Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Lithium-Associated Generalized Ostraceous Psoriasis Treated with Adalimumab: A Case Report
Received 11 February 2023
Accepted for publication 31 March 2023
Published 6 April 2023 Volume 2023:16 Pages 947—950
DOI https://doi.org/10.2147/CCID.S408245
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Yao Yang,1,2 Xiaoyan Lyu1,2
1Department of Dermatology, West China Hospital, Sichuan University, Chengdu, People’s Republic of China; 2Laboratory of Dermatology, Clinical Institute of Inflammation and Immunology, Frontiers Science Center for Disease-Related Molecular Network, West China Hospital, Sichuan University, Chengdu, People’s Republic of China
Correspondence: Xiaoyan Lyu, West China Hospital, Sichuan University, #37 Guoxue Alley, Wuhou District, Chengdu, Sichuan, 610041, People’s Republic of China, Tel +86 18980601897, Fax +86-028-85422560, Email [email protected]
Abstract: Ostraceous psoriasis is a rare variant of psoriasis, characterized by severe hyperkeratotic lesions resembling an oyster shell. Adalimumab is a biological agent that antagonizes tumor necrosis factor (TNF) and is used clinically in plaque psoriasis. Some medications such as lithium carbonate (LC) may aggravate or trigger psoriasis. Here, we describe a case of generalized ostraceous psoriasis associated with lithium carbonate, whose lesions completely improved after discontinuing lithium carbonate and using adalimumab.
Keywords: ostraceous psoriasis, adalimumab, lithium carbonate
Introduction
The term ostraceous psoriasis was first published by Deutsch as “atypical psoriasis” in 1898.1 It is a rare variant of psoriasis characterized by severe hyperkeratotic lesions resembling an oyster shell.1,2 There are many factors that can trigger and exacerbate psoriasis, such as infections and medications.3 Some drugs can induce psoriasis or exacerbate pre-existing psoriasis, such as beta-blockers, lithium, synthetic antimalarials, non-steroidal anti-inflammatory drugs and tetracyclines.3,4 Adalimumab is a monoclonal anti-tumor necrosis factor (TNF) that blocks the interaction with p55 on epithelial cells and p75 on the endothelial cells, thereby suppressing the TNF-mediated inflammation.5 Here, we describe a case of generalized ostraceous psoriasis in a patient associated with lithium carbonate (LC) who experienced complete improvement in lesions after discontinuation of LC and use of adalimumab.
Case Presentation
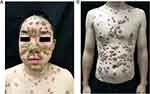
An 18-year-old male came to our hospital with a generalized hyperkeratotic lesion associated with pruritus for 12 months, which was exaggerated for 4 months. Physical examination revealed generalized firmly demarcated erythematous circular plaques covered with thick adherent greasy yellowish scales resembling oyster shells over his scalp, face, trunk, upper and lower extremities, groin, and buttocks (Figure 1A and B). The baseline psoriasis activity severity index (PASI) and body surface area (BSA) scores were 22.8% and 14% respectively. The patient had a history of bipolar disorder for 1.5 years for which he was taking LC. After the dose of LC increased, his condition significantly deteriorated. He denied any infectious disease or arthritis. The patient was treated in the local hospital for neurodermatitis, for which topical steroids were given with no improvement.
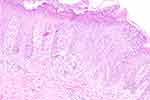
Tuberculosis, HIV, hepatitis, syphilis and other related laboratory screenings showed no obvious abnormalities, except for elevation of C-reactive protein levels (12.00 mg/L). Histopathology revealed psoriasiform hyperplasia of the epidermis with confluent parakeratosis, neutrophils infiltration in the stratum corneum, and a moderate amount of lymphocytes around small vessels in the superficial dermis (Figure 2).
The patient was started on adalimumab subcutaneous injection of 80 mg initial dose then 40 mg every 2 weeks. On the 9-week follow-up, a small improvement was seen. With his history of taking LC, which can exacerbate psoriasis, we advised him to stop taking it under the guidance of a psychiatrist. He gradually stopped taking LC and switched to olanzapine tablets and magnesium valproate after consulting a psychiatrist. After 18 weeks of treatment and stopping of LC, there was a marked improvement (Figure 3A and B). On reassessment, his PASI and BSA scores were 0.3% and 0.5%, respectively.
