of appearance of IgG, IgM, and IgE antibodies specific for Loa loa in Gabonese children. Microbiol Immunol. 1984;28(7):787–792. | ||
Kobayashi T, Hayakawa K, Mawatari M, et al. Loiasis in a Japanese traveler returning from Central Africa. Trop Med Health. 2015;43(2):149–153. | ||
Akue JP, Egwang TG, Devaney E. High levels of parasite-specific IgG4 in the absence of microfilaremia in Loa loa infection. Trop Med Parasitol. 1994;45(3):246–248. | ||
Toure FS, Egwang TG, Millet P, et al. IgG4 serology of loiasis in three villages in an endemic area of south-eastern Gabon. Trop Med Int Health. 1998;3(4):313–317. | ||
Dupont A, Zue-N’dong J, Pinder M. Common occurrence of amicrofilaraemic Loa loa filariasis within the endemic region. Trans R Soc Trop Med Hyg. 1988;82(5):730. | ||
Klion AD, Vijaykumar A, Oei T, Martin B, Nutman TB. Serum immunoglobulin G4 antibodies to the recombinant antigen, Ll-SXP-1, are highly specific for Loa loa infection. J Infect Dis. 2003;187(1):128–133. | ||
Burbelo PD, Ramanathan R, Klion AD, Iadarola MJ, Nutman TB. Rapid, novel, specific, high-throughput assay for diagnosis of Loa loa infection. J Clin Microbiol. 2008;46(7):2298–2304. | ||
Pedram B, Pasquetto V, Drame PM, et al. A novel rapid test for detecting antibody responses to Loa loa infections. PLoS Negl Trop Dis. 2017;11(7):e0005741. | ||
Jaoko WG. Loa loa antigen detection by ELISA: a new approach to diagnosis. East Afr Med J. 1995;72(3):176–179. | ||
Walker-Deemin A, Ferrer A, Gauthier F, et al. Identification and specificity of a 38 kDa Loa loa antigenic fraction in sera from high-microfilaraemic Gabonese patients. Parasitol Res. 2004;92(2):128–132. | ||
Bakajika DK, Nigo MM, Lotsima JP, et al. Filarial antigenemia and Loa loa night blood microfilaremia in an area without bancroftian filariasis in the Democratic Republic of Congo. Am J Trop Med Hyg. 2014;91(6):1142–1148. | ||
Wanji S, Amvongo-Adjia N, Koudou B, et al. Cross-reactivity of filariais ICT cards in areas of contrasting endemicity of Loa loa and Mansonella perstans in Cameroon: implications for shrinking of the lymphatic filariasis map in the Central African Region. PLoS Negl Trop Dis. 2015;9(11):e0004184. | ||
Samuel W, Amvongo-Adjia N, Njouendou AJ, et al. Further evidence of the cross-reactivity of the Binax NOW® Filariasis ICT cards to non-Wuchereria bancrofti filariae: experimental studies with Loa loa and Onchocerca ochengi. Parasit Vectors. 2016;9:217. | ||
Ajuh PM, Akue JP, Boutin P, Everaere S, Egwang TG. Loa loa: structural diversity of a 15-kDa repetitive antigen. Exp Parasitol. 1995;81(2):145–153. | ||
Toure FS, Bain O, Nerrienet E, et al. Detection of Loa loa-specific DNA in blood from occult-infected individuals. Exp Parasitol. 1997;86(3):163–170. | ||
Toure FS, Mavoungou E, Kassambara L, et al. Human occult loiasis: field evaluation of a nested polymerase chain reaction assay for the detection of occult infection. Trop Med Int Health. 1998;3(6):505–511. | ||
Jiménez M, González LM, Carranza C, et al. Detection and discrimination of Loa loa, Mansonella perstans and Wuchereria bancrofti by PCR-RFLP and nested-PCR of ribosomal DNA ITS1 region. Exp Parasitol. 2011;127(1):282–286. | ||
Fink DL, Kamgno J, Nutman TB. Rapid molecular assays for specific detection and quantitation of Loa loa microfilaremia. PLoS Negl Trop Dis. 2011;5(8):e1299. | ||
Notomi T, Okayama H, Masubuchi H, et al. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000;28(12):E63. | ||
Fernández-Soto P, Mvoulouga PO, Akue JP, et al. Development of a highly sensitive loop-mediated isothermal amplification (LAMP) method for the detection of Loa loa. PLoS One. 2014;9(4):e94664. | ||
Drame PM, Fink DL, Kamgno J, Herrick JA, Nutman TB. Loop-mediated isothermal amplification for rapid and semi-quantitative detection of Loa loa infection. J Clin Microbiol. 2014;52(6):2071–2077. | ||
Drame PM, Meng Z, Bennuru S, Herrick JA, Veenstra TD, Nutman TB. Identification and validation of Loa loa microfilaria-specific biomarkers: a rational design approach using proteomics and novel immunoassays. MBio. 2016;7(1):e02132–15. | ||
Rush EAE. Analyse des complexes immuns circulants et des protéines urinaires des personnes infectées par Loa loa à l’aide des sous classes d’immunoglobuline G1 et G4. [Analysis of circulating immune complexes and urinary proteins from Loa loa infected individuals using subclasses of immunoglobulin G1 and G4]. [master’s thesis]. Burkina Faso: Université de Ouagadougou; 2015. | ||
Sayre R, Comer P, Hak J, et al. A New Map of Standardized Terrestrial Ecosystems of Africa. NatureServe. Washington, DC: Association of American Geographers; 2013. Available from: http://www.natureserve.org/sites/default/files/africa_map.gif. Accessed February 22, 2018. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.