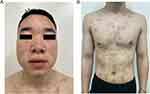 |
Figure 3 The skin lesions on the patient’s face (A), trunk, and limbs (B) have improved significantly after 18 weeks of treatment. |
Discussion
Psoriasis is an immune-mediated chronic inflammatory disease that primarily involves the skin and joints. The clinical classification is based on the morphologic evaluation or the anatomical location includes plaque psoriasis, drippy psoriasis, erythrodermic psoriasis, psoriatic arthritis and other types.6 Psoriasis may present as intensely hyperkeratotic lesions that include ostraceous, rupioid, and elephantine forms.2,7 The ostraceous psoriasis is typically characterized by lesions with firm adherent thick scales and a surface resembling an oyster shell, while the rupioid form is characterized by hyperkeratotic cone-shaped lesions.7 Most hyperkeratotic psoriasis including the ostraceous variety is resistant to topical therapy, while systemic drugs, such as acitretin, cyclosporine, methotrexate, and biologics have been tried with varying results.7 Adalimumab is a monoclonal antibody against TNF that has been widely used in the treatment of moderate-to-severe chronic plaque psoriasis in adults, especially in patients with psoriatic arthritis.5 A case showed complete resolution of lesions within 14 weeks after the addition of adalimumab to treat a case of ostraceous psoriasis.8 In addition, there are various factors that can trigger or exacerbate psoriasis, of which drug ingestion is an important one.3 The most common drugs that induce or exacerbate psoriasis are beta-blockers, lithium, synthetic antimalarials, non-steroidal anti-inflammatory drugs and tetracyclines.3,4 In addition to medication, emotional stress can also exacerbate many chronic skin diseases, including psoriasis, urticaria and eczema, which are considered psychodermatological disorders.9 Majority of psychodermatological disorders can be treated with cognitive-behavioral psychotherapy, psychotherapeutic stress-and-anxiety-management techniques and psychotropic drugs.9 There are also some cases that have reported some biological agents, such as Brodalumab and Guselkumab have achieved good results in the treatment of patients with psoriasis combined with psychiatric comorbidities.10,11 Our patient’s condition deteriorated significantly after the dose of lithium carbonate was increased. Also, there was no significant improvement in the first 9 weeks of treatment, and only after stopping lithium carbonate at the later stage did his lesions improve significantly. The etiology of psoriasis is complex and may be influenced by many factors. The influence of bipolar disorder cannot be completely ruled out in this patient, but lithium carbonate is more likely to be related in this case.
Conclusion
Although one case cannot provide adequate evidence, this is a rare variant of psoriasis that highlights the dramatic improvement with adalimumab and discontinuation of lithium. We believe it will help physicians to choose the right treatment and adalimumab could be a good choice. It also alerts physicians to pay attention to the medication history of psoriasis patients, especially lithium carbonate.
Ethics Approval and Consent for Publication
This case report has been performed in accordance with the principles stated in the Declaration of Helsinki. A written informed consent, provided by the Department of Dermatology and Venereology, West China Hospital, Sichuan University for publication of this case report and including photography and medical information, was signed by the patient. Institutional approval was not required to publish the case details.
Acknowledgment
The authors would like to thank the patient recruited for participation in this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
No specific funding was received from any bodies in the public, commercial or not-for-profit sectors to carry out the work described in this article.
Disclosure
The authors have declared no conflicts of interest in this work.
References
1. Sérgio M. Psoríase ostrácea: relato de caso = Ostraceous psoriasis: case report. An Bras Dermatol. 2002;2002:207–210.
2. Estrada BD, Azevedo PM, Tamler C, et al. Dermatologia comparativa: psoríase hiperceratósica. Comparative dermatology: hyperkeratotic psoriasis. An Bras Dermatol. 2007;82(4):369–371. doi:10.1590/s0365-05962007000400013
3. Basavaraj KH, Ashok NM, Rashmi R, et al. The role of drugs in the induction and/or exacerbation of psoriasis. Int J Dermatol. 2010;49(12):1351–1361. doi:10.1111/j.1365-4632.2010.04570.x
4. Jafferany M. Lithium and skin: dermatologic manifestations of lithium therapy. Review. Int J Dermatol. 2008;47(11):1101–1111. doi:10.1111/j.1365-4632.2008.03873.x
5. Chiricozzi A, Zangrilli A, Bavetta M, et al. Real-life 9-year experience with Adalimumab in psoriasis and psoriatic arthritis: results of a single-centre, retrospective study. J Eur Acad Dermatol Venereol. 2017;31(2):304–311. doi:10.1111/jdv.13771
6. Raychaudhuri SK, Maverakis E, Raychaudhuri SP. Diagnosis and classification of psoriasis. Autoimmun Rev. 2014;13(4–5):490–495. doi:10.1016/j.autrev.2014.01.008
7. Ip KH, Cheng HS, Oliver FG. Rupioid Psoriasis. JAMA Dermatol. 2021;157(7):859. doi:10.1001/jamadermatol.2021.0451
8. Arias-Santiago SA, Naranjo-Sintes R. Generalized ostraceous psoriasis. N Engl J Med. 2010;362(2):155. doi:10.1056/NEJMicm0810475
9. Situm M, Kolic M, Buljan M. PSYCHODERMATOLOGY.; Review. Acta Clin Croat. 2016;70(Suppl 1):35–38.
10. Rivera-Oyola R, Stanger R, Litchman GH, et al. The use of brodalumab in three patients with psoriasis and psychiatric comorbidities. J Clin Aesthet Dermatol. 2020;13(12):44–48.
11. Bernardini N, Skroza N, Prevete E, et al. Guselkumab for the treatment of severe plaque psoriasis in a schizophrenia patient. Dermatol Rep. 2022;14(4):9476. doi:10.4081/dr.2022.9476
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